Abstract
Antenatal steroid administration is associated with alterations in fetal kidney development and hypertension. However, a causal relationship between nephron deficit and hypertension has not been established. In this study, we measured nephron number, renal function, and blood pressure in sheep exposed antenataly to betamethasone. Pregnant sheep were given 2 betamethasone doses (0.17 mg/kg) or vehicle at 80 and 81 days gestational age and allowed to deliver at term. Data were obtained from a fetal cohort and 2 adult cohorts and were analyzed by analysis of variance (ANOVA) and/or 2 sample t test. Antenatal betamethasone induced a 26% reduction in the number of nephrons in both males and females in the absence of intrauterine growth restriction and/or prematurity. Adult males presented a reduction in glomerular filtration rate (GFR; 132 ± 12.7 vs 114 ± 7.0 mL/min, P < .05). Betamethasone administration was also associated with an increase in arterial blood pressure of similar magnitude in male (mean arterial pressure [MAP] in mm Hg; 98 ± 2.7 vs 105 ± 2.4) and female (96 ± 1.9 vs 105 ± 2.4) adult sheep and the increase in blood pressure preceded the decrease in GFR in the males. Furthermore, we found no significant association between the magnitude of the decrease in nephron number and the magnitude of the increase in arterial blood pressure. Our data thus support the conclusion that exposure to glucocorticoids at a time of rapid kidney growth is associated with an elevation in blood pressure that does not appear related solely to the reduction in nephron number.
Keywords: antenatal steroids, nephron endowment, hypertension, glomerular filtration rate, betamethasone, clinical doses, sheep
Antenatal steroid administration for accelerating fetal lung maturation has been in use for over 30 years1; however, controversy still exists regarding potential long-term side effects.2–4 Antenatal steroid exposure is associated with alterations in fetal kidney development, in particular a reduction in nephron number and the development of hypertension.5,6 Animal studies have also linked a reduction in nephron number with elevations in arterial blood pressure.7–13 Although a causal relationship between nephron deficit and hypertension has not been definitively established, one of the mechanisms thought to be involved is an effect on single nephron hyperfiltration caused by the reduction in nephron number.9 Nephrons can be observed as early as 14 weeks in the human fetus, with peak nephrogenesis occurring at about 24 weeks (0.6 of gestation) and ending at 32 weeks (0.8 of gestation).14 Very similar to humans, sheep nephrogenesis is an early fetal event, beginning by 30 days of gestational age (dGA), peaking at 0.6 gestation (80 dGA) and ending at 0.8 of gestation.15 These similarities in renal development prompted us to evaluate the effects of antenatal exposure to glucocorticoids at a gestational age and dose regime equivalent to that currently used in clinical practice. We recently reported that a single course of betamethasone induced a 25% decrease in nephron number in late gestation fetuses and an elevation in arterial blood pressure in the 6-month-old offspring.16,17 The aim of the current study was to assess the importance of the decrease in nephron number in the development of hypertension, following exposure to glucocorticoids during peak nephrogenesis. We measured nephron number, indices of renal function, and blood pressure in sheep exposed antenatally to betamethasone and evaluated the existence of an association between these variables. Data were obtained from 3 different groups of animals treated with either vehicle or betamethasone; a fetal cohort in which we only evaluated nephron number, a cohort ranging in age from 6 to 32 months in which we evaluated indices of renal function, and an adult cohort in which we measured blood pressure, indices of renal function, and number of nephrons within a 2-week period.
MATERIALS AND METHODS
Mixed Western breed sheep were mated during the reproductive season during spontaneous estrus. Pregnant sheep received 2 doses of 0.17 mg/kg of a 1:1 mixture of betamethasone acetate and betamethasone phosphate (Celestone Soluspan). The total dose never exceeded 12 mg/d, which is equivalent to the dose a 70-kg pregnant women would receive. Intramuscular injections were given 24 hours apart at 80 and 81 days of gestation (term gestation is 145 days). Offspring were studied as either part of the fetal cohort (control n = 21, betamethasone n = 14), the renal function cohort (control n = 23, betamethasone n = 20), or the blood pressure cohort (control n = 24, betamethasone n = 18). Pregnant sheep were maintained with free access to food and water in open pasture. To obtain fetal tissues, sheep were brought to the laboratory at 130 days gestation for cesarean section delivery of the fetus at 135 days gestation. For the adult studies, lambs were weaned at 3 months of age after spontaneous term delivery, placed in sex-specific enclosures and brought to the laboratory at different times starting at 6 months of age. In all adults, a nonocclusive femoral artery and vein catheters were placed under general isofluorane anesthesia using strict aseptic procedures as previously described.16 Sheep were housed in a study cart into which they were placed after the surgical procedure and experiments were conducted at least 5 days after surgery. All procedures were approved by the Institutional ACUC (Animal Care and Use Committee).
Determination of Nephron Number
In the fetal cohort, fetuses were delivered by cesarean section performed under general anesthesia and euthanized with 4 mL of Euthasol, dried, and body weight obtained before harvesting the kidneys. In the adult cohort, sheep were euthanized by exsanguination under general inhalation anesthesia and the kidneys removed. Kidneys were weighed, and the caudal half (adult) or the entire right kidney (fetus) was used to count the number of glomeruli. Nephron counting was performed in a blinded fashion using the acid maceration method.16 Briefly, the right kidney was weighed and minced, and two 0.8 g aliquots were digested at room temperature in 10 mL of 8 N HCl for 2 hours in a rocking platform. From each aliquot, fifteen 10 μL samples were placed on a grided microscope slide and glomeruli counted under phase contrast. In addition, in a subset of the adults, glomerular density, and diameter were evaluated on 5 μm sections of kidney tissues from a transverse formalin-fixed specimen obtained at the level of the kidney hilum. Glomerular density and diameter were measured on digital 20× microphotographs of hematoxylin and eosin-stained kidney sections using Sigma Scan (Systat Software Inc, New Jersey). The number of glomeruli within the measuring grid on a random area of the specimen was used to determine the density, and the average of the 2 major axes on each glomerulus was used to determine diameter. The number of glomeruli examined for each animal ranged from 60 to 100.
Blood Pressure recording
The arterial catheter and an open tip catheter filled with saline were connected to pressure transducers (World Precision Instruments, Sarasota, Florida). Pressure was recorded continuously for a minimum of 2 days using Windaq Pro+ data acquisition software (DATAQ Instruments, Inc, Akron, Ohio). The output of the amplifiers was sampled at 150 Hz, digitized, and stored on the hard disk of a desktop computer. Pressure recorded from the open tip catheter was used as a reference to subtract pressure changes related to changes in the animal’s position. One minute averages of heart rate, systolic, mean, and diastolic pressure were calculated using custom designed software (Cruncher, Instrument Concepts Inc., Debert, Nova Scotia, Canada). The variable used for statistical purposes was the computed mean of 2400 1-minute pressure averages over 2 days.
Inulin and Para aminohippuric Acid Clearance
Renal function was evaluated by measuring glomerular filtration rate and effective renal plasma flow (ERPF) using inulin and para aminohippuric acid (PAH), respectively (Sigma Chemical, St Louis, Missouri). A bolus of 850 mg inulin and 225 mg PAH was followed by a 10 mg/min and 11 mg/min continuous infusion for 3 hours, respectively. Samples of arterial blood were obtained after 80-min equilibrium time; clearances were calculated using the average of 4 samples taken 15 minutes apart.16
Statistical Analysis
Data are expressed as mean ± SEM and were analyzed by either 2-way ANOVA or 2 sample t test.
RESULTS
Fetal Studies
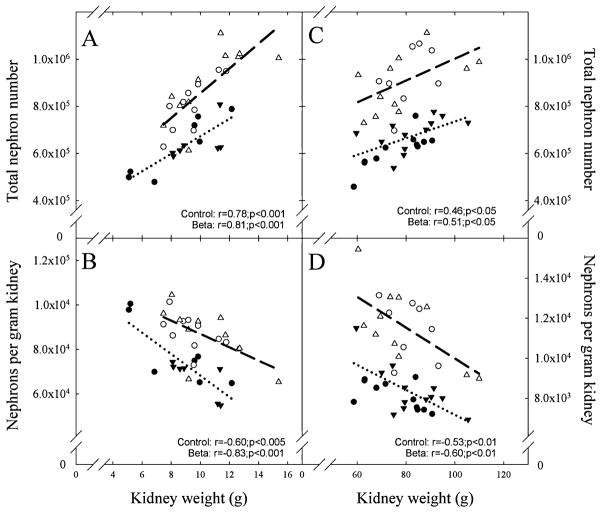
Betamethasone administration at 80 dGA reduced glomerular number by approximately 25% at 135 dGA in both male and female fetuses (Table 1). The decrease in nephron number occurred without a significant decrease in either fetal or kidney weight. A statistically significant correlation between the kidney weight and the total number of glomeruli, which was positive, and between kidney weight and glomeruli per gram of kidney weight that was negative was observed in both the control and the steroid exposed groups (Figure 1, panels A and B). Similarly, kidney weight (r = .7, P < .001; r = .8, P < .001) and nephrons per gram of kidney (r = −.7, P < .01; r = −.9, P < .001) were highly correlated to body weight in control and betamethasone groups, respectively. In contrast, total nephron number was not associated with fetal body weight in either group.
Table 1.
Fetal Number, Body Weight, Kidney Weight, and Number of Glomeruli in 135 Days Gestational Age Fetal Sheep Treated With Either Vehicle or Betamethasone at 80 Days Gestational Age
| N | Singleton | Twins | Body Weight (kg) | Kidney Weight (g) | NGlom | ||
|---|---|---|---|---|---|---|---|
| Male | BC | 11 | 1 | 10 | 3.9 ± 0.22 | 10.6 ± 0.73 | 896814 ± 45772 |
| B | 7 | 2 | 5 | 4.0 ± 0.13 | 9.6 ± 0.36 | 767330 ± 27715a | |
| Female | BC | 10 | 0 | 10 | 3.9 ± 0.11 | 9.3 ± 0.45 | 808140 ± 34634 |
| B | 7 | 1 | 6 | 3.4 ± 0.35 | 8.4 ± 1.01 | 630616 ± 49019a |
Abbreviations: BC, betamethasone control; B, betamethasone 0.17 mg/kg maternal weight.
P < .05 by t test within sex group.
Figure 1.
Total nephron number (A and C) and nephron number per gram of kidney (B and D) in fetal and adult male and female sheep exposed antenataly to either vehicle (○ males; fetus n = 11, adult n = 11, △ females fetus n = 10, adult n = 8) or betamethasone (● males fetus n = 7, adult n = 12, ▲ females fetus n = 7, adult n = 12) at 80 days of gestation. A significant correlation between kidney weight and either total nephron number or nephrons per gram of kidney was present in both treatment groups in the fetal and adult cohorts.
Adult Studies
Inulin and PAH clearance
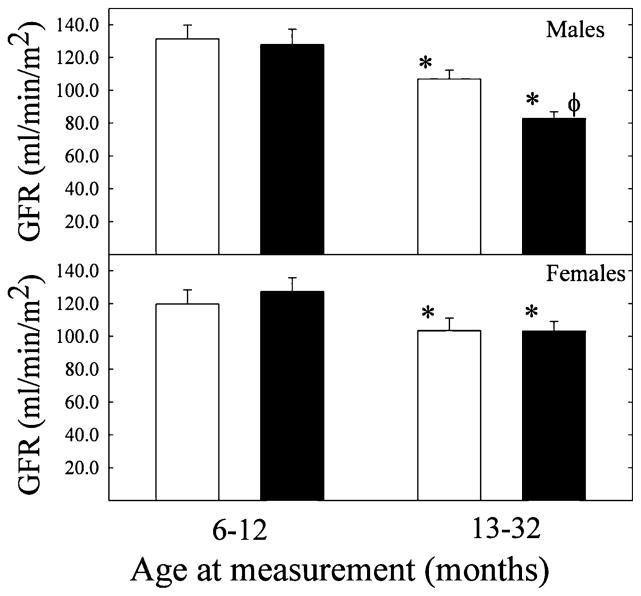
To account for sex and age differences in weight, clearance data were normalized for body surface area. Glomerular filtration rate was not significantly different between control and steroid exposed sheep by 1 year of age (Figure 2). In older animals, GFR was significantly lower compared to sheep up to a year of age, in control and steroid-exposed groups of both sexes (Figure 2). In addition, in this older group, a significant betamethasone effect was observed only in males (Figure 2). Similarly, in the cohort in which we have contemporaneous measurements of blood pressure, GFR and nephron number at an average age of 17 months, a significant decrease in GFR from 132.0 ± 12.7 to 114.0 ± 7.0 mL/min was present only in the male betamethasone-exposed group (Table 2). No sex, age, or treatment differences were observed in ERPF (data not shown).
Figure 2.
Normalized glomerular filtration rate (GFR) in male (top panel) and female (lower panel) adult sheep exposed antenataly to either vehicle (□) or betamethasone (■) at 80 days of gestation. No significant decrease in GFR was observed in animals younger than 12 months. ★Significant age effect P < .05 by ANOVA; φ significant treatment effect P < .05 by ANOVA. [Control: n = 12 and n = 17 in young and old males and n = 10 and n = 11 in young and old females; betamethasone n = 10 and n = 10 in young and old males, n = 12 and n = 14 in young and old females].
Table 2.
Birth Weight, Body Weight, Number of Fetuses, Kidney Weight, and Number of Glomeruli in Adult Sheep Treated With Either Vehicle or Betamethasone at 80 Days Gestational Age
| N | Birth Weight (Kg) | Singleton | Twins | Age (months) | Body Weight (kg) | Kidney Weight (g) | GFR (ml/min) | NGlom | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | BC | 15 | 5.0 ± 0.28 | 4 | 11 | 17.0 ± 2.1 | 58.0 ± 4.4 | 84.0 ± 4.9 | 132 ± 12.7 | 896612 ± 36808 |
| B | 14 | 4.5 ± 0.27 | 5 | 9 | 17.0 ± 1.5 | 61.0 ± 2.3 | 83.0 ± 3.7 | 114 ± 7.0a | 681669 ± 20339a | |
| Female | BC | 13 | 4.9 ± 0.32 | 5 | 8 | 18.0 ± 2.0 | 64.0 ± 5.1 | 77.0 ± 2.9 | 134 ± 9.2 | 923010 ± 44548 |
| B | 13 | 4.7 ± 0.32 | 2 | 11 | 16.0 ± 1.0 | 60.0 ± 2.8 | 76.0 ± 3.2 | 141 ± 6.9 | 640026 ± 31966a |
Abbreviations: BC, betamethasone control; B, betamethasone 0.17 mg/kg maternal weight; GFR, glomerular filtration rate.
P < .05 by t test within sex group.
Blood pressure, GFR, and nephron number
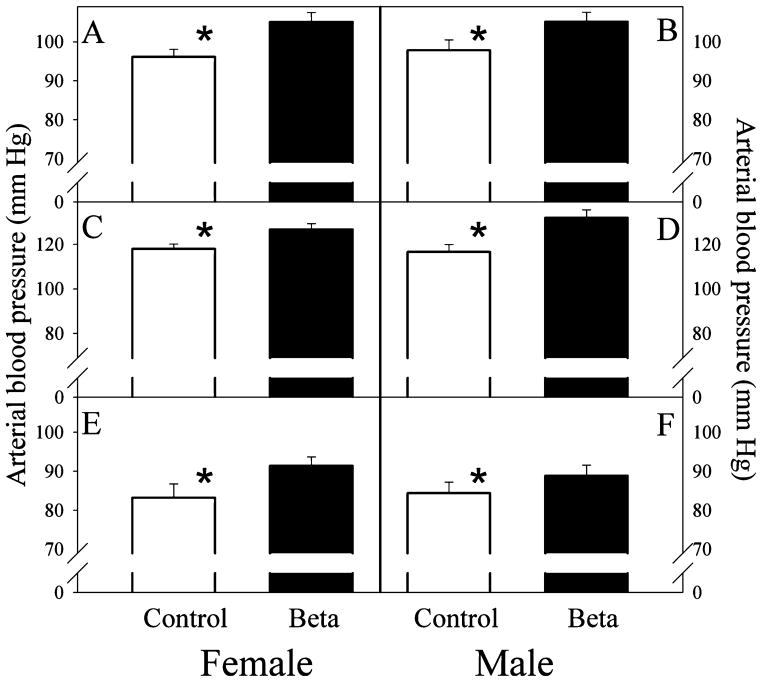
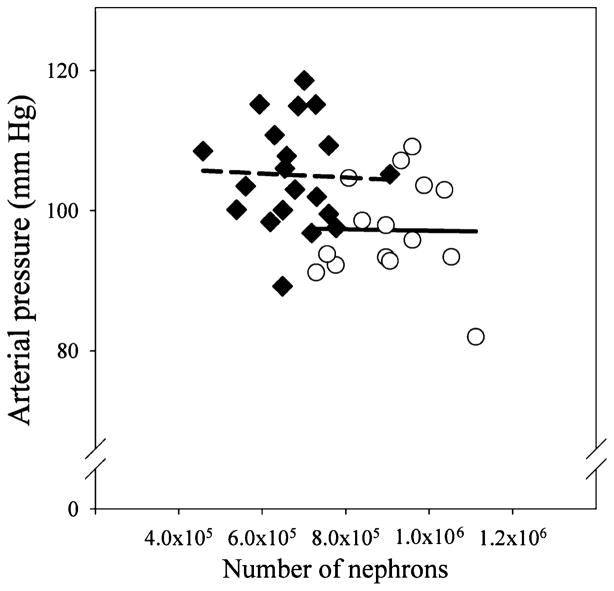
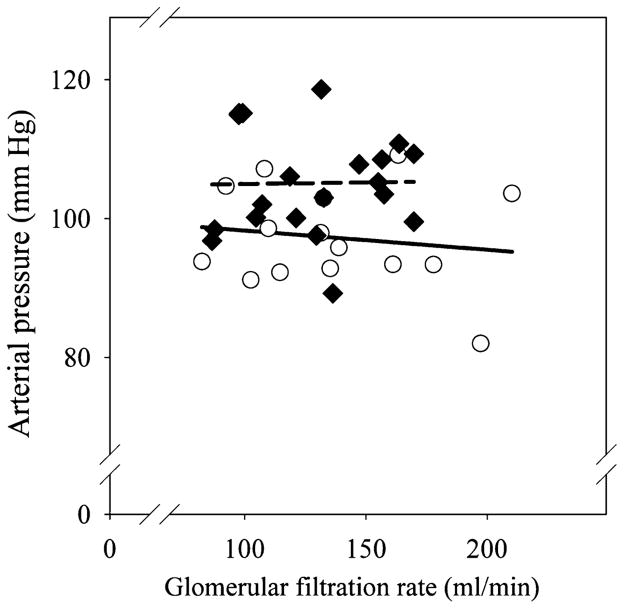
Measurements of arterial blood pressure, GFR, and nephron number were obtained at similar ages in both groups (females 16.0 ± 1.0 vs 18.0 ± 2.0; males 17.0 ± 2.1 vs 17.0 ± 1.5; age in months betamethasone vs control). We did not observe sex or betamethasone treatment effects on birth weight and on kidney or body weights at the time of tissue harvest (Table 2). The number of glomeruli in the adult kidney was significantly reduced in both male and female sheep exposed to antenatal betamethasone (Table 2). Both the absolute number as well as the magnitude of the decrease (~25%) were in the same range as those found in the fetal cohort at 135 dGA. We found no differences in glomerular density or diameter between the 2 groups; density in arbitrary units 23.0 ± 1.0 vs 22.0 ± 1.6 and diameter in μm 148.0 ± 2.4 vs 145.0 ± 2.5 for control (n = 8) and betamethasone, respectively (n = 9). Similar to the findings in the fetal cohort, the weight of the adult kidney also exhibited a statistically significant correlation with either the total number of glomeruli (positive) or glomeruli per gram of kidney weight (negative) in both the control and the steroid exposed groups (Figure 1, panels C and D). Total nephron number was not associated with birth weight (data not shown). In contrast to the fetal cohort, adult kidney weight was not correlated to either birth or adult body weight. As shown in Figure 3, there was no trend for a progressive decrease in nephron number with advancing age in either the control or the betamethasone-treated groups. Arterial blood pressure was significantly elevated in both female and male adult sheep exposed antenataly to betamethasone (Figure 4). As in the case of nephron number, there were no sex differences in either the absolute value or the magnitude of the betamethasone effect in arterial blood pressure. Figure 5 shows a scatter plot of the individual values of mean arterial blood pressure as a function of total nephron number in male and female sheep. There was no statistically significant association between the number of nephrons and the arterial blood (mean, systolic or diastolic) pressure in either the control or the steroid exposed groups. Similarly, there was no statistically significant association between GFR and arterial blood pressure in either the control or the steroid-exposed group (Figure 6) and ERPF (data not shown).
Figure 3.
Total nephron number plotted against age at which the tissue was obtained in fetal and adult sheep of both sexes exposed antenataly to either vehicle (○) or betamethasone (◆) at 80 days of gestation. No systematic trend in the number of glomeruli with advancing age was present.
Figure 4.
Arterial blood pressure in adult sheep of both sexes exposed antenataly to either vehicle (□; n = 5 females, n = 10 males) or betamethasone (■; n = 8 females, n = 12 males) at 80 days of gestation. Panels A and B; mean arterial pressure, Panels C and D; systolic arterial pressure and Panels E and F; diastolic arterial blood pressure. ★P < .05 treatment effect by analysis of variance (ANOVA).
Figure 5.
Mean arterial blood pressure in adult sheep of both sexes exposed antenataly to either vehicle (○) or betamethasone (◆) at 80 days of gestation. No systematic trend in the number of glomeruli with advancing age was present.
Figure 6.
Mean arterial blood pressure versus glomerular filtration rate (GFR) in adult sheep of both sexes exposed antenataly to either vehicle (○) or betamethasone (◆) at 80 days of gestation. No significant correlation was present between arterial pressure and GFR.
DISCUSSION
The purpose of the current study was to assess the importance of the decrease in nephron number by evaluating the relationship between nephron number, blood pressure, and indices of renal function in adult sheep that were exposed to betamethasone before birth. We found that antenatal administration of clinically relevant doses of betamethasone during peak nephrogenesis induces a reduction in the number of nephrons in both males and females in the absence of intrauterine growth restriction and/or prematurity. In adult males, but not females, the reduction in nephron number was accompanied by a reduction in GFR. We also demonstrated that betamethasone administration was associated with an increase in arterial blood pressure of similar magnitude in male and female adult sheep and the increase in blood pressure precedes any decrease in GFR in the males. Furthermore, we found no significant association between the magnitude of the decrease in nephron number and the magnitude of the increase in arterial blood pressure. Our data thus support the conclusion that exposure to glucocorticoids at a time of rapid kidney growth is associated with an elevation in blood pressure that does not appear related solely to the reduction in nephron number.
In sheep as in humans, nephrogenesis takes place during intrauterine life and is complete well before normal term delivery. We administered betamethasone to fetal sheep at the time of peak nephrogenesis that would be comparable to a gestational age of about 24 weeks in humans. The time was chosen because more than 50% of pregnant women receiving antenatal steroids are treated between 23 and 28 weeks of gestation.18,19 As in our previous report,16 we observed a decrease in the number of glomeruli of about 25% without any decrease in fetal body or kidney weights. In addition, betamethasone treatment did not alter the linear relationship between kidney weight and body weight or total number of nephrons and kidney weight. The lack of any detectable effect on fetal or postnatal growth indicates the steroid-induced reduction in nephron number can occur in the absence of alterations in growth. This finding is highly relevant as antenatal steroid administration is still the only effective treatment to decrease the incidence of acute respiratory distress in newborns of less than 32 weeks gestation. More than 90% of women threatened with premature labor receive at least 1 course of antenatal steroids.18,19
The estimation of total number of glomeruli using the acid maceration technique compares very well to the unbiased fractional dissector technique. The total number of nephrons for the control group in both the fetal and the adult cohorts are consistent with the number reported for this species and agrees very well with those reports using the same technique.20–27 The 2-fold range in the number of nephrons that we observed in both the control and the betamethasone-exposed groups is in keeping with reports of high variability in the number of glomeruli within a given species. In fact, sheep seem to have a smaller variability when compared to humans where an 8-fold range was observed in individuals with no known renal pathology.28 As reported for humans and other species, we found that total nephron number is highly correlated with kidney weight. However, we did not find a significant correlation of nephron number with either fetal weight in the 135 dGA group or birth weight in the adult cohort. In humans, several studies have reported that nephron number is directly correlated with birth weight.29,28 The correlation is stronger in those studies in which the sample included individuals with birth weights outside of what is normally considered adequate for gestational age (<2.5 kg at term).29,28 In a more recent study, although a relationship between birth weight and nephron number was also found, the authors suggest that it was heavily influenced by the existence of extreme values.30 The lack of a steroid effect on fetal or birth weight in the offspring and the lack of correlation of nephron number with fetal birth weight suggests that such a correlation exist only if the group in question includes small-for-gestational age or intrauterine-restricted individuals. Nevertheless, it is important to note that in the case of IUGR the reduction in nephron number is associated with a decrease in kidney weight, representing a proportional decrease in number and size of renal structures. In contrast, a decrease in nephron number without a concomitant decrease in kidney size and/or weight, as the one in the current study, would suggest a compensatory growth of parts of the nephron or other renal structures. Although we did not find differences in glomerular density or size, we cannot rule out that possibility, as we examined only tissue representing the middle section of the kidney and we did not look for changes in elements of the kidney medulla.
The data presented herein suggest that it is unlikely the reduction in nephron endowment is the main mechanism for the elevation in blood pressure in the betamethasone-exposed adult sheep. This view has also been advanced by others in the case of protein restriction-induced nephron deficit.31 The magnitude of the blood pressure increase did not change with advancing age, and there was no significant correlation between the decrease in the number of glomeruli and the increase in arterial blood pressure. A 50% reduction of the nutritional allowance during early ovine pregnancy has been shown to be associated with a decrease in nephron number in the offspring ranging from 11% to 44%.27,26 Interestingly, a persistent elevation in blood pressure was found only in those animals in which the reduction in nephron mass was 11%.27 In animals with a 44% decrease in nephron number, an elevated blood pressure was found only under fasting conditions.26 Brenner et al32 have proposed that a congenital deficit in nephron number, as the one observed in association with IUGR, increases the susceptibility to develop hypertension. The hypothesis states that in the setting of low nephron endowment there is compensatory hypertrophy of the remaining glomeruli and hyperfiltration at the level of each nephron. Hypertension develops as a consequence of the progressive decrease in nephron number due to glomerulosclerosis.33 We saw a significant increase in arterial blood pressure as early as 6 months of age16 and neither the decrease in nephron number nor arterial blood pressure show progression with advancing age. During the first year of age, GFR was normal in these animals with reduced number of nephron, indicating that single nephron hyperfiltration must have developed. However, no further decrease in nephron number was observed in older animals, and we found no evidence of glomerular hypertrophy or severe glomerular damage in a subset of animals. In humans born with a single kidney, the changes associated with hyperfiltration are thought to take two to three decades.33 Therefore, the relatively short follow-up time in our studies would preclude us from finding extensive nephron damage. Reports by us and others on alternative mechanisms for the development of hypertension following exposure to antenatal steroids notwithstanding,34–36,6,37 a permissive effect of the reduction in nephron number cannot be ruled out. Administration of dexamethasone to sheep early in gestation is also associated with hypertension in the adult offspring. Although a reduction in nephron number of similar magnitude has been reported in adult females, the authors suggest that an increase in cardiac output is the most likely mechanism for the elevation in blood pressure.6 In contrast, upregulation of AT1 angiotensin II receptor expression in the brain seems to mediate the observed hypertension in males.37
Animal studies have clearly shown an association between antenatal steroid exposure and the development of hypertension later in life, and in all cases low nephron endowment was present.38,5,39–41 In contrast, data on the long-term effects of antenatal steroids in humans are contradictory and also have the confounding effects of prematurity, very low birth weight and differences in the stage of gestation at which steroids were given. For example, a small but significant increase in arterial blood pressure was reported in 14-year-old children born prematurely3 who received steroids, and an increased odds ratio for hypertension was found in infants exposed to steroids before birth.42 However, other studies have failed to find elevations in blood pressure associated with antenatal steroid treatment43,44 although one did note a reduction in GFR in the steroid group.44 The fact that questions remain regarding the long-term effects of antenatal steroids on blood pressure and renal function in adulthood and that the use of antenatal steroids has become standard of care in women with premature labor makes the use of animal models to study the consequences of prenatal steroid exposure both necessary and important.
In conclusion, this study establishes the long-term effects of antenatal glucocorticoids on nephron number, arterial blood pressure, and GFR in the same animals when used at a dose and gestational age comparable to clinical usage. An important finding is the lack of support for the decrease in nephron number being the underlying mechanism of the elevation in blood pressure. Additional work is needed to further our understanding of the mechanisms leading to the development of hypertension in the adult exposed antenatally to glucocorticoids.
Acknowledgments
This study was funded by NIH HL 68728; HD P01 HD04784.
References
- 1.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525. [PubMed] [Google Scholar]
- 2.Leviton LC, Goldenberg RL, Baker CS, et al. Methods to encourage the use of antenatal corticosteroid therapy for fetal maturation: a randomized controlled trial. JAMA. 1999;281(1):46–52. doi: 10.1001/jama.281.1.46. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci. 2000;98(2):137–142. [PubMed] [Google Scholar]
- 4.Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 5.Celsi G, Kistner A, Aizman R, et al. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res. 1998;44(3):317–322. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol (Lond) 2003;549(3):929–935. doi: 10.1113/jphysiol.2003.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64(11):965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 8.Hellmann H, Davis JM, Thurau K. Glomerulus number and blood pressure in the Prague hypertensive rat. Kidney Int Suppl. 1998;67:S211–S212. doi: 10.1046/j.1523-1755.1998.06750.x. [DOI] [PubMed] [Google Scholar]
- 9.Mackenzie HS, Lawler EV, Brenner BM. Congenital oligonephropathy: the fetal flaw in essential hypertension? Kidney Int. 1996;55:S30–S34. [PubMed] [Google Scholar]
- 10.Skov K, Nyengaard JR, Korsgaard N, Mulvany MJ. Number and size of renal glomeruli in spontaneously hypertensive rats. J Hypertens. 1994;12(12):1373–1376. [PubMed] [Google Scholar]
- 11.Fassi A, Sangalli F, Maffi R, et al. Progressive glomerular injury in the MWF rat is predicted by inborn nephron deficit. J Am Soc Nephrol. 1998;9(8):1399–1406. doi: 10.1681/ASN.V981399. [DOI] [PubMed] [Google Scholar]
- 12.Cullen-McEwen LA, Kett MM, Dowling J, Anderson WP, Bertram JF. Nephron number, renal function, and arterial pressure in aged GDNF heterozygous mice. Hypertension. 2003;41(2):335–340. doi: 10.1161/01.hyp.0000050961.70182.56. [DOI] [PubMed] [Google Scholar]
- 13.Poladia DP, Kish K, Kutay B, Bauer J, Baum M, Bates CM. Link between reduced nephron number and hypertension: studies in a mutant mouse model. Pediatr Res. 2006;59(4 pt 1):489–493. doi: 10.1203/01.pdr.0000202764.02295.45. [DOI] [PubMed] [Google Scholar]
- 14.Crocker JF, Brown DM, Vernier RL. Developmental defects of the kidney. A review of renal development and experimental studies of maldevelopment. Pediatr Clin North Am. 1971;18(2):355–376. doi: 10.1016/s0031-3955(16)32556-1. [DOI] [PubMed] [Google Scholar]
- 15.Gimonet V, Bussieres L, Medjebeur AA, Gasser B, Lelongt B, Laborde K. Nephrogenesis and angiotensin II receptor subtypes gene expression in the fetal lamb. Am J Physiol. 1998;274(6 pt 2):F1062–F1069. doi: 10.1152/ajprenal.1998.274.6.F1062. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res. 2005;58(3):510–515. doi: 10.1203/01.PDR.0000179410.57947.88. [DOI] [PubMed] [Google Scholar]
- 17.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53(2):404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirtschafter DD, Danielsen BH, Main EK, et al. Promoting antenatal steroid use for fetal maturation: results from the California Perinatal Quality Care Collaborative. J Pediatr. 2006;148(5):606–612. doi: 10.1016/j.jpeds.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 19.Wapner RJ, Sorokin Y, Mele L, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357(12):1190–1198. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 20.Bains RK, Sibbons PD, Murray RD, Howard CV, Van Velzen D. Stereological estimation of the absolute number of glomeruli in the kidneys of lambs. Res Vet Sci. 1996;60(2):122–125. doi: 10.1016/s0034-5288(96)90005-3. [DOI] [PubMed] [Google Scholar]
- 21.Brandon AE, Boyce AC, Lumbers ER, Zimanyi MA, Bertram JF, Gibson KJ. Glomerular hypertrophy in offspring of subtotally nephrectomized ewes. Anat Rec (Hoboken) 2008;291(3):318–324. doi: 10.1002/ar.20651. [DOI] [PubMed] [Google Scholar]
- 22.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. AJP—Reg Int Comp Phys. 2005;288(1):R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 23.Zohdi V, Moritz KM, Bubb KJ, et al. Nephrogenesis and the renal renin-angiotensin system in fetal sheep: effects of intrauterine growth restriction during late gestation. AJP—Reg Int Comp Phys. 2007;293(3):R1267–R1273. doi: 10.1152/ajpregu.00119.2007. [DOI] [PubMed] [Google Scholar]
- 24.Gray SP, Kenna K, Bertram JF, et al. Repeated ethanol exposure during late gestation decreases nephron endowment in fetal sheep. AJP—Reg Int Comp Phys. 2008;295(2):R568–R574. doi: 10.1152/ajpregu.90316.2008. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell EK, Louey S, Cock ML, Harding R, Black MJ. Nephron endowment and filtration surface area in the kidney after growth restriction of fetal sheep. Pediatr Res. 2004;55(5):769–773. doi: 10.1203/01.PDR.0000120681.61201.B4. [DOI] [PubMed] [Google Scholar]
- 26.Gopalakrishnan GS, Gardner DS, Dandrea J, et al. Influence of maternal pre-pregnancy body composition and diet during early-mid pregnancy on cardiovascular function and nephron number in juvenile sheep. Br J Nutr. 2005;94(6):938–947. doi: 10.1079/bjn20051559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension, decreased nephron number and altered renal RAS expression in offspring at nine months. J Physiol. 2005;565(1):137–147. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughson M, Farris AB, III, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63(6):2113–2122. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 29.Mañalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58(2):770–773. doi: 10.1046/j.1523-1755.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 30.Hughson MD, Gobe GC, Hoy WE, Manning RD, Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis. 2008;52(1):18–28. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Bagby SP. Maternal nutrition, low nephron number, and hypertension in later life: pathways of nutritional programming. J Nutr. 2007;137(4):1066–1072. doi: 10.1093/jn/137.4.1066. [DOI] [PubMed] [Google Scholar]
- 32.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1(4 pt 1):335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 33.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49(6):1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 34.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53(2):404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang L, Carey LC, Bi J, et al. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol. 2009;296(2):R309–R317. doi: 10.1152/ajpregu.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massmann GA, Zhang J, Rose JC, Figueroa JP. Acute and long-term effects of clinical doses of antenatal glucocorticoids in the developing fetal sheep kidney. J Soc Gynecol Investig. 2006;13(3):174–180. doi: 10.1016/j.jsgi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Dodic M, McAlinden AT, Jefferies AJ, et al. Differential effects of prenatal exposure to dexamethasone or cortisol on circulatory control mechanisms mediated by angiotensin II in the central nervous system of adult sheep. J Physiol. 2006;571(pt 3):651–660. doi: 10.1113/jphysiol.2005.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64(6):412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- 39.Dodic M, Tangalakis K, Moritz K, McFarlane A, Wintour EM. Fluid abnormalities occur in the chronically cannulated mid-gestation but not late gestation ovine fetus. Pediatr Res. 1998;44(6):894–899. doi: 10.1203/00006450-199812000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Dodic M, Abouantoun T, O’Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002;40(5):729–734. doi: 10.1161/01.hyp.0000036455.62159.7e. [DOI] [PubMed] [Google Scholar]
- 41.Dodic M, Hantzis V, Duncan J, et al. Programming effects of short prenatal exposure to cortisol. FASEB J. 2002;16(9):1017–1026. doi: 10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- 42.Seliem WA, Falk MC, Shadbolt B, Kent AL. Antenatal and postnatal risk factors for neonatal hypertension and infant follow-up. Pediatr Nephrol. 2007;22(12):2081–2087. doi: 10.1007/s00467-007-0603-2. [DOI] [PubMed] [Google Scholar]
- 43.Dessens AB, Haas HS, Koppe JG. Twenty-year follow-up of antenatal corticosteroid treatment. Pediatrics. 2000;105(6):E77–E84. doi: 10.1542/peds.105.6.e77. [DOI] [PubMed] [Google Scholar]
- 44.Finken MJ, Keijzer-Veen MG, Dekker FW, et al. Antenatal glucocorticoid treatment is not associated with long-term metabolic risks in individuals born before 32 weeks of gestation. Arch Dis Child Fetal Neonatal Ed. 2008;93(6):F442–F447. doi: 10.1136/adc.2007.128470. [DOI] [PubMed] [Google Scholar]