Abstract
Controlled gene silencing technologies have significant, unrealized potential for use in tissue regeneration applications. The design described herein provides a means to package and protect siRNA within pH-responsive, endosomolytic micellar nanoparticles (si-NPs) that can be incorporated into nontoxic, biodegradable, and injectable polyurethane (PUR) tissue scaffolds. The si-NPs were homogeneously incorporated throughout the porous PUR scaffolds, and they were shown to be released via a diffusion-based mechanism for over three weeks. The siRNA-loaded micelles were larger but retained nano particulate morphology of approximately 100 nm diameter following incorporation into and release from the scaffolds. PUR scaffold releasate collected in vitro in PBS at 37°C for 1–4 days was able to achieve dose-dependent siRNA-mediated silencing with approximately 50% silencing achieved of the model gene GAPDH in NIH3T3 mouse fibroblasts. This promising platform technology provides both a research tool capable of probing the effects of local gene silencing and a potentially high-impact therapeutic approach for sustained, local silencing of deleterious genes within tissue defects.
1. Introduction
The discovery of RNA interference [1] motivated extensive efforts toward harnessing gene-silencing biomacromolecules for clinical therapeutic use. Small-interfering RNA (siRNA) has rapidly advanced into clinical trials for indications such as macular degeneration [2], skin disorders [3], and targeted delivery to melanoma [4–6]. The current work focuses on development of a platform technology to be used for the controlled, local delivery for regenerative medicine, which is a less mature but promising application area for siRNA [7].
Effective delivery has been the primary limitation to more rapid and widespread adoption of siRNA for clinical use due to its susceptibility to nucleases and poor intracellular cytosolic delivery [8]. A variety of strategies have been developed to protect siRNA and improve intracellular delivery including electrostatic complexation with cationic lipids, polymers, and polysacaccharides, as well as conjugation to cell-penetrating/fusogenic peptides, dendrimers, antibodies, vitamins, and nanoparticles [9–19]. Controlled polymerization techniques such as reversible addition-fragmentation chain transfer (RAFT) polymerization offer a promising approach to designing synthetic polymers that are monodispersed, and contain spatially-defined functionalities [20, 21], and the current work employs a RAFT-synthesized, pH-responsive polymer-based micellar nanoparticle (si-NP) recently optimized for efficient intracellular siRNA delivery [22, 23].
The polyplex, bioconjugate, and nanoparticulate siRNA carriers that have advanced to in vivo preclinical testing have been primarily delivered intravenously or through local injection (i.e., intratumoral) in PBS. For tissue regeneration applications, it is anticipated that it will be desirable for siRNA activity to be locally sustained and mediated from a non-cytotoxic and biodegradable tissue template. Because siRNA activity is typically transient and can be exhausted by one week in rapidly dividing cells [24, 25], natural materials including alginate, collagen and agarose have been applied for sustained delivery of siRNA [26–29]. Pre-fabricated synthetic scaffolds made from ε-caprolactone and ethyl ethylene phosphate copolymer (PCLEEP) nanofibers have also been pursued for the release of siRNA/transfection reagent (TransIT-TKO) complexes and have been shown to achieve sustained delivery of bioactive siRNA for 28 days [30].
Porous, non-cytotoxic, and biodegradable polyester polyurethanes (PUR) comprise a promising class of synthetic injectable biomaterials that can provide both mechanical support and also controlled drug release to regenerating tissues [31]. Several drugs, including insulin-like growth factor-1 (IGF-1), hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), recombinant human bone morphogenetic protein 2 (rhBMP-2), platelet-derived growth factor (PDGF), and the antibiotic vancomycin have been incorporated into and delivered from PUR scaffolds [32–36]. Additionally, PURs support the ingrowth of cells in excisional cutaneous wounds [35] and bone defects [34, 36]. Further advantages of PURs are that they adhere to tissue, do not stimulate inflammation [35], and biodegrade into nontoxic side products at rates that can be tuned based on the polyester triol and isocyanate precursor compositions [37]. Importantly, the use of lysine-derived polyisocyanates in the PUR scaffolds makes them more clinically translatable because they can be synthesized using a two-component foaming process that allows a short manipulation time for filling of any shape or size defect, followed by rapid curing in situ [38, 39].
The current study pursues a application of PURs to deliver pH-responsive micellar si-NPs designed for the intracellular delivery of siRNA. This investigation validates homogenous loading of siRNA nanocarriers within the PUR scaffold, sustained, diffusion-controlled release of intact nanoparticles, and maintenance of gene silencing bioactivity of the released si-NPs.
2. Methods
2.1. Materials
All chemicals were purchased from Sigma-Aldrich (Milwaukee, WI, USA) except the following. Purchase of siRNA was from Applied Biosciences (Ambion), LDH cytotoxicity kit from Roche, Hiperfect transfection reagent (positive control) from Qiagen, and PD10 desalting columns from GE healthcare. Lysine Triisocyanate (LTI) was purchased from Kyowa Hakko Kogyo Co., Ltd. (Tokyo, Japan). DMAEMA, and butyl methacrylate (BMA) were vacuum distilled prior to use. 2,2′-Azobis(2-methylpropionitrile) (AIBN) was recrystallized twice with methanol.
2.2 Synthesis of 4-cyano-4-(ethylsulfanylthiocarbonyl) sulfanylpentanoic acid (ECT)
The RAFT chain transfer agent ECT was synthesized following protocols previously described by Convertine et al. [22] adapted from Moad et al. [40]. Briefly, Ethanethiol (76 mmol, 4.72 g) was reacted with carbon disulfide (79 mmol, 6.0 g) in the presence of sodium hydride (79 mmol, 3.15 g) in diethyl ether for 1h. The resulting sodium S-ethyl trithiocarbonate was further reacted with iodine (25 mmol, 6.3 g) to obtain bis(ethylfulfanythiocarbonyl) disulfide, which was further refluxed with 4,4′-azobis(4-cyanopentanoic acid) in ethylacetate for 18 h. The crude ECT was purified by column chromatography using silica gel as the stationary phase and ethyl acetate:hexane (50:50) as the mobile phase. 1H NMR (400MHz, CDCl3): δ 1.36 t (SCH2CH3); δ 1.88 s (CCNCH3); δ 2.3–2.65 m (CH2CH2); δ 3.35 q (SCH2CH3).
2.3. Synthesis of 2-propyl acrylic acid (PAA)
The synthesis of PAA was adapted from existing methods [41]. In brief, diethyl propylmalonate (200 mmol, 40.45 g) was stirred in 1M KOH in 95% ethanol and acifidied with HCl to yield 2-carbopropoxybutyric acid, which was reacted with diethylamine (200 mmol, 14.62 g) and formalin (200 mmol, 16.11 g) at room temperature for 24h, followed by reflux at 60°C for 8 hours. After acidification, the resulting 2-propylacrylate was refluxed in 2M KOH for 20 h to yield 2-propyl acrylic acid, which was extracted, dried, and vacuum distilled under vacuum to yield a colorless oil. 1H NMR (400 MHz, CDCl3) δ 0.97 t (CH3CH2); δ 1.55 m (CH3CH2CH2); δ 2.31 t (CH3CH2CH2); δ 5.69–6.32 q (CH2=C); δ 12 s (CCOOH)
2.4. Synthesis and characterization of pDMAEMA macro CTA
The synthesis of the poly[2-(diethylamino)ethyl methacrylate] (pDMAEMA) macro chain transfer agent (mCTA) was conducted by RAFT polymerization using conditions adapted from [22]. Based on a polymerization kinetics experiments (Supplementary Figure 1), the RAFT polymerization was conducted at 70 °C under a nitrogen atmosphere for eight hours with 1,4-dioxane as the solvent (70% by weight), an initial monomer to CTA ratio of 100, and a CTA to initiator ratio of 10. The pDMAEMA mCTA was isolated by precipitation into n-hexane (×3) and dried overnight. The polymer was analyzed by gel permeation chromatography (GPC, Shimadzu Crop., Kyoto, Japan) with an inline Wyatt miniDAWN TREOS light scattering detector (Wyatt Technology Corp., Santa Barabara, CA) and 1H nuclear magnetic resonance spectroscopy (NMR, Bruker 400Mhz Spectrometer equipped with 9.4 Tesla Oxford magnet) for molecular weight and polydispersity.
2.5. Synthesis and characterization of DMAEMA-b-(PAA-co-BMA-co-DMAEMA)
RAFT polymerization was utilized to synthesize the second block as previously described [22]. Additional monomers BMA, PAA, and DMAEMA were added to the pDMAEMA mCTA chain with an initial monomer to mCTA ratio of 250 in stoichiometric quantities of 50% BMA, 25% PAA, and 25% DMAEMA. The initiator AIBN was used with a mCTA to initiator ratio of 5. The polymerization was conducted for 18 hours under a nitrogen atmosphere at 70°C. The resulting polymer was isolated by precipitation into chilled 50:50 ether:pentane, redissolved in acetone and precipitated into chilled pentane twice, and vacuum dried overnight. The polymer was then dissolved in a minimal amount of ethanol, diluted into dH2O, and further purified using PD10 desalting columns (GE Healthcare). The eluent was frozen and lyophilized yielding a pure polymer powder. The polymer was analyzed by GPC for number average molecular weight (Mn) and polydispersity. 1H NMR in CDCl3 and D2O was used to determine composition and verify the formation of micelles with a DMAEMA corona. Transmission Electron Microscopy (TEM, Philips CM20 Transmission Electron Microscope, EO, Netherlands) and Dynamic Light Scattering (DLS, Zetasizer nano-ZS Malvern Instruments Ltd, Worcestershire, U.K.) were used to confirm presence and size of micelles, to determine the critical micelle concentration, and to characterize micelle pH-responsiveness. Carbon TEM grids (Ted Pella Inc. Redding, CA) were spotted with 5 μL of polymer solution (~50 μg/mL) and dried under vacuum for 24 hours.
2.6. Formation and characterization of siRNA-loaded micellar nanoparticles
siRNA was dissolved in nuclease free water, and si-NPs were formed by injecting siRNA in nuclease free polypropelene tubes, diluting with PBS, adding polymer in PBS, and incubating at room temperature for 30 minutes. si-NPs were formulated based on the charge ratio defined as the number of positively charged tertiary amines (assumed to be 50% at physiologic pH) on the DMAEMA block (N) to the number of negatively charged phosphate groups on the backbone of siRNA (P). Complexes were formed anywhere between 0.5 and 8 N/P. A 2% agarose gel was prepared with 0.5 μg/mL ethidium bromide and allowed to gel at room temperature. si-NPs and controls were run for 40 minutes at 100 V. This experiment was also conducted after pre-incubating the si-NPs in 50% serum to verify serum stability. Dynamic light scattering and ζ–potential were used for physicochemical characterization of the si-NPs, and TEM was used to further verify si-NP size and morphology.
2.7 Synthesis of si-NP-loaded PUR scaffolds
Polyester triols were synthesized as previously described from a glycerol starter targeting 900 Da and a backbone comprising 60 wt% ε-caprolactone, 30 wt% glycolide, and 10 wt% D,L-lactide [35, 42, 43]. si-NPs were synthesized as described above using an N/P of 4 and 4 nmol of fluorescently labeled (6-FAM) siRNA against GAPDH or non-labeled siRNA with a scrambled sequence. si-NPs were frozen and lyophilized and the resulting powder was rigorously mixed into 134 μmol of the polyol component of PUR using a Hauschild DAC 150 FVZ-K SpeedMixer (FlackTek, Inc., Landrum, SC). A slight excess of lysine triisocyanate (387 μmol) was then added and scaffolds were allowed to cure at room temperature forming a porous PUR foam over approximately 10 minutes. 134 μmol of water was included in the polyol because it reacts with LTI to produce CO2 which acts as a blowing agent and creates pores in the scaffold. The resulting 200 mg foams were sectioned into discs with a diameter of 13 mm and a thickness of approximately 3 mm.
2.8. PUR characterization
Confocal microscopy (Zeiss LSM 510Meta) equipped with differential interference contrast (DIC) was used to analyze the distribution of si-NPs in the scaffold. The 13 mm diameter by 3 mm cylindrical foams were immersed in 1 mL of PBS in a 24 well plate. Releasate was collected at regular intervals approximating an infinite sink condition, and release data were fit to the Weibull function [36, 44]. Releasate was analyzed by TEM and DLS for presence and size of released si-NPs.
2.9. Cell culture and siRNA knockdown
Mouse Embryonic Fibroblasts (NIH3T3) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco Cell Culture, Carlsbad, CA) supplemented with 5% Bovine Calf Serum (BCS, Gibco), and 1% penicillin-streptomycin (Gibco). For gene silencing experiments, NIH3T3 mouse embryo fibroblasts were seeded at a density of 12,500 cells/cm2 in a 12 well plate and allowed to adhere overnight. Fresh NPs or released NPs were added in fresh media with a final concentration of 6.25 nM to 50 nM siRNA and allowed to incubate for 24 hours. Each group was analyzed with three biological replicates and 3 technical replicates during qRT-PCR. The cells were lysed and homogenized with QIAshredder (Qiagen), and RNA was purified using the RNeasy® Mini Kit (Qiagen). RNA quantity and quality was assessed with a nanodrop spectrophotometer ND-1000 (Thermo Scientific). cDNA was synthesized with iScript™ cDNA synthesis kit (BIO-RAD) on a C1000™ thermal cycler. Quantitative PCR was done using IQ™ Real Time SYBR Green PCR Supermix on a quantitative thermal cycler (Bio-Rad iCycler iQ). GAPDH expression was normalized to β-Actin expression using the Δ ΔCT method. Primers used were: β-actin Forward 5′-CTACGAGGGCTATGCTCTCCC-3′, β-actin backward 5′-CGTCCTCATGCTACTCAGGCC-3′, GAPDH Forward 5′-CTCACTCAAGATTGTCAGCAATG-3′, GAPDH Backward 5′-GAGGGAGATGCTCAGTGTTGG-3′.
2.10 Imaging of cell uptake of si-NPs post-release from PUR scaffolds
NIH3T3s were seeded at 12,500 cells/cm2 in 8 well chamber slides and incubated for 4 hours with FAM labeled siRNA containing si-NPs released from PUR scaffolds. The media was removed, and the cells were washed 3x with PBS and fixed in 4% paraformaldehyde for 30 minutes. After 2 washes in PBS, cell nuclei were counterstained with Hoechst 33258 (5 μg/mL, Sigma) and then washed an additional 3x. Images were acquired on a fluorescent microscope.
2.11. Cytotoxicity
NIH3T3 cells were seeded at a density of 12,500 cells/cm2 in a 96 well plate and allowed to adhere overnight. si-NPs were then added in fresh media and allowed to incubate for 24 hours. The cells were then lysed and analyzed for intracellular LDH with a Cytotoxicity Detection Kit (Roche Applied Science) as previously described [45], and a plate reader (Infinite F500, Tecan Group Ltd., Mannedorf, Switzerland) set for absorbance at 492 nm with reference at 595 nm.
2.12. Statistical analysis
All data are reported as mean ± standard error of the mean (SEM). Analysis of Variance (ANOVA) was used to determine treatment effects and p<0.05 was considered significant.
3. Results
3.1. Polymer synthesis and characterization
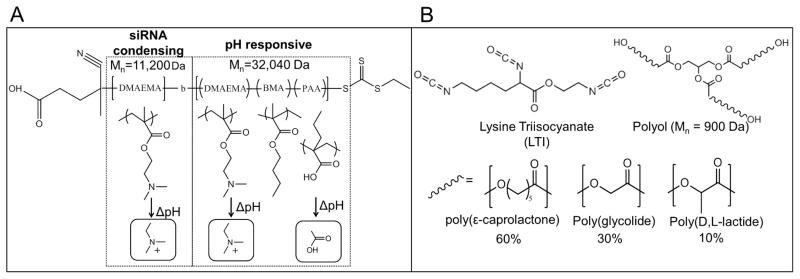
4-cyano-4-(ethylsulfanylthiocarbonyl) sulfanylvpentanoic acid (ECT) was synthesized as previously described [22]. 2-propyl acrylic acid (PAA) was synthesized using established methods [41]. RAFT polymerization was used to synthesize a mCTA of DMAEMA (Mn = 11200g/mol, PDI = 1.40, (Supplementary Fig 2). The pDMAEMA mCTA was used to polymerize a second block with a resultant Mn of 32040 g/mol for a total Mn of 43240 g/mol (PDI = 1.41) as shown in Supplementary Fig 2. 1H-NMR was used to confirm the percent composition of the second block which was determined to be 30%PAA, 25%DMAEMA, and 45%BMA (Supplementary Fig 3a). When dissolved in D2O, 1H-NMR peaks from the core-forming terpolymer are suppressed, verifying the formation of micelles in an aqueous environment. (Supplementary Fig 3b). The polymer structure is depicted in Fig 1.
Figure 1. Chemical composition of materials used for siRNA delivery.
A) Chemical structure of the micelle-forming, pH–responsive diblock copolymer used for siRNA packaging and intracellular delivery. The homo-DMAEMA first block was designed for siRNA condensation due to the positive charge on the tertiary amines. The second block is pH-responsive and tuned for endosomal escape due to micelle destabilization and endosomolytic activity triggered by protonation of PAA and DMAEMA. B) Chemical structure of polyurethane precursors. LTI reacts with the –OH groups of the polyol to form urethane bonds and create the PUR network.
3.2 si-NP synthesis and characterization
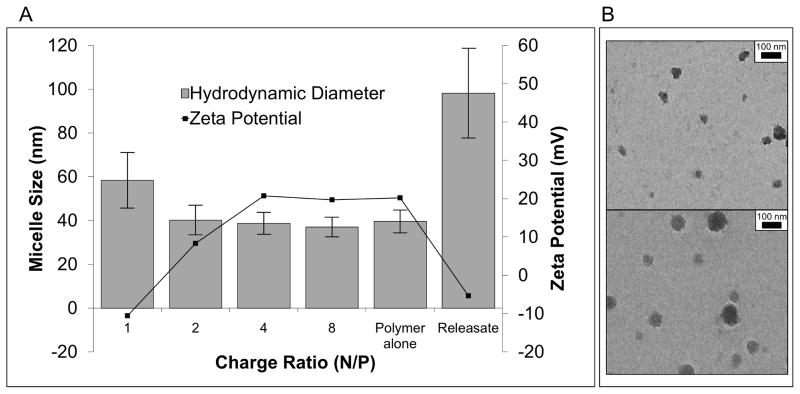
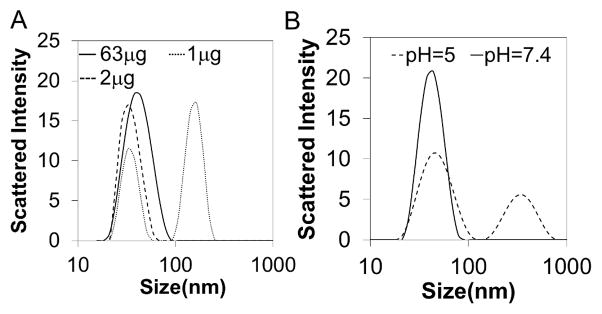
Micellar nanoparticles were self-assembled in an aqueous environment and characterized for size and morphology by DLS and TEM respectively. TEM and DLS (Fig 2) report similar diameters of 31 nm and 39.6 nm respectively, with the smaller diameter seen with TEM being due to micelle dehydration. DLS of serially diluted samples revealed a critical micelle concentration (CMC) below 2 μg/mL, based on a DLS-detected loss of micelle stability (Fig 3a). DLS was also used to demonstrate the dependency of the CMC on pH. The results confirm that micelle structure was destabilized at pH 5 at a concentration of 100 μg/mL, which is important for micelle endosomolytic behavior (Fig 3b) [23]. Gel electrophoresis determined serum stable complexation of siRNA into si-NPs across a range of N:P ratios (Supplementary Fig 4).
Figure 2. Physicochemical characterization of freshly prepared and PUR-released si-NPs.
A) Dynamic light scattering demonstrated that si-NP diameter was around 40-nm at N/P ratios of 2 or greater, and at N/P = 1, the charge neutrality caused the NPs to be less stable and larger. This is further represented by the ζ–potential, which was slightly negative at N/P of 1, 8.3 mV at N/P of 2, and approximately 20 mV at all N/P of 4 or greater. B) The TEM image confirmed the micellar architecture and size of fresh si-NPs. Releasate si-NPs had a larger diameter of approximately 100 nm as shown both by DLS and TEM (B, bottom), and PUR-release si-NPs also had significantly reduced ζ–potential that was approximately charge neutral.
Figure 3. Micelle stability is dependent on concentration and pH.
A) critical micelle concentration (CMC) determination using DLS demonstrated disruption of micelles occurred at 2 μg/mL. B) DLS also revealed pH-dependent destabilization of the micelles at pH = 5 at a concentration of 100 μg/mL.
3.3. si-NP-loaded PUR scaffolds
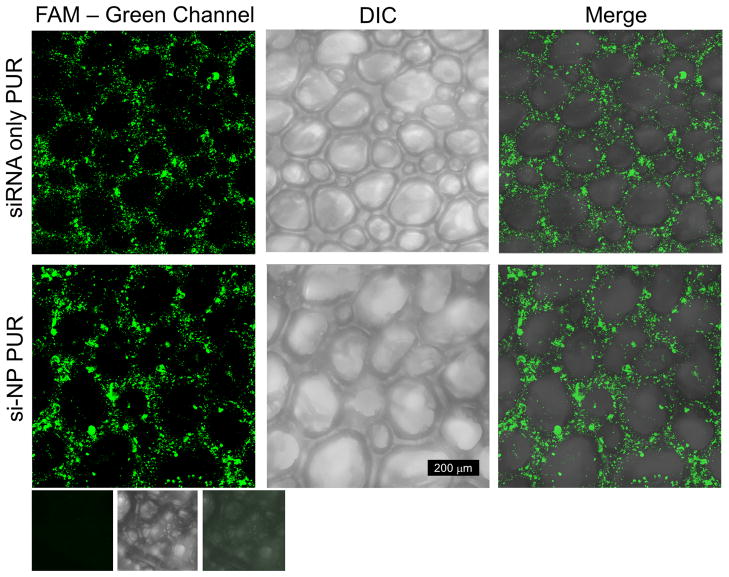
PUR foams were synthesized by reacting polyester triols (polyol) with lysine triisocyanate forming the porous polyurethane foam (Fig 1b). Differential interference contrast microscopy (DIC) of PUR scaffolds revealed an intact, connected porous structure (Figure 4b,e) with a mean pore diameter of 150 μm ± 64 μm. Confocal microscopy shows a relatively homogenous distribution of fluorescently labeled siRNA containing NPs throughout the PUR matrix (Fig 4a–c) comparable to the distribution seen in the PUR containing naked siRNA (Fig 4d–f).
Figure 4. FAM labeled siRNA and si-NPs distribution within the PUR scaffold.
Comparison of fluorescent confocal images of PUR scaffolds loaded with FAM-labeled siRNA or si-NPs. Row 1 is a scaffold loaded with naked siRNA. Row 2 is a scaffold loaded with si-NPs. The 3rd row is an empty scaffold to verify that there is no green autofluorescence of the PUR scaffold. Note that scaffold pores contain no fluorescence, and the distribution between naked siRNA and si-NPs is similar.
3.4. siRNA-NP release kinetics and modeling
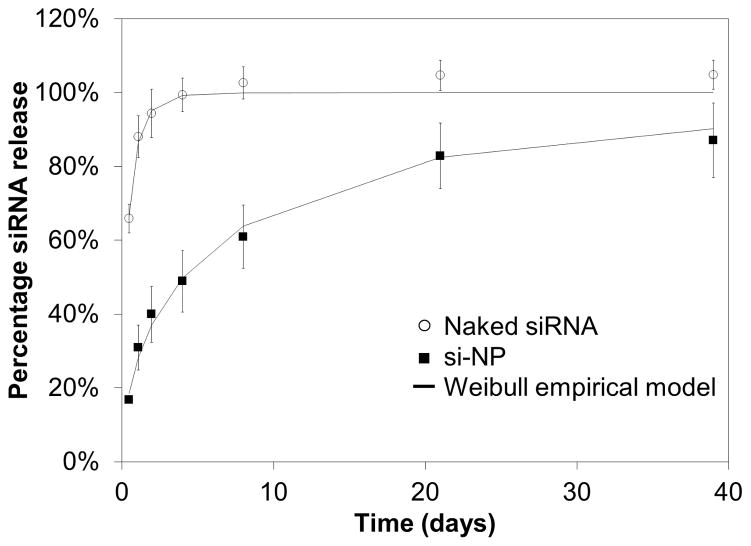
Release from the scaffold was quantitatively assessed using si-NPs made with fluorescently labeled siRNA. Approximately 20% of the payload was released in the first 12 hours followed by a sustained release approaching 80% cumulative release by 21 days (Fig 5). Conversely, the much smaller naked siRNA diffuses from the scaffold much faster than si-NPs, reaching nearly 100% in 3 days. Importantly, TEM and DLS of releasate demonstrated that intact si-NPs, although of larger diameter than fresh si-NPs (approximately 100 nm), were delivered from the PUR scaffolds (Fig 2).
Figure 5. Release of siRNA and si-NPs from PUR scaffolds is diffusion controlled.
The Weibull empirical model equation best-fit was determined and is overlaid here for each data set. Naked siRNA is rapidly released with an initial burst of over 60% at 12 hours and is entirely released by 3 days. si-NPs have a slower rate of release with a burst release of less than 20% during the first 12 hours, followed by sustained release that approaches 80% by 21 days.
The Weibull function has been previously used to evaluate the drug release mechanisms of drug eluting matrices that efficiently release their payload (cumulative release exceeding 60%) [36, 44]. The release of si-NPs was fit to the Weibull empirical model in Equation 1:
| Eqn 1 |
where Mt is the mass of si-NPs released at time t, M∞ is the total mass of si-NPs, a is a constant based on the system, and b is a constant based on the release kinetics. Previous reports suggest that values of b < 0.75 indicate that Fickian diffusion is the dominant release mechanism [36, 44]. The values obtained from the best fit were found to be a=1.892, b=0.699, R2=0.995 for siRNA only and a=0.317, b=0.560, with R2 = .996 for si-NPs.
For additional evidence supporting diffusion-controlled release of siRNA, we performed a scaling analysis to compare the predicted and measured initial release rates. The Stokes-Einstein equation (eqn 2) and the Higuchi equation [46] (eqn 3) were utilized together to further validate the diffusion-controlled release mechanism. These equations, where D is the diffusivity, Mt is the rate of mass transfer, and r is the radius of the particle, provide relationships that allow the initial mass transfer rate to be related to the inverse of the square root of the radius of the solute:
| Eqn 2 |
| Eqn 3 |
Assuming all conditions except hydrodynamic diameter are maintained constant between the two samples except yields the following scaling prediction:
| Eqn 4 |
This analysis was completed assuming a hydrodynamic diameter of 2.56 nm for the siRNA, which was the value suggested by Barone et al. for a 28 mer duplex RNA [47]. The hydrodynamic diameter of 38.69 nm that was experimentally determined using DLS for a charge ratio of 4/1 for si-NPs was used. Based on the measured initial release of 17% for si-NPs and 66% for naked siRNA, the left side of Eqn. 4 reduces to 3.88 and the right side 3.87. Thus the scaling analysis is consistent with the notion that the release of siRNA from the scaffolds is governed by Fickian diffusion.
3.5. PUR-released si-NP
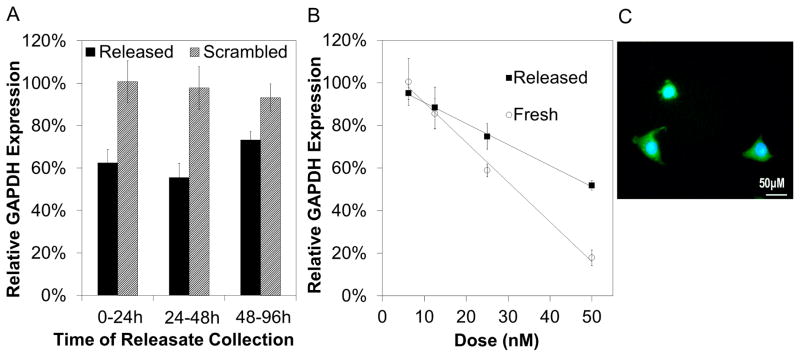
Cytotoxicity experiments showed that the si-NPs were cytocompatible at the doses used (Supplementary Fig 5). Gene expression analyzed by qRT-PCR showed significant reduction (p<0.05) in mRNA levels for GAPDH mediated by releasate collected between 0–24h, 24–48h, and 48–96h, while controls containing scrambled siRNA showed no activity (Fig 6a). Further experimentation showed that PUR-released si-NPs produced dose dependent silencing of GAPDH expression with the highest dose of 50 nM producing approximately 50% gene knockdown (Fig 6b) while freshly prepared si-NPs produced a dose dependent silencing with 83% reduction at 50 nM. The finding that there was a strong correlation between dose and gene silencing, indicated a siRNA dependent effect, and importantly, scrambled siRNA controls had no observable activity. The microscopic observation of diffuse fluorescent siRNA in the cytoplasm of cultured cells confirmed the maintenance of endosomolytic behavior, cytoplasmic delivery, and bioactivity of the PUR-released si-NPs (Fig 6c).
Figure 6. Fresh and released si-NPs are delivered intercellularly and mediate gene specific silencing.
qRT-PCR was used to measure expression of the model gene GAPDH relative to β-actin and then normalized to no treatment controls. A) Bioactivity of freshly prepared and PUR-released si-NPs collected during the defined timeframes 0–24h, 24–48h, and 48–96h indicates that bioactivity of si-NPs released from the PUR is not significantly altered over time. Statistical significance relative to scrambled control siRNA containing si-NPs was noted at all time points (p<0.05). B) Dose response of PUR-released si-NPs demonstrated a linear relationship (R2= 0.999) between siRNA dose and silencing activity, suggesting an siRNA-dependent gene silencing effect. Minor reduction in siRNA bioactivity was apparent in PUR-released si-NPs relative to fresh si-NPs. C) Diffuse green fluorescence is noted in the cytoplasm of NIH3T3s after 4 hours of incubation with PUR-released si-NPs. This presence of FAM-labeled siRNA in the cytoplasm confirmed effective siRNA cytoplasmic delivery.
4. Discussion
Technologies that enable the efficient and sustained delivery of siRNA are a high-impact but relatively unmet need. This is primarily due to the number and complexity of the delivery barriers that exist. Here, a platform is presented that is capable of both sustained and effective delivery of siRNA from a PUR scaffold capable of providing a non-cytotoxic and biodegradable tissue template that can be cured in situ using a clinically-translatable injectable formulation. Due to nuclease susceptibility and membrane impermeability of naked siRNA, little success has been found with carrier-free siRNA delivery methodologies, and thus, the siRNA was first loaded into the pH-responsive micellar si-NPs prior to formulation with the biomaterial matrix.
The RAFT synthesized polymer shown in Fig 1A is the basis for the si-NPs and was specifically designed for improved cytoplasmic uptake, siRNA protection, and endosome escape [22, 23]. Toxicity of typically-utilized polyplexes made with cationic polymers [48] and the limitations associated with inefficient bioactivity due to lysosomal degradation or extracellular clearance [49] motivated the development of the polymer. si-NPs are formulated at positive charge ratios (typically 4:1) providing a net positive charge which facilitates efficient cell uptake by most cell types [50, 51]. Once in the endosome, decreasing pH destabilizes the micelle structure due to protonation of PAA and DMAEMA monomers and exposes the membrane-disruptive core [52]. The configuration of the second block (Fig. 1A) is finely tuned to provide a sharp pH response at the desired pH by incorporating appropriate amounts of hydrophobic BMA [53] and pH responsive DMAEMA and PAA. Fig 3B confirmed the pH-dependent micelle destabilization using DLS and it is hypothesized that this destabilization allows the hydrophobic 2nd block to penetrate and disrupt endosomal membranes and facilitate siRNA delivery to the cytoplasm [54–57]. Once internalized, siRNA may be competitively dissociated from the polymer through interactions within the cytoplasm by other ionic molecules [50] thus gaining access to the RNAi machinery in the cytoplasm.
Recently, biomaterials have been pursued for sustained siRNA delivery, with natural materials such as alginate, collagen and agarose being mostly used in these applications due to their biocompatibility [26–29]. However, these natural materials generally lack tunability and have been limited to rapid burst release of siRNA. The best sustained delivery to date has been achieved using PCLEEP nanofiber scaffolds, however, the manufacture of the scaffold requires complex equipment (electrospinning apparatus) and must be pre-made to a defined size and geometry [30]. Therefore, there still remains a significant need for a more clinically translatable biomaterial that can conform to tissue defects of varied sizes and shapes where it will cure in situ and deliver siRNA locally in a sustained manner.
PUR scaffolds provide multiple advantages as a biomaterial for controlled drug delivery to tissue defects for several reasons. PUR scaffolds can be easily adapted to be injectable making clinical use easier and requiring no additional fabrication equipment [38, 39]. After injection, PURs react in situ to form a non-cytotoxic and biodegradable tissue scaffold with inter-connected pores that effectively serves as a template for cell influx and tissue formation and remodeling [35]. The mechanism of degradation includes hydrolytic degradation (on the order of months) and macrophage-mediated oxidative degradation by reactive oxygen species (ROS) secretion (on the order of weeks) that is ideal for the timescale of wound healing [37]. Finally, PUR has been shown to deliver biologics efficiently, typically delivering as much as 80% of the payload [34–36]. However, a previous study has reported that 50-μm PLGA microspheres with rhPDGF bound to the surface supported <10% release over 21 days, suggesting that primary amines in the protein reacted with the polyisocyanate, resulting in loss of activity [35]. The present study has confirmed for the first time that nanoparticulate carriers incorporated in reactive PUR scaffolds support high-efficiency, diffusion-controlled release as seen in Fig 5. The release data demonstrates cumulative release of si-NPs approaching 80% over 21 days compared to naked siRNA which was released rapidly, approaching 100% delivery of the payload in three days. The mechanism of release for both free siRNA and si-NPs was found to be diffusion-controlled based on the Weibull model. Further, scaling analysis with the Stokes-Einstein and Higuchi equations demonstrated that the initial release rates of siRNA and si-NPs scales appropriated to the hydrodynamic diameter of the solute. The diffusion-based release suggests that an additional level of control exists by altering si-NP diffusivity in the PUR matrix to tune the rate of release by varying the nanoparticle size.
It is hypothesized that, in many applications, sustained delivery of siRNA into tissue defects will be ideal for producing a therapeutic effect since siRNA produces relatively transient gene silencing activity [58]. It is hypothesized that when the formulation tested here is translated in vivo, the initial burst release will establish gene silencing while the continual, slower siRNA delivery over the next few weeks will sustain the initial effect over a few weeks. Importantly, several approaches exist for tuning PUR-based drug delivery to be more rapid or more sustained [34].
Fig 6A demonstrates that the activity of released NPs is not significantly reduced over the time frames tested (0–24, 24–48, 48–96 hours). Sustained delivery of active complexes is critical to compensate for transiency of siRNA in a highly proliferative environment (i.e. tissue regeneration). Fig 6B demonstrates that the siRNA-mediated reduction in GAPDH of PUR-released si-NPs is dose dependent. However, it is evident that there is partial loss of bioactivity post-release from the scaffold compared to fresh si-NPs. It is possible that this reduction in silencing is due to reorganization of the micelle structure or a partial si-NP aggregation during lyophilization and incorporation into the PUR. There was a detectable difference in size revealed by TEM and DLS (Fig 2) of fresh micelles versus PUR-released micelles, and the ζ–potential of PUR-releasate si-NPs was also found to be reduced. It could also be possible that unreacted components in the PUR specifically adsorb to the surface of the released si-NPs, thereby reducing the ζ-potential of the si-NPs resulting in aggregation. Our unpublished data have shown that 1–2% of the PUR mass leaches from the reactive material during the first 45 minutes of cure when incubated in serum medium. The primary components in the leachates include polyester triol, dipropylene glycol, and triethylene diamine. Hydrolytic degradation of the cured scaffolds releases α-hydroxy acids [37], which could bind electrostatically to the positive surface of the si-NPs. However, further studies will be necessary to better understand and overcome the alteration of the si-NPs during processing, and excipients such as agarose and sucrose may provide one route for improving their stability during lyophilization [59].
5. Conclusions
Injectable poly(ester urethane) foams were successfully utilized for sustained release of bioactive si-NPs for an extended period of 21 days. The si-NPs synthesized using RAFT were found to remain intact and bioactive following incorporation into and release from PUR scaffolds, although changes in si-NP size and bioactivity were evident relative to fresh si-NPs. As a platform technology, the combination of PUR scaffolds and pH-responsive micellar siRNA carriers provides a logical approach to basic scientific studies of long-term siRNA-mediated gene silencing at local, pathological or healing tissue sites. The described system also has the potential to be applied to control cell phenotype and fate in tissue constructs developed in vitro. Finally, as a therapeutic, the described approach may be applied to reduce expression of deleterious genes and improve regeneration in tissue defects.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Jeffery Davidson for constructive conversations on experimental design. Confocal Imaging was performed using a Zeiss LSM 510 Inverted Confocal Microscope in part through the use of the VUMC Cell Imaging Shared Resources, (supported by NIH Grants CA68485, DK20593, DK58404, HD15052, DK59637, and Ey008126). Dynamic light scattering and TEM were conducted through the use of the core facilities of the Vanderbilt Institute of Nanoscale Sciences and Engineering (VINSE). qRT-PCR was conducted at the Vanderbilt University Molecular Cell Biology Resource Core. This work was supported by a Vanderbilt Discovery Grant, NIH R21EB012750, and NIH 1R01AR056138-01A2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Kaiser PK, Symons RC, Shah SM, Quinlan EJ, Tabandeh H, Do DV, et al. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol. 2010;150:33–9. e2. doi: 10.1016/j.ajo.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Leachman SA, Hickerson RP, Schwartz ME, Bullough EE, Hutcherson SL, Boucher KM, et al. First-inhuman mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Mol Ther. 2010;18:442–6. doi: 10.1038/mt.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6:659–68. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 6.Ribas A, Zuckerman JE, Hsueh T, Koya RC, Davis ME. siRNA knockdown of ribonucleotide reductase inhibits melanoma cell line proliferation alone or synergistically with temozolomide. J Invest Dermatol. 2011;131:453–60. doi: 10.1038/jid.2010.310. [DOI] [PubMed] [Google Scholar]
- 7.Yao Y, Wang C, Varshney RR, Wang DA. Antisense makes sense in engineered regenerative medicine. Pharm Res. 2009;26:263–75. doi: 10.1007/s11095-008-9772-3. [DOI] [PubMed] [Google Scholar]
- 8.White PJ. Barriers to successful delivery of short interfering RNA after systemic administration. Clin Exp Pharmacol P. 2008;35:1371–6. doi: 10.1111/j.1440-1681.2008.04992.x. [DOI] [PubMed] [Google Scholar]
- 9.Gao K, Huang L. Nonviral methods for siRNA delivery. Mol Pharm. 2009;6:651–8. doi: 10.1021/mp800134q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal A, Min DH, Singh N, Zhu H, Birjiniuk A, von Maltzahn G, et al. Functional delivery of siRNA in mice using dendriworms. ACS Nano. 2009;3:2495–504. doi: 10.1021/nn900201e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Convertine AJ, Benoit DS, Duvall CL, Hoffman AS, Stayton PS. Development of a novel endosomolytic diblock copolymer for siRNA delivery. J Control Release. 2009;133:221–9. doi: 10.1016/j.jconrel.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jafari M, Chen P. Peptide mediated siRNA delivery. Curr Top Med Chem. 2009;9:1088–97. doi: 10.2174/156802609789630839. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Green JJ, Love KT, Sunshine J, Langer R, Anderson DG. Gold, poly(beta-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9:2402–6. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishina K, Unno T, Uno Y, Kubodera T, Kanouchi T, Mizusawa H, et al. Efficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherol. Mol Ther. 2008;16:734–40. doi: 10.1038/mt.2008.14. [DOI] [PubMed] [Google Scholar]
- 15.Qi L, Gao X. Quantum dot-amphipol nanocomplex for intracellular delivery and real-time imaging of siRNA. ACS Nano. 2008;2:1403–10. doi: 10.1021/nn800280r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe K, Harada-Shiba M, Suzuki A, Gokuden R, Kurihara R, Sugao Y, et al. In vivo siRNA delivery with dendritic poly(L-lysine) for the treatment of hypercholesterolemia. Mol Biosyst. 2009;5:1306–10. doi: 10.1039/b900880b. [DOI] [PubMed] [Google Scholar]
- 17.Wu SY, McMillan NA. Lipidic systems for in vivo siRNA delivery. AAPS J. 2009;11:639–52. doi: 10.1208/s12248-009-9140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia CF, Boado RJ, Pardridge WM. Antibody-mediated targeting of siRNA via the human insulin receptor using avidin-biotin technology. Mol Pharm. 2009;6:747–51. doi: 10.1021/mp800194y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosn B, Kasturi SP, Roy K. Enhancing polysaccharide-mediated delivery of nucleic acids through functionalization with secondary and tertiary amines. Curr Top Med Chem. 2008;8:331–40. doi: 10.2174/156802608783790947. [DOI] [PubMed] [Google Scholar]
- 20.Moad G, Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules. 1998;31:5559–62. [Google Scholar]
- 21.Boyer C, Bulmus V, Davis TP, Ladmiral V, Liu J, Perrier S. Bioapplications of RAFT polymerization. Chem Rev. 2009;109:5402–36. doi: 10.1021/cr9001403. [DOI] [PubMed] [Google Scholar]
- 22.Convertine A, Benoit D, Duvall C, Hoffman A, Stayton P. Development of a novel endosomolytic diblock copolymer for siRNA delivery. Journal Control Release. 2009;133:221–9. doi: 10.1016/j.jconrel.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Convertine AJ, Diab C, Prieve M, Paschal A, Hoffman AS, Johnson PH, et al. pH-responsive polymeric micelle carriers for siRNA drugs. Biomacromolecules. 2010;11:2904–2911. doi: 10.1021/bm100652w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10:766–71. doi: 10.1261/rna.5239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13:541–52. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]
- 26.Krebs MD, Jeon O, Alsberg E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J Am Chem Soc. 2009;131:9204–6. doi: 10.1021/ja9037615. [DOI] [PubMed] [Google Scholar]
- 27.Vinas-Castells R, Holladay C, di Luca A, Diaz VM, Pandit A. Snail1 down-regulation using small interfering RNA complexes delivered through collagen scaffolds. Bioconjugate Chem. 2009;20:2262–9. doi: 10.1021/bc900241w. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen PD, Tutela JP, Thanik VD, Knobel D, Allen RJ, Chang CC, et al. Improved diabetic wound healing through topical silencing of p53 is associated with augmented vasculogenic mediators. Wound Repair Regen. 2010;18:553–9. doi: 10.1111/j.1524-475X.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JW, Tutela JP, Zoumalan RA, Thanik VD, Nguyen PD, Varjabedian L, et al. Inhibition of Smad3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch Otolaryngol. 2010;136:714–9. doi: 10.1001/archoto.2010.107. [DOI] [PubMed] [Google Scholar]
- 30.Rujitanaroj PO, Wang YC, Wang J, Chew SY. Nanofiber-mediated controlled release of siRNA complexes for long term gene-silencing applications. Biomaterials. 2011;32:5915–23. doi: 10.1016/j.biomaterials.2011.04.065. [DOI] [PubMed] [Google Scholar]
- 31.Guelcher SA. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Eng Pt B-Rev. 2008;14:3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- 32.Guan J, Stankus JJ, Wagner WR. Biodegradable elastomeric scaffolds with basic fibroblast growth factor release. J Control Release. 2007;120:70–8. doi: 10.1016/j.jconrel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson DM, Baraniak PR, Ma Z, Guan J, Mason NS, Wagner WR. Controlled release of IGF-1 and HGF from a biodegradable polyurethane scaffold. Pharm Res. 2011;28:1282–93. doi: 10.1007/s11095-011-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Yoshii T, Hafeman AE, Nyman JS, Wenke JC, Guelcher SA. The effects of rhBMP-2 released from biodegradable polyurethane/microsphere composite scaffolds on new bone formation in rat femora. Biomaterials. 2009;30:6768–79. doi: 10.1016/j.biomaterials.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 35.Li B, Davidson JM, Guelcher SA. The effect of the local delivery of platelet-derived growth factor from reactive two-component polyurethane scaffolds on the healing in rat skin excisional wounds. Biomaterials. 2009;30:3486–94. doi: 10.1016/j.biomaterials.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B, Brown KV, Wenke JC, Guelcher SA. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J Control Release. 2010;145:221–30. doi: 10.1016/j.jconrel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Hafeman AE, Zienkiewicz KJ, Zachman AL, Sung HJ, Nanney LB, Davidson JM, et al. Characterization of the degradation mechanisms of lysine-derived aliphatic poly(ester urethane) scaffolds. Biomaterials. 2011;32:419–29. doi: 10.1016/j.biomaterials.2010.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adolph EJ, Hafeman A, Davidson J, Nanney L, Guelcher S. Injectable polyurethane composite scaffolds delay wound contraction and support cellular infiltration and remodeling in rat excisional wounds. J Biomed Mater Res A. doi: 10.1002/jbm.a.33266. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hafeman AE, Li B, Yoshii T, Zienkiewicz K, Davidson JM, Guelcher SA. Injectable biodegradable polyurethane scaffolds with release of platelet-derived growth factor for tissue repair and regeneration. Pharm Res. 2008;25:2387–99. doi: 10.1007/s11095-008-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moad G, Chong YK, Postma A, Rizzardo E, Thang SH. Advances in RAFT polymerization: the synthesis of polymers with defined end-groups. Polymer. 2005;46:8458–68. [Google Scholar]
- 41.Ferrito M, Tirrell DA. Poly(2-ethylacrylic acid) Macromol Synth. 1992;11:59–62. [Google Scholar]
- 42.Guelcher SA, Patel V, Gallagher KM, Connolly S, Didier JE, Doctor JS, et al. Synthesis and in vitro biocompatibility of injectable polyurethane foam scaffolds. Tissue Eng. 2006;12:1247–59. doi: 10.1089/ten.2006.12.1247. [DOI] [PubMed] [Google Scholar]
- 43.Guelcher S, Srinivasan A, Hafeman A, Gallagher K, Doctor J, Khetan S, et al. Synthesis, in vitro degradation, and mechanical properties of two-component poly(ester urethane)urea scaffolds: effects of water and polyol composition. Tissue Eng. 2007;13:2321–33. doi: 10.1089/ten.2006.0395. [DOI] [PubMed] [Google Scholar]
- 44.Papadopoulou V, Kosmidis K, Vlachou M, Macheras P. On the use of the Weibull function for the discernment of drug release mechanisms. Int J Pharm. 2006;309:44–50. doi: 10.1016/j.ijpharm.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 45.Duvall CL, Convertine AJ, Benoit DS, Hoffman AS, Stayton PS. Intracellular delivery of a proapoptotic peptide via conjugation to a RAFT synthesized endosomolytic polymer. Mol Pharm. 2010;7:468–76. doi: 10.1021/mp9002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siepmann J, Peppas NA. Higuchi equation: derivation, applications, use and misuse. Int J Pharm. 2011;418:6–12. doi: 10.1016/j.ijpharm.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 47.Barone F, Cellai L, Matzeu M, Mazzei F, Pedone F. DNA, RNA and hybrid RNA-DNA oligomers of identical sequence: structural and dynamic differences. Biophys Chem. 2000;86:37–47. doi: 10.1016/s0301-4622(00)00157-5. [DOI] [PubMed] [Google Scholar]
- 48.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–32. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medina-Kauwe LK, Xie J, Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther. 2005;12:1734–51. doi: 10.1038/sj.gt.3302592. [DOI] [PubMed] [Google Scholar]
- 50.Cho YW, Kim JD, Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J Pharm Pharmacol. 2003;55:721–34. doi: 10.1211/002235703765951311. [DOI] [PubMed] [Google Scholar]
- 51.van der Aa MA, Huth US, Hafele SY, Schubert R, Oosting RS, Mastrobattista E, et al. Cellular uptake of cationic polymer-DNA complexes via caveolae plays a pivotal role in gene transfection in COS-7 cells. Pharm Res. 2007;24:1590–8. doi: 10.1007/s11095-007-9287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones RA, Cheung CY, Black FE, Zia JK, Stayton PS, Hoffman AS, et al. Poly(2-alkylacrylic acid) polymers deliver molecules to the cytosol by pH-sensitive disruption of endosomal vesicles. Biochem J. 2003;372:65–75. doi: 10.1042/BJ20021945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Sayed ME, Hoffman AS, Stayton PS. Rational design of composition and activity correlations for pH-sensitive and glutathione-reactive polymer therapeutics. J Control Release. 2005;101:47–58. doi: 10.1016/j.jconrel.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 54.Thomas JL, Barton SW, Tirrell DA. Membrane solubilization by a hydrophobic polyelectrolyte: surface activity and membrane binding. Biophys J. 1994;67:1101–6. doi: 10.1016/S0006-3495(94)80575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas JL, Tirrell DA. Polyelectrolyte-sensitized phospholipid-vesicles. Accounts Chem Res. 1992;25:336–42. [Google Scholar]
- 56.Borden KA, Eum KM, Langley KH, Tirrell DA. Interactions of synthetic-polymers with cell-membranes + model membrane systems .13. on the mechanism of polyelectrolyte-induced structural reorganization in thin molecular films. Macromolecules. 1987;20:454–6. [Google Scholar]
- 57.Hoffman AS, Lackey CA, Press OW, Stayton PS. A biomimetic pH-responsive polymer directs endosomal release and intracellular delivery of an endocytosed antibody complex. Bioconjugate Chem. 2002;13:996–1001. doi: 10.1021/bc010053l. [DOI] [PubMed] [Google Scholar]
- 58.Krebs MD, Alsberg E. Localized, targeted, and sustained siRNA Delivery. Chem-Eur J. 2011;17:3054–62. doi: 10.1002/chem.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei Y, Rahim M, Ng Q, Segura T. Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery. J Control Release. 2011;153:255–61. doi: 10.1016/j.jconrel.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.