Abstract
Objective
Granulocytopenia frequently occurs in alcohol abusers suffering from severe bacterial infection, which strongly correlates with poor clinical outcome. Knowledge of the molecular mechanisms underlying the granulopoietic response to bacterial infection remains limited. This study investigated the involvement of Stem Cell Antigen-1 (Sca-1) expression by granulocyte lineage-committed progenitors in the granulopoietic response to septicemia and how alcohol affected this response.
Design
Laboratory investigation.
Setting
University laboratory.
Subjects
Male Balb/c mice.
Interventions
Thirty minutes after intraperitoneal injection of alcohol (20% ethanol in saline at 5g of ethanol/Kg) or saline, mice received intravenous Escherichia coli (E.coli) challenge.
Measurements and Main Results
E. coli septicemia activated Sca-1 expression by marrow immature granulocyte differentiation antigen-1 (Gr1)lo precursors which correlated with an increase in proliferation, CFU-GM production, and expansion of this granulopoietic precursor cell pool. Acute alcohol treatment suppressed Sca-1 activation and inhibited the infection-induced increases in proliferation, CFU-GM production, and expansion the of Gr1lo cell population. Consequently, recovery of the marrow mature Gr1hi cell population following E.coli challenge was impaired. Sca-1 was induced in sorted Gr1+Sca1- cells by LPS-stimulated JNK activation that was also inhibited by alcohol. Furthermore, Sca-1 knockout (KO) mice failed to expand the marrow Gr1lo cell pool and demonstrated fewer newly produced granulocytes in the circulation following E.coli challenge.
Conclusions
Alcohol suppresses the Sca-1 response in granulocyte lineage-committed precursors and restricts granulocyte production during septicemia, which may serve as a novel mechanism underlying impaired host defense in alcohol abusers.
Keywords: Hematopoietic Progenitor, Granulopoiesis, Sca-1, Septicemia, Immunosuppression, Neutrophil
Introduction
Septicemia is a severe bacterial infection that causes over 34,000 deaths each year in the United States (1). Excessive alcohol consumption impairs immune function predisposing the host to bacterial infections, particularly septicemia. Patients who abuse alcohol comprise up to 39% of all ICU admissions, and suffer higher rates of sepsis and septic shock. (2). In alcoholic patients, septicemia can develop from various origins including trauma, wound infection, peritonitis, pneumonia, urinary tract infection, biliary infection, colonization of intravenous catheters, and bacterial translocation of gut flora (3–5). A prominent feature of alcohol abusers with septicemia is that they frequently present with granulocytopenia, which is an indicator of increased mortality (3). Clinical studies have shown that mortality rates exceed 80% in alcoholic patients with septicemia and granulocytopenia (6).
Representing the largest population of phagocytes in the systemic circulation, polymorphonuclear leukocytes (PMNs, neutrophils, or granulocytes) are the first line of host defense against blood-borne pathogens. These cells constantly patrol the circulatory system and readily eliminate invading microbes by phagocytosis and intracellular killing. During systemic bacterial infection, granulocyte turnover in the circulation is accelerated. The bone marrow concomitantly increases production of granulocytes in order to sustain and/or reinforce phagocytic defense in the bloodstream (7, 8). Failure to develop an adequate granulopoietic response results in poor infection control with increases in morbidity and mortality.
Current knowledge about mechanisms underlying the granulopoietic response to bacterial infection remains superficial. Studies have shown that tissues with infection and inflammation increase production of certain granulopoietic mediators including granulocyte colony-stimulating factor (G-CSF) and CXC chemokines. These mediators can stimulate bone marrow granulocyte maturation and release (9, 10). Previous studies from our group have revealed that conversion of marrow LKS- [lineage (lin)-stem cell growth factor receptor (c-kit)+stem cell antigen-1 (Sca-1)−] to LKS+ (lin-c-kit+Sca-1+) cells through enhancement of Sca-1 expression plays an essential role in directing lineage commitment of primitive precursors towards myeloid lineage development in mice following severe bacterial infection (11, 12). Alcohol treatment impairs this initial step of the granulopoietic response (12). Our current study extended this line of investigation by exploring the significance of enhanced Sca-1 expression by downstream granulocyte lineage-committed (granulocyte differentiation antigen-1 positive or Gr1+) progenitor populations in the host defense against septicemia. The surface expression of Gr1 is representative of the maturation status of granulocytes (13). Immature granulopoietic precursors express low levels of Gr1 (Gr1lo). With terminal differentiation, the expression of Gr1 increases such that mature granulocytes express the highest level of Gr1 (Gr1hi). We focused on determining how enhanced Sca-1 expression related to expansion of the granulopoietic precursor pool in the bone marrow during bacterial infection. The underlying molecular mechanism as well as the adverse effect of alcohol treatment on this host defense process was delineated.
Materials and Methods
Animals
Male Balb/c mice (7–10 weeks old; Charles River Laboratories) with a body weight of 20.9 +/− 1.3 g were fed a standard laboratory diet and housed in a specific pathogen free facility with a 12 h light/dark cycle. Acute alcohol treatment was conducted by intraperitoneal (i.p.) injection of 20% ethanol in saline at a concentration of 5g/kg. Blood alcohol levels were determined to be 132.8 ± 4.5, 122.4 ± 1.9, and 61.4 ± 6.8 mM, respectively, at 90 min, 3 h, and 6 h post alcohol administration (N = 5 at each time point). Control animals received an equal volume of i.p. saline. Thirty minutes following i.p. injection, mice under inhaled isoflurane anesthesia were intravenously (i.v.) challenged with live Escherichia coli (E.coli) (E11775 from American Type Culture Collection, 1 × 106 or 5 × 107 CFUs in 100uL/mouse) via penile vein injection. Control animals received an equal volume of i.v. saline. Sca-1 KO mice (14) which are considered congenic on the C57BL/6 background (15) were bred under specific pathogen free conditions in the Animal Care Facility of Louisiana State University Health Sciences Center. Experiments with Sca-1 KO mice were performed using male, 7–8 week old animals with age and gender matched C57BL/6 background controls (Charles River Laboratories). Animals received i.v. challenge with either 108 CFU E.coli or saline. Animals were sacrificed at specific timepoints following i.v. challenge as indicated in each figure legend. In a subgroup of animals, i.v. BrdU (1mg in 100uL of PBS/mouse; BD Biosciences) was administered during E. coli challenge 24 h prior to sacrifice. During sacrifice, heparinized blood was obtained by cardiac puncture and differential white blood cell counts were performed using Wright-Giemsa stain. Peripheral granulocyte counts following 24 and 48 h E.coli challenge are included in Supplemental Figure 1 (Supplemental Digital Content #1). Bone marrow cells were flushed from femurs and tibias as published previously (12). The studies described here were performed in adherence with the National Institute of Health guidelines on the use of experimental animals. Approval of the Animal Care and Use Committee of Louisiana State University Health Sciences Center was obtained prior to initiating these experiments.
Flow cytometric analysis
Nucleated bone marrow cells suspended in RPMI 1640 (Invitrogen) containing 10% FCS (2× 106 cells in 100uL media) were added to monoclonal antibodies against CD3ε (clone 500A2, eBiosciences), CD19 (clone 1D3, BD Biosciences), F4-80 (clone BM8, eBiosciences), Gr1 (Ly6G/Ly6C, clone RB6-8CF, BD Biosciences), Sca-1 (Ly6A/E, clone D7, BD Biosciences), TER-119 (clone TER-119, eBiosciences) or isotype control antibodies. Cells were incubated in the dark for 20 min at room temperature and then washed in cold PBS. BrdU incorporation was measured with further processing using a BD BrdU Flow Kit (BD Biosciences) following the manufacturer’s protocol. Analysis was performed on a FACSAria or LSR-II flow cytometer using FACSDiva software (BD Biosciences). A total of 300,000 cells were analyzed for each sample. Cell samples stained with isotype control antibodies were used to establish the baseline for positive staining of all analyses. Data are expressed as a proportion of cells in the bone marrow. Quantified changes in the marrow Sca-1+, Gr1lo, and Grhi cell populations are included in Supplemental Figure 2 (Supplemental Digital Content #2).
FACS Sorting of bone marrow Gr1+Sca-1−, Gr1loSca-1−, and Gr1loSca-1+ cell populations
Nucleated bone marrow cells were suspended in StemSpan serum-free medium (StemCell Technologies) and the staining procedure for cell surface markers was repeated as described above. Sorting of bone marrow Gr1+Sca-1−, Gr1loSca-1−, and Gr1loSca-1+ cells was performed using a FACSAria flow cytometer with FACSDiva software. The purity of sorted cell populations was 97–99%.
Morphological analysis
Bone marrow Gr1loSca-1− and Gr1loSca-1+ cells were stained and isolated by FACS sorting as described above. Cytospin preparations of 100,000 cells were Wright-Geimsa stained and imaged at 60X magnification using an inverted Nikon microscope and SPOT software (Diagnostics Inc., Sterling Heights, MI).
Colony Forming Unit (CFU) assays for mitotic activity
CFU assays of sorted bone marrow Gr1loSca-1− and Gr1loSca-1+ cells were performed by culturing cells in Methocult GF 3534 medium (StemCell Technologies). One milliliter of medium containing 100 sorted cells was plated on a 35-mm Nunclon dish (Nunc). Each sample was cultured in triplicate for 7 days at 37°C in an atmosphere of 5% CO2. Colonies containing 50 or more cells were enumerated.
In vitro culture of bone marrow Gr1+Sca-1+Sca-1− cells
Sorted Gr1+Sca-1− cells from naïve mice were plated into a 96-well tissue culture plate with 5 × 104 cells/well in a total volume of 100 uL of StemSpan serum-free medium. Cells were treated with vehicle or 25 ng/mL LPS (E. coli 0111:B4, List Biological Laboratories, Inc.) and cultured in the absence or presence of different alcohol concentrations for 12 h at 37°C and 5% CO2. Alcohol exposure included one hour incubation prior to LPS addition. Where indicated, the JNK inhibitor SP600125 (Sigma Aldrich), was added to cultured cells at 20 μM concentration. At the end of culture, cells were stained with fluorochrome-conjugated anti-Gr1 and anti-Sca-1 antibodies. Flow cytometric analysis of live (propidium iodide negative) cells was conducted on a LSR-II flow cytometer with FACSDiva Software.
Western blot analysis
Nucleated bone marrow cells were isolated from mice following i.v. challenge with 5 × 107 E. coli for 8 h in the absence or presence of acute alcohol treatment. Western blot analysis of phospho-JNK in these cells was performed as previously described (16). Twenty micrograms of protein from each sample were resolved on an 8% SDS-PAGE gel. The primary and secondary antibodies used were anti-phospho-JNK (1:200 dilution, Santa Cruz) and horseradish peroxidase-conjugated horse anti-mouse IgG (1:1000 dilution, Cell Signaling Technology, Danvers, MA), respectively. Semi-quantification was performed using the Kodak Gel Logic 2,200 Imaging System. Data are presented as the normalized mean intensity ratio (MI ratio).
Statistical analysis
Data are presented as mean ± SEM. The sample size is indicated in the legend of each figure. Statistical analyses of data were conducted using unpaired Student’s t test(for comparison between two groups), one-way ANOVA followed by Student-Newman-Keuls test, or Proc Mixed (SAS Institute; 2004)two-way ANOVA (for comparisons among multiple groups) followed by Tukey post hoc test. Asterisks and bars with different letters in each panel are statistically different (p < 0.05). Regression analysis was performed with the Microsoft Excel Analysis ToolPak. Differences were considered statistically significant at p < 0.05.
Results
Alteration of marrow hematopoietic cell constitution following septicemia
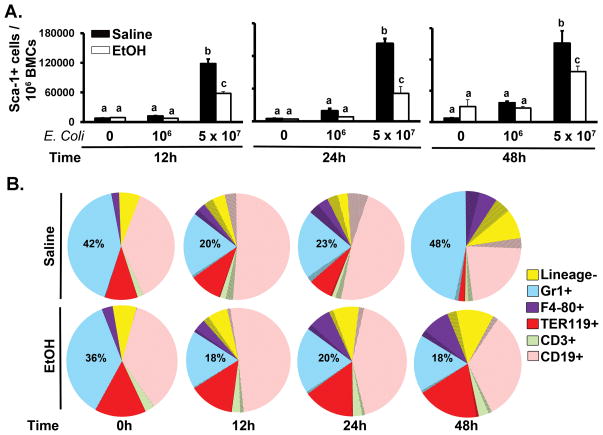
As shown in Figure 1A, a very small fraction of nucleated bone marrow cells express Sca-1 in control animals. Intravenous challenge with 5 × 107 E. coli caused a marked increase in Sca-1 expression by bone marrow cells at 12, 24, and 48 h post challenge. Acute alcohol treatment suppressed this up-regulation of Sca-1 expression. Figure 1B demonstrates changes in the distribution of Sca-1 expressing cells (area with shading) in the bone marrow following i.v. challenge with 5 × 107 E. coli. Septicemia caused a significant increase in the proportion of Sca-1+ cells within the lin- and all subtypes of lin+ marrow cell compartments. This Sca-1 response was associated with a dynamic change in the bone marrow granulocyte (Gr1+) population. At 12 and 24 h post E. coli challenge, the marrow granulocyte fraction was reduced (Fig. 1B). This decrease in the marrow pool of granulocytes during the early stage of septicemia may reflect accelerated mobilization of granulocytes from the bone marrow into the systemic circulation (17–19). By 48 h post E. coli challenge, the marrow granulocyte fraction recovered and even exceeded the level of saline treated control animals (Fig. 1B). The lineage negative fraction of hematopoietic precursors and monocyte (F4-80+) subpopulation also increased significantly at this time point. Recovery of the marrow granulocyte fraction at 48 h post E. coli challenge was associated with a reduction in erythroid precursor (TER119+), T lymphocyte (CD3+), and B lymphocyte (CD19+) proportions in the bone marrow. Alcohol treatment inhibited recovery of the marrow granulocyte pool following septicemia.
Figure 1.
Enhanced Sca-1 expression and granulocyte recovery in the bone marrow during septicemia. (A) Increase in the proportion of Sca-1+ bone marrow cells following E.coli septicemia. (B) Constitution of hematopoietic cells in the bone marrow after 5 × 107 E.coli challenge. Proportion of cells expressing Sca-1 is shaded. BMCs: bone marrow cells. Data are mean ± SEM (N=5–8). Bars with different letters are statistically different (p < 0.05).
Sca-1 expression in granulocyte lineage cells in response to septicemia
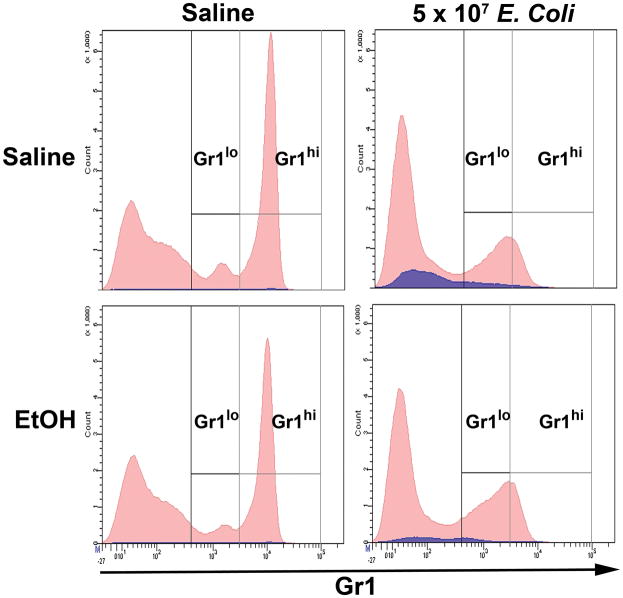
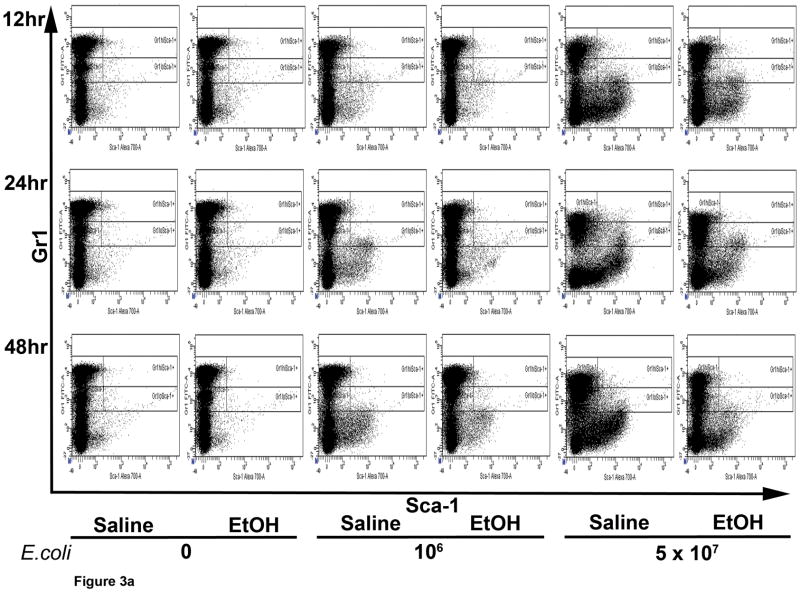
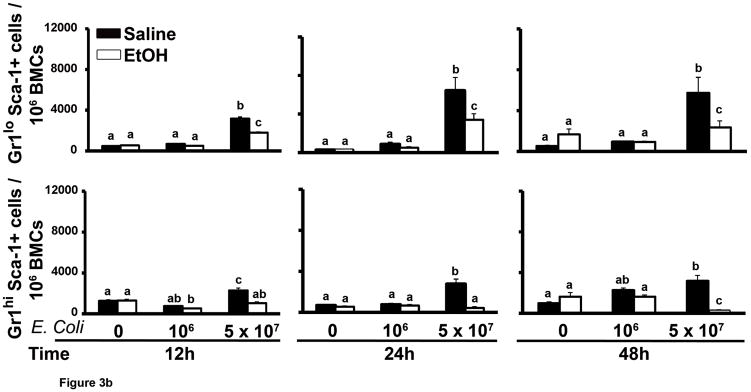
The proportion of Gr1hi cells predominated over Gr1lo cells in the marrow Gr1+ cell population of control animals (Fig. 2). However, fewer mature granulocytes remained in the bone marrow 24 h post challenge with 5 × 107 E. coli, leading to a proportionate increase in the number of Gr1lo cells. E. coli septicemia stimulated Sca-1 expression (blue color in histogram) in the bone marrow that included the Gr1+ cell compartment (Fig. 2). Following 12, 24, and 48 h of 5 × 107 E. coli challenge, the proportion of Sca-1+ cells increased in both Gr1lo and Gr1hi cell populations (Fig. 3A and B). A lower dose of E. coli (106 CFU) challenge did not significantly affect Sca-1 expression in the Gr1+ cell compartment. Acute alcohol treatment impaired the up-regulation of Sca-1 expression by cells in the granulocyte lineage compartment following 5 × 107 E. coli challenge (Fig. 3B). This inhibition occurred in both Gr1lo and Gr1hi cell subtypes.
Figure 2.
Representative histograms of changes in the marrow Gr1+ cell population after 24 h E.coli (5 × 107) challenge. Bone marrow cells expressing Sca-1 are shown in blue.
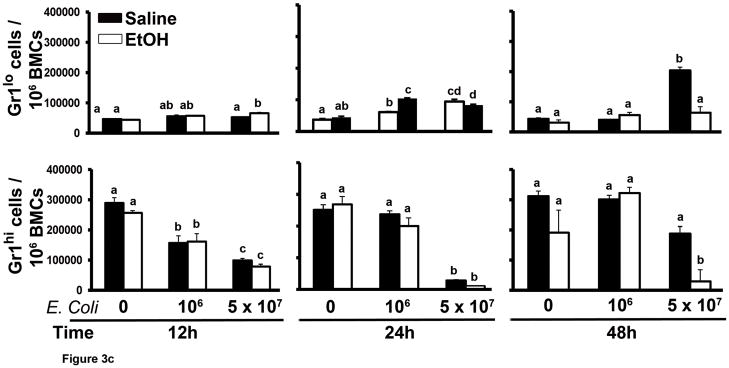
Figure 3.
The marrow Gr1+Sca-1+ cell response to septicemia. (A) Representative dot plots of AF700-Sca-1 (x axis) and FITC-Gr1 (y axis) expression by bone marrow cells during septicemia. Gated populations include Gr1hiSca-1−, Gr1loSca-1−, Gr1hiSca-1+, and Gr1loSca-1+ cells. (B and C) Quantification of the results. Data are mean ± SEM (N=5–8). Bars with different letters are statistically different (p < 0.05).
Bone marrow Gr1+ cell development during septicemia
During bacterial infection, the bone marrow accelerates production of granulocytes to support the increase in mobilization of these phagocytes into the circulation (17–19). As shown in Figure 3C, the proportion of Gr1hi cells in the bone marrow was decreased at 12 h of E. coli challenge in an E. coli dose-dependent manner. The marrow Gr1hi cell population in mice challenged with 106 E. coli recovered to control levels by 24 h post challenge. However, the level of Gr1hi cells in the bone marrow of mice challenged with 5 × 107 E. coli was further reduced at 24 h post challenge. By 48 h post i.v. challenge with 5 × 107 E. coli, the marrow Gr1hi cell proportion tended to recover. These alterations of the marrow Gr1hi cell population paralleled changes in the total bone marrow granulocyte pool during septicemia (Fig. 1). Marrow Gr1hi cells (mature granulocytes) develop from Gr1lo cells (granulopoietic precursors). We found that the proportion of marrow Gr1lo cells increased significantly 48 h after 5 × 107 E. coli challenge. Alcohol treatment inhibited the increase in marrow Gr1lo cells 48 h after 5 × 107 E. coli challenge. Accordingly, recovery of the marrow Gr1hi cell population and total granulocyte pool was impaired in these animals.
Granulopoietic cell proliferation during septicemia
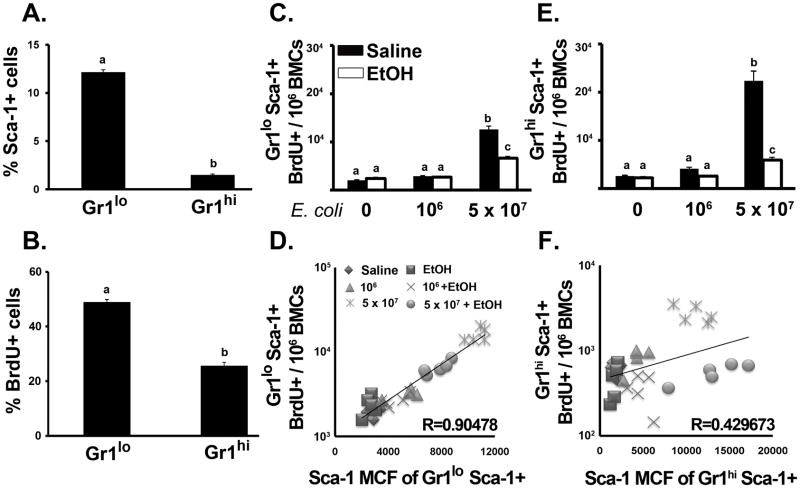
To define how enhanced Sca-1 expression affects cell proliferation in the Gr1+ cell compartment, we employed BrdU incorporation analysis. During granulocyte development, the ability to replicate is lost as granulopoietic precursor cells reach the myelocyte stage (20). As shown in Figure 4A, the percentage of Sca-1+ cells in the Gr1lo cell population was higher than in the Gr1hi cell population of control mice. Similarly, BrdU incorporation into Gr1lo cells was also higher than in Gr1hi cells in saline treated animals (Fig. 4B). During E. coli challenge, the proliferation of marrow granulocytes was enhanced. The proportion of Gr1loSca-1+BrdU+ and Gr1hiSca-1+BrdU+ (Fig. 4C and E) cells increased significantly following 24 h of 5 × 107 E. coli challenge. Alcohol treatment suppressed these increases. Further analysis of the relationship between Sca-1 expression and BrdU incorporation in Gr1+ cells showed that the increase in surface density of Sca-1 expression (Sca-1 Mean Channel Fluorescence; Sca-1 MCF) by Gr1loSca-1+ cells during infection closely correlated with an increase in BrdU incorporation into these cells (r = 0.905, p < 0.01) (Fig. 4D). This correlation also existed in Gr1hiSca-1+ cells (r = 0.430, p < 0.05) (Fig. 4F). These data suggest a strong correlation between Sca-1 expression and the proliferation of granulopoietic precursors during septicemia.
Figure 4.
Marrow granulocyte proliferation. Percent of (A) Sca-1+ and (B) BrdU+ cells in marrow Gr1+ populations from control mice. Number of BrdU+ cells in (C) Gr1loSca-1+ and (E) Gr1hiSca-1+ populations after 24 h E.coli (5 × 107) challenge. Correlation between Sca-1 MCF and BrdU+ cell number in (D) Gr1loSca-1+ and (F) Gr1hiSca-1+ cells. Data are mean ± SEM (N=5). Bars with different letters are statistically different (p < 0.05).
CFU-GM activity in granulopoietic cells during septicemia
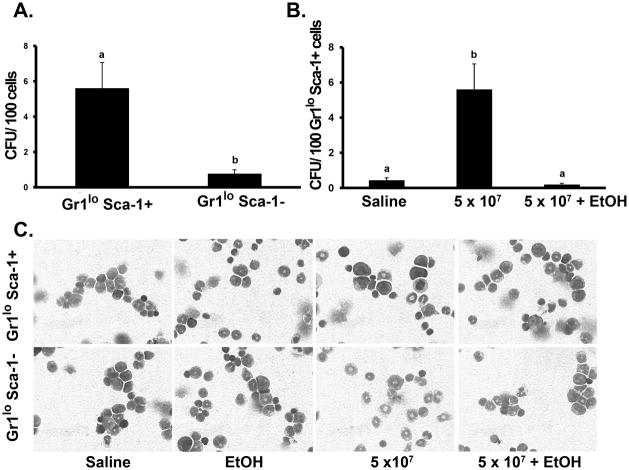
As shown in Figure 5A, Gr1loSca-1+ cells demonstrated greater CFU-GM production than Gr1loSca-1− cells following 24 h of 5 × 107 E. coli challenge. Gr1loSca-1+ cells isolated from alcohol treated animals had significantly less CFU-GM activity following the E. coli challenge than those from saline treated animals (Fig. 5B). Gr1loSca1+ cells also demonstrated more immature, blast-like nuclear morphology following E. coli challenge than Gr1loSca-1− cells (Fig. 5C). Alcohol treatment did not alter the nuclear morphology of either cell type.
Figure 5.
Enhanced CFU-GM activity of Gr1lo cells during septicemia. (A) Colony Forming Unit (CFU) assay of marrow Gr1loSca-1+/− cells after 24 h E.coli (5 × 107) challenge. (B) CFU assay of marrow Gr1loSca-1+ cells from mice receiving E.coli challenge with and without alcohol intoxication. (C) Representative cytospins (60X objective) of marrow Gr1loSca-1+/− cells. Data are mean ± SEM (N=5). Bars with different letters are statistically different (p < 0.05).
LPS-stimulated Sca-1 expression by Gr1+Sca-1− cells
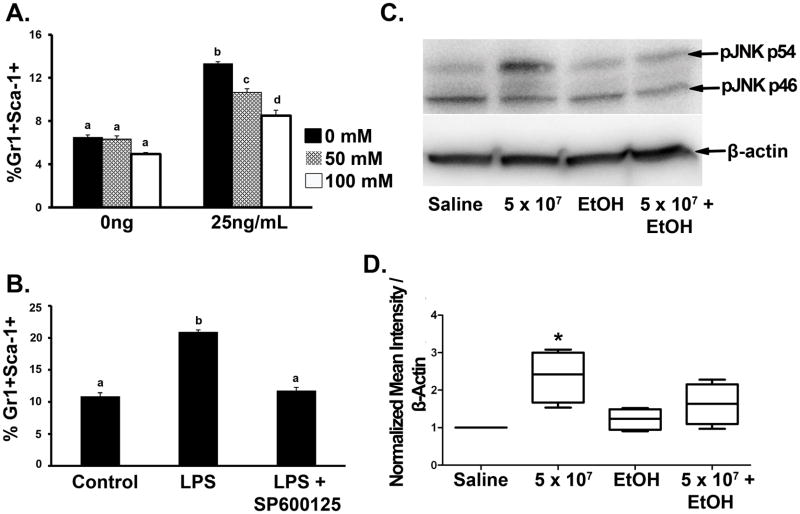
Culture of sorted Gr1+Sca-1− cells with LPS for 12 h stimulated a significant up-regulation of Sca-1 expression by these cells (Fig. 6A). Alcohol dose-dependently inhibited this LPS-stimulated enhancement of Sca-1 expression. Previous studies from our group have shown that the alteration of Sca-1 expression by hematopoietic precursors in response to bacterial infection is regulated at the transcriptional level (11, 12). After analyzing the promoter region of the Sca-1 gene, we have found 20 AP-1 binding sites with an optimization (OPT) value between 0.87–0.94 in the sequence of −1 to −13743 bp upstream of the Sca-1 gene transcription start site. C-Jun is the most potent transcriptional activator in the AP-1 family (21). Hematopoietic precursors express Toll-like receptors (TLRs) and their co-receptors (22). Engagement of LPS to TLR4 activates c-Jun amino terminal kinase (JNK) and consequently enhances the transcriptional activity of c-Jun by phosphorylation of its N-terminal activation domain. As shown in Figure 6B, addition of the JNK inhibitor SP600125 to the culture system blocked LPS-stimulated Sca-1 expression by sorted marrow Gr1+Sca1- cells. Additionally, i.v. challenge with 5 × 107 E. coli for 8 h caused a significant increase in JNK phosphorylation, particularly of JNK p54, in nucleated bone marrow cells (Fig. 6C and D). Alcohol treatment inhibited this JNK activation in bone marrow cells following septicemia.
Figure 6.
LPS-stimulated Sca-1 expression by marrow Gr1+Sca-1− cells. LPS-stimulated Sca-1 expression in sorted Gr1+Sca-1− cells was inhibited by (A) alcohol exposure and (B) JNK inhibition. (C) Western blot of phospho-JNK expression by bone marrow cells after 8 h E.coli challenge. (D) Mean intensity of phospho-JNK p54 expression normalized to β-actin. Data are mean ± SEM (N=4–5). Bars with different letters are statistically different (p < 0.05).
Participation of Sca-1 in the regulation of granulocyte production during septicemia
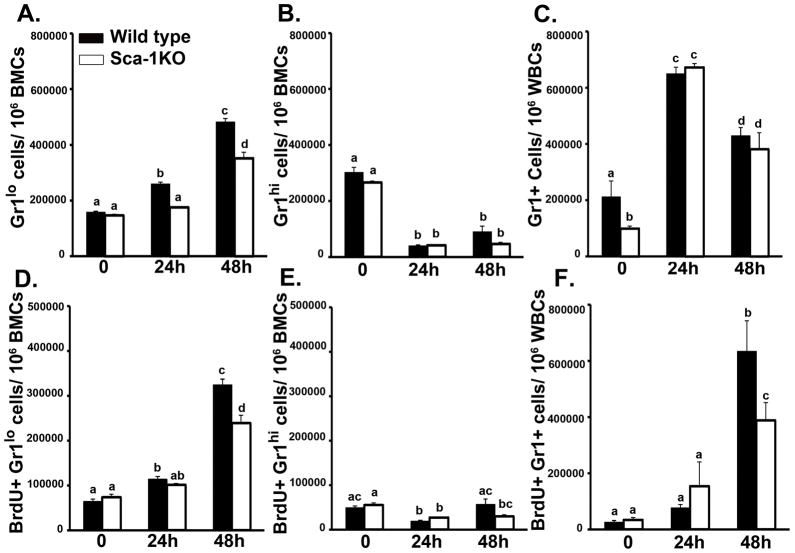
To further evaluate the role of enhanced Sca-1 expression in increasing marrow granulocyte production during septicemia, we compared the granulopoietic response of wild type C57BL/6 and Sca-1 KO mice following i.v. E. coli challenge. As shown in Figure 7A, the increase in the proportion of marrow Gr1lo cells 24 and 48 h post 108 E. coli challenge was significantly attenuated in Sca-1 KO mice. While there was a trend for wild type marrow Gr1hi cells to recover towards baseline after 48 h, Sca-1 KO animals had no significant difference in the proportion of marrow Gr1hi cells following E. coli challenge (Fig. 7B). Additionally, while Gr1+ granulocytes in the peripheral circulation were fewer in saline treated Sca-1 KO mice, no difference was seen in the number of circulating granulocytes 24 or 48 h following E. coli challenge (Fig.7C). In wild type mice, BrdU incorporation into marrow Gr1lo cells was significantly increased 24 and 48 h post E. coli challenge (Fig. 7D). This increase in BrdU incorporation by marrow Gr1lo cells was attenuated in Sca-1 KO animals. Sca-1 KO mice did not show differences in BrdU incorporation by marrow Gr1hi cells following E. coli challenge (Fig. 7E). In the systemic circulation, the frequency of BrdU positive granulocytes was significantly increased 48 h post E. coli challenge in wild type animals (Fig. 7F). Sca-1 KO mice showed an attenuation of this increase.
Figure 7.
Marrow granulocyte production is attenuated in Sca-1 KO mice during septicemia. (A) Gr1lo and (B) Gr1hi cell numbers and BrdU incorporation (D and E) in the bone marrow after 108 E.coli challenge. (C) Gr1+ and (F) BrdU+Gr1+ cell numbers in white blood cells (WBCs) during challenge. Data are mean ± SEM (N=4–10). Bars with different letters are statistically different (p < 0.05).
Discussion
Sca-1 is an 18-kDa, glycophosphatidylinositol (GPI)-anchored cell surface protein of the lymphocyte activation protein-6 (Ly6) gene family (23). Studies with Sca-1 KO mice have clarified the cellular and physiological functions of Sca-1, demonstrating its role in hematopoietic and mesenchymal stem cell self-renewal and myeloid progenitor cell production (15, 24). Additionally, greater Sca-1 expression has been directly linked with myeloid lineage commitment in multipotent hematopoietic cell lines (25, 26). Our previous studies have revealed that Sca-1 re-expression by LKS- precursors during bacterial infection drives rapid expansion of the marrow LKS+ cell population. This enlarged LKS+ cell pool serves as a platform where primitive hematopoietic precursor cells reprogram their commitment towards myeloid lineage development (11, 12).
Sca-1 antigen is expressed in enriched mouse hematopoietic stem cells (27, 28). In our current study, the bone marrow expression of Sca-1 was significantly up-regulated in response to septicemia. This enhancement of Sca-1 expression was not limited to the upstream lin- primitive precursor cell compartment. Instead, virtually all of the lineage-committed cell populations examined increased expression of Sca-1. Our current research focus is to determine how the up-regulation of Sca-1 expression by granulocyte lineage-committed cells relates to enhancement of granulocyte production during systemic bacterial infection and how alcohol impairs this host defense mechanism. During the initial response to septicemia, the bone marrow storage pool of granulocytes was markedly reduced, reflecting the rapid release of these phagocytes into the systemic circulation. Enhanced Sca-1 expression by marrow Gr1+ cells correlated with this change and was accompanied by recovery of the marrow granulocyte pool in the later stage of septicemia. Alcohol treatment suppressed Sca-1 expression by Gr1+ cells, which may serve as a potential mechanism underlying impaired recovery of the marrow granulocyte population in intoxicated hosts.
In the mouse bone marrow, a small fraction of cells express both Gr1 and F4-80 antigens. The lineage commitment of these cells is not yet defined. In our current study, we observed that approximately 80% of the cells in this subpopulation are positive for myeloperoxidase and had a nuclear morphology consistent with myeloneutrophil series development (unpublished data). Therefore, this cell subtype was included in our analysis. In this study, the initial reduction of the marrow granulocyte pool essentially resulted from the loss of mature Gr1hi cells following E. coli infection. In the later stage of septicemia, the increase in Gr1lo cells initiated recovery of the marrow granulocyte pool. Alcohol treatment inhibited expansion of the marrow Gr1lo cell fraction and impaired marrow granulocyte recovery. These observations highlight the detrimental effects of alcohol on the dynamic alterations in the marrow microenvironment that support the granulopoietic response to systemic bacterial infection.
Enhanced Sca-1 expression by Gr1lo precursors during septicemia was associated with an increase in the activities of proliferation and CFU-GM formation in these cells, suggesting the potential role of Sca-1 in promoting marrow granulocyte production. In order to clarify whether the increase in Sca-1 expression by marrow Gr1+ cells was simply due to carry-over of the Sca-1 surface marker by upstream lin- precursor cells during the accelerated process of granulocyte lineage development, we sorted Gr1+Sca-1- cells from naïve mice and cultured them in the presence of LPS stimulation. Our results provide direct evidence that marrow granulocyte lineage-committed cells are able to express Sca-1 in response to LPS stimulation. Alcohol inhibits this Sca-1 response.
Our data also demonstrate that activation of JNK signaling plays a key role in mediating the up-regulation of Sca-1 expression in marrow Gr1+ cells following E. coli challenge. Bone marrow cells from E. coli challenged mice significantly increased JNK phosphorylation; however, alcohol treatment suppressed activation of JNK signaling. The JNK inhibitor SP600125, at a concentration of 20 μM, has been reported to sufficiently inhibit LPS-induced JNK activation in myeloid cells (29, 30). In our experiments, blocking activation of the JNK pathway abolished LPS-stimulated Sca-1 expression by cultured marrow Gr1+Sca1− cells. LPS, a cell wall component of gram negative bacteria, is known to be a major ligand for TLR4. Ligand engagement of TLR4 in various cell types activates the JNK pathway during host defense (31). Previous observations have reported that TLR4 activation increases hematopoietic stem cell proliferation and polarization towards myeloid lineage development during bacterial infection (22). Alcohol has been shown to impair LPS-stimulated JNK activation by multiple mechanisms including the modification of lipid rafts and impairment of TLR4 downstream signaling (32–34).
To further define the role of Sca-1 expression in enhancing granulopoietic precursor proliferation during bacterial infection, we used the Sca-1 KO mouse model. We predicted that similar to alcohol treated animals, Sca-1 KO mice would not adequately expand marrow granulocyte production during septicemia. Our results support this prediction, showing impaired expansion of the marrow Gr1lo cell fraction and an attenuated increase in Gr1+ cell proliferation in Sca-1 KO mice following septicemia. Unlike our results in wild type Balb/c mice, recovery of the marrow Gr1hi cell compartment did not occur 48 h post challenge in experiments using the C57Bl/6 strain. This outcome likely resulted from using a higher dose of E.coli challenge for C57Bl/6 mice to elicit the granulopoietic response. The impaired proliferative response in Sca-1 KO mice appears to be specific to myeloid lineage development as splenocytes from Sca-1 KO animals proliferate normally in response to LPS stimulation (14). In fact, Sca-1−/− T cells are hyper-proliferative in response to antigens that act through the T cell receptor (14). Thus, these data suggest that enhanced Sca-1 expression during septicemia was necessary to foster increased granulopoietic precursor proliferation and production of mature granulocytes in the bone marrow. Suppression of infection-induced Sca-1 expression by alcohol may serve as a novel mechanism by which alcohol inhibits the granulopoietic response to bacterial infection.
The role of Sca-1 in host defense has been investigated with different focuses by other laboratories. Our work continues to support the concept that Sca-1 acts as a “molecular switch,” to stimulate the proliferation of early hematopoietic precursors (35). Previous studies from our group and others have shown that a number of mediators including components of microbes, cytokines, and synthesized molecules are able to stimulate Sca-1 expression by hematopoietic cells (11, 26, 36, 37). These results indicate that multiple mechanisms exist for Sca-1 induction and subsequent triggering of this “molecular switch” for proliferation (11). Since the LPS/TLR4 pathway generates essential proximal signaling for initiating the granulopoietic cell response to E. coli septicemia, it warrants the critical role of this signaling cascade in induction of Sca-1 expression by marrow granulocyte lineage-committed cells. Other mediators generated from infected tissues may subsequently participate in the continued induction of Sca-1 expression by hematopoietic cells during septicemia.
Although it has not yet been identified, there is strong evidence that a human orthologue of Sca-1 exists and plays a significant role in hematopoietic precursor proliferation and myeloid cell development (38). Additionally, human hematopoietic precursors express other Ly6 family proteins, of which Sca-1 is a member, including Sca-2, CD59, and other GPI-linked proteins. Because Sca-1 plays a significant role in murine hematopoietic activity during infection, human Ly6 family proteins may provide a novel area for investigating molecular targets to strengthen host defense in critically ill patients.
It has long been recognized that alcohol abusers with severe bacterial infection have an increased incidence of developing granulocytopenia, an indicator of poor outcomes (3). Excessive alcohol consumption has been shown to injure myeloid progenitor cells and impair granulopoietic activity in the bone marrow (39, 40). In vitro observations have documented that exposure of bone marrow cells to alcohol at concentrations commonly observed in intoxicated patients suppresses granulocyte colony formation (41, 42). Our investigations using a mouse model demonstrate that alcohol suppresses Sca-1 induction in granulopoietic cells and impairs their proliferation following septicemia. Since hematopoietic progenitors transverse a stage termed “transit amplification,” in which precursors undergo maximal mitotic expansion during the process of maturation (43), our current study indicates that alcohol impairs Sca-1-associated signaling and restricts maximal expansion of the granulopoietic cell pool during the host response to septicemia. These observations reveal a novel mechanism underlying impairment of the granulopoietic response to severe bacterial infection in hosts who excessively consume alcohol.
Supplementary Material
Supplemental Digital Content 1. Peripheral granulocyte counts during septicemia. Changes in the number of circulating granulocytes (A) 24 h and (B) 48 h post E.coli challenge. Data are mean ± SEM (N=5–8). Bars with different letters are statistically different (p < 0.05).
Supplemental Digital Content 2. Quantified changes in marrow cell populations during septicemia. Changes in the number of marrow Sca-1+, Gr1lo, and Gr1hi cells after E.coli challenge. Data are mean ± SEM (N=5–8). Bars with different letters are statistically different (p < 0.05).
Acknowledgments
This study was funded, in part, by the National Institutes of Health and the Canadian Institutes of Health Research.
Financial Support: This work was supported by Public Health Service Grants AA017494, AA019676, AA09803, AA07577, and AA019586.
We thank Amy B. Weinberg, Joseph S. Soblosky Ph.D., Meredith Booth, and Jane A. Schexnayder for technical assistance. We also thank Connie P. Porretta for expert assistance with flow cytometric analysis and cell sorting. Additionally, we thank Howard Blakesly for help with data analysis.
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Kung HC, Hoyert DL, Xu J, et al. Deaths: Final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 2.O’Brien JM, Jr, Lu B, Ali NA, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35:345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- 3.MacGregor RR, Louria DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- 4.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- 5.Plurad D, Demetriades D, Gruzinski G, et al. Motor vehicle crashes: The association of alcohol consumption with the type and severity of injuries and outcomes. J Emerg Med. 2010;38:12–17. doi: 10.1016/j.jemermed.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Perlino CA, Rimland D. Alcoholism, leukopenia, and pneumococcal sepsis. Am Rev Respir Dis. 1985;132:757–760. doi: 10.1164/arrd.1985.132.4.757. [DOI] [PubMed] [Google Scholar]
- 7.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolb-Maurer A, Weissinger F, Kurzai O, et al. Bacterial infection of human hematopoietic stem cells induces monocytic differentiation. FEMS Immunol Med Microbiol. 2004;40:147–153. doi: 10.1016/S0928-8244(03)00305-5. [DOI] [PubMed] [Google Scholar]
- 9.Wengner AM, Pitchford SC, Furze RC, et al. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–49. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P, Quinton LJ, Gamble L, et al. The granulopoietic cytokine response and enhancement of granulopoiesis in mice during endotoxemia. Shock. 2005;23:344–352. doi: 10.1097/01.shk.0000158960.93832.de. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Nelson S, Bagby GJ, et al. The lineage-c-Kit+Sca-1+ cell response to escherichia coli bacteremia in Balb/c mice. Stem Cells. 2008;26:1778–1786. doi: 10.1634/stemcells.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P, Welsh DA, Siggins RW, 2nd, et al. Acute alcohol intoxication inhibits the lineage-c-kit+ sca-1+ cell response to escherichia coli bacteremia. J Immunol. 2009;182:1568–1576. doi: 10.4049/jimmunol.182.3.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu S, Hodgson G, Katz M, et al. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- 14.Stanford WL, Haque S, Alexander R, et al. Altered proliferative response by T lymphocytes of ly-6A (sca-1) null mice. J Exp Med. 1997;186:705–717. doi: 10.1084/jem.186.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito CY, Li CY, Bernstein A, et al. Hematopoietic stem cell and progenitor defects in sca-1/Ly-6A-null mice. Blood. 2003;101:517–523. doi: 10.1182/blood-2002-06-1918. [DOI] [PubMed] [Google Scholar]
- 16.Siggins RW, Melvan JN, Welsh DA, et al. Alcohol suppresses the granulopoietic response to pulmonary streptococcus pneumoniae infection with enhancement of STAT3 signaling. J Immunol. 2011 doi: 10.4049/jimmunol.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh JC, Boggs DR, Cartwright GE, et al. Neutrophil kinetics in acute infection. J Clin Invest. 1967;46:1943–1953. doi: 10.1172/JCI105684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann DW, Entringer MA, Robinson WA, et al. Regulation of granulopoiesis and distribution of granulocytes in early phase of bacterial infection. J Cell Physiol. 1981;109:17–24. doi: 10.1002/jcp.1041090103. [DOI] [PubMed] [Google Scholar]
- 19.Terashima T, Wiggs B, English D, et al. Polymorphonuclear leukocyte transit times in bone marrow during streptococcal pneumonia. Am J Physiol. 1996;271:L587–92. doi: 10.1152/ajplung.1996.271.4.L587. [DOI] [PubMed] [Google Scholar]
- 20.Theilgaard-Monch K, Jacobsen LC, Borup R, et al. The transcriptional program of terminal granulocytic differentiation. Blood. 2005;105:1785–1796. doi: 10.1182/blood-2004-08-3346. [DOI] [PubMed] [Google Scholar]
- 21.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–6. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 22.Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumley TP, McKenzie IF, Sandrin MS. Tissue expression, structure and function of the murine ly-6 family of molecules. Immunol Cell Biol. 1995;73:277–296. doi: 10.1038/icb.1995.45. [DOI] [PubMed] [Google Scholar]
- 24.Bonyadi M, Waldman SD, Liu D, et al. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in sca-1/Ly-6A null mice. Proc Natl Acad Sci U S A. 2003;100:5840–5845. doi: 10.1073/pnas.1036475100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang HH, Hemberg M, Barahona M, et al. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Essers MA, Offner S, Blanco-Bose WE, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 27.Ma X, Robin C, Ottersbach K, et al. The ly-6A (sca-1) GFP transgene is expressed in all adult mouse hematopoietic stem cells. Stem Cells. 2002;20:514–521. doi: 10.1634/stemcells.20-6-514. [DOI] [PubMed] [Google Scholar]
- 28.Okada S, Nakauchi H, Nagayoshi K, et al. In vivo and in vitro stem cell function of c-kit- and sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 29.Arndt PG, Suzuki N, Avdi NJ, et al. Lipopolysaccharide-induced c-jun NH2-terminal kinase activation in human neutrophils: Role of phosphatidylinositol 3-kinase and syk-mediated pathways. J Biol Chem. 2004;279:10883–10891. doi: 10.1074/jbc.M309901200. [DOI] [PubMed] [Google Scholar]
- 30.Utsugi M, Dobashi K, Ono A, et al. JNK1 and JNK2 differently regulate IL-12 production in THP-1 macrophage cells. Cytokine. 2010;51:127–131. doi: 10.1016/j.cyto.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 32.Oak S, Mandrekar P, Catalano D, et al. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- 33.Dai Q, Pruett SB. Ethanol suppresses LPS-induced toll-like receptor 4 clustering, reorganization of the actin cytoskeleton, and associated TNF-alpha production. Alcohol Clin Exp Res. 2006;30:1436–1444. doi: 10.1111/j.1530-0277.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 34.Szabo G, Dolganiuc A, Dai Q, et al. TLR4, ethanol, and lipid rafts: A new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243–1249. doi: 10.4049/jimmunol.178.3.1243. [DOI] [PubMed] [Google Scholar]
- 35.Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10:201–209. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- 36.Singh P, Yao Y, Weliver A, et al. Vaccinia virus infection modulates the hematopoietic cell compartments in the bone marrow. Stem Cells. 2008;26:1009–1016. doi: 10.1634/stemcells.2007-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Ren G, Liang L, et al. Brief report: Interferon-gamma induces expansion of lin(−)sca-1(+)C-kit(+) cells. Stem Cells. 2010;28:122–126. doi: 10.1002/stem.252. [DOI] [PubMed] [Google Scholar]
- 38.Bradfute SB, Graubert TA, Goodell MA. Roles of sca-1 in hematopoietic stem/progenitor cell function. Exp Hematol. 2005;33:836–843. doi: 10.1016/j.exphem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 39.MacGregor RR. Alcohol and immune defense. JAMA. 1986;256:1474–1479. [PubMed] [Google Scholar]
- 40.Meagher RC, Sieber F, Spivak JL. Suppression of hematopoietic-progenitor-cell proliferation by ethanol and acetaldehyde. N Engl J Med. 1982;307:845–849. doi: 10.1056/NEJM198209303071402. [DOI] [PubMed] [Google Scholar]
- 41.Imperia PS, Chikkappa G, Phillips PG. Mechanism of inhibition of granulopoiesis by ethanol. Proc Soc Exp Biol Med. 1984;175:219–225. doi: 10.3181/00379727-175-41792. [DOI] [PubMed] [Google Scholar]
- 42.Tisman G, Herbert V. In vitro myelosuppression and immunosuppression by ethanol. J Clin Invest. 1973;52:1410–1414. doi: 10.1172/JCI107314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Attar EC, Scadden DT. Regulation of hematopoietic stem cell growth. Leukemia. 2004;18:1760–1768. doi: 10.1038/sj.leu.2403515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Peripheral granulocyte counts during septicemia. Changes in the number of circulating granulocytes (A) 24 h and (B) 48 h post E.coli challenge. Data are mean ± SEM (N=5–8). Bars with different letters are statistically different (p < 0.05).
Supplemental Digital Content 2. Quantified changes in marrow cell populations during septicemia. Changes in the number of marrow Sca-1+, Gr1lo, and Gr1hi cells after E.coli challenge. Data are mean ± SEM (N=5–8). Bars with different letters are statistically different (p < 0.05).