Abstract
Background and the purpose of the study
Heat Shock Protein 90 (Hsp90) is typically the most abundant chaperone in the eukaryotic cell cytoplasm, and its expression is essential for loading immunogenic peptides onto major histocompatibility complex molecules for presentation to T-cells. Therefore, it may act as a good candidate as an adjuvant molecule in vaccine technology.
Methods
Initially the human Hsp90β gene was cloned into the heat inducible expression vector pGP1-2 and then the recombinant protein was isolated by ion exchange chromatography. After intradermal injection of confirmed purified band of protein to rabbits and isolation of the serum IgG antibody, for its affinity purification, the rabbit's purified Hsp90 specific IgG was coupled to the cyanogen bromide-activated Sepharose 4B.
Results
The recovery of the purified protein of interest by affinity chromatography was 50%.
Conclusion
This research enabled purification of human heat shock protein by a laboratory prepared column chromatography.
Keywords: Head shock protein 90(Hsp90), CNBr activated Sepharose 4B, Rabbit IgG, pGP1-2
INTRODUCTION
Hsp90 (heat shock protein 90) is a molecular chaperone that modulates the stability and/or transport of a diverse set of critical cellular regulatory including metabolism, organization, and signaling proteins. Binding to Hsp90 is required for normal function of many proteins (1). In addition purified heat shock proteins (Hsps) can be applied in several aspects of biology, medicine and protein biotechnology. In mammalian cells, there are two genes encoding cytosolic Hsp90 homologues with the human Hsp90α which show 85% sequence identity to Hsp90β (2, 3). The protein has been implicated in loading immunogenic peptides onto major histocompatibility complex molecules for presentation to T cells(4,5). They are immunogenic and can be used for anticancer vaccines6.
Immunoaffinity purification is a highly specific and reversible technique7 which was used in this investigation to produce the recombinant Hsp90 without using any chemicals as a convenient adjuvant and chaperone in human vaccines.
MATERIAL AND METHODS
Cell line and Plasmids
The MDA-MB468 cell line was obtained from the Pasteur Institute of Iran. Escherichia coli strains NovaBlue and DH5α were purchased from Invitrogen, USA. pTZ57R (Fermentas, Germany) and pGP1-2 (donated by Dr. Bagher Yhakhchali NIGEB, Tehran, Iran) were plasmids which were used in this study. Human Hsp90 polyclonal antibody was purchased from Abcam, UK.
Cell culture and stimulation
The MDA- MB468 cell line was maintained in RPMI (Roswell Park Memorial Institute, Sigma, Germany) containing 2mM L-glutamine (Sigma, Germany), 100 U/ml penicillin (Sigma, Germany), and 100µg/ml streptomycin (Sigma, Germany) supplemented with 10% fetal bovine serum(Gibco,UK)8. The plateau level for these cultures was about one million viable cells/ml. The sufficient flasks were induced by 50 ng of phorbol myristate acetate (PMA)( Sigma, Germany) without any physical manipulation9.
RT –PCR and Cloning into the pGP1-2 vector
The MDA-MB468 cell line was analyzed for Hsp90 expression. The total RNA was extracted by RNeasy mini kit (Qiagen, USA, 74104) and the generation of cDNA and PCR was carried out by C.therm Polymerase One-Step RT-PCR System (Roche, Germany, 12016338001). Specific primers were designed by Generunner software: H90F, 5′TC CGG ACC TGA GGA AGT GCA CCA3′ and H90R, 5′ GGT NAC CCT AAT CGA CTT CTT CCA TGC 3′ (10). The Hsp90 gene PCR product was cloned into the pTZ57R T-vector (Fermentas, Lituvania) as described previously11. The recombinant plasmid was constructed by using the Kpn2I and BstEII restriction enzymes (Fermentas, Lituvania).
Expression, primary purification and analysis of the human Hsp90β subunit
The Nova Blue strain of E. coli was transformed using above construct and protein expression was performed in LB (Lorian Bertani) medium containing 15µg/ml of kanamycine (Fermentas, Lituvania) (12, 13). The heat induced culture of expressed Novablue strain of Ecoli with pGpHsp (pGP1-2 & human Hsp90β subunit) was prepared and analyzed as described previously (14).
Polyclonal antibody production in Rabbits
The purified Hsp90β (10 µg) prepared from acrylamide as described previously (15) was used for immunization of rabbits subcutaneously. The serum antibody response was determined after secondary immunization by double diffusion test and also following booster injection (16).
Preparation of the Hsp90β -specific IgG Sepharose 4B column
In this stage the common carotid artery of injected rabbits was used to collect large amount of blood after anesthetizing by 100-150 mg/kg ketamine (Merck, Germany). The IgG antibody of rabbits sera was isolated by column chromatography using 3ml protein A resin (17). Rabbit's purified IgG was used as ligand to designe the affinity chromatography column, as follow. For affinity purification of antibodies, immunoadsorbent columns were prepared with Hsp90β coupled to cyanogen bromide-activated Sepharose 4B (GE Healthcare Bio-sciences, Sweden, 17-0430-01) (www6.gelifesciences.com).The above procedure allowed coupling of all specific IgG antibody (IgG pI 3-9) against HSP90 protein which were coupled to cyanogen bromide-activated Sepharose 4B.
Final Hsp90β purification by Hsp-specific IgG Sepharose 4B column
The heat induced culture was harvested and the extracted protein was used for purification of the Hsp90 by the prepared affinity column as described above. The proceed lysate (10ml) was loaded onto the immunoadsorbent column at a flow rate of 0.5 ml/min. Bound proteins were eluted using a glycine-HCl (0.1M) and 2M NaCl at pH 5 (www6.gelifesciences.com) and confirmed by the double diffusion test and western blotting using a human Hsp90 polyclonal antibody.
RESULTS
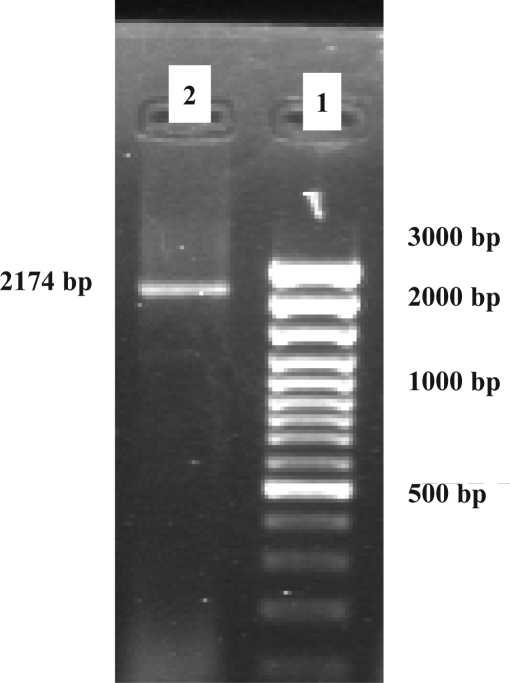
The present study demonstrates solation of the recombinant human Hsp90β subunit by ion exchange chromatography after cloning and expression of the RT-PCR product (Fig 1). Figure 1 shows the 2174 bp PCR product representing the human Hsp90 gene.
Figure 1.
PCR product of Hsp90 on 1% agarose gel
Lane 1: 100 bp DNA Ladder (Fermentas, Germany)
Lane 2: 2174 bp Hsp90 PCR product
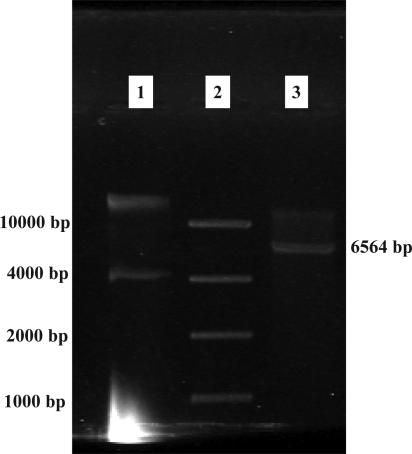
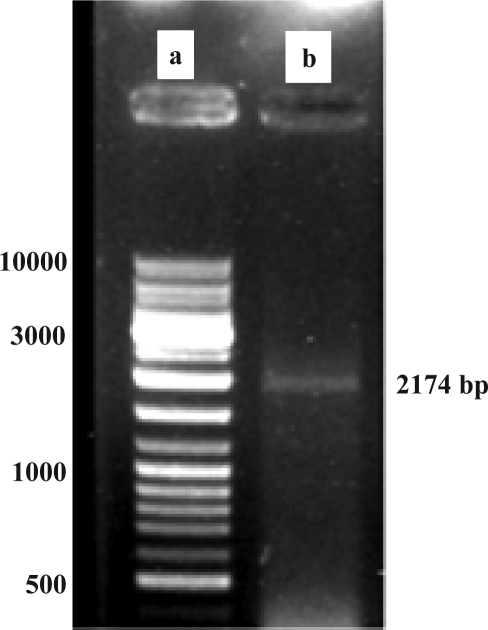
Figure 2 shows confirmation of subcloning of Hsp90β segment by DraII restriction enzyme digestion. Also, this vector contained the kanamycine resistance gene and after transformation, bacteria could grow on medium supplemented with 30µg/ml kanamycine. The resulting clone was amplified using Hsp90 specific primers (Fig 3).
Figure 2.
pGp1-2 subcloning confirmation
Lane 1: Undigested cloned plasmid
Lane 2: Gene Ruler 1kb DNA Ladder (Fermentas, Germany)
Lane 3: Digested plasmid by DraII (linear form)
Figure 3.
Confirmation of Hsp90 Cloning into the pGP1-2 by specific PCR
Lane a: Gene Ruler 1kb DNA Ladder (Fermentas, Germany)
Lane b: Direct PCR product of cloned plasmid
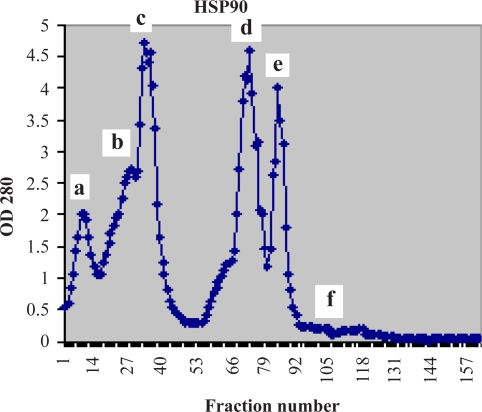
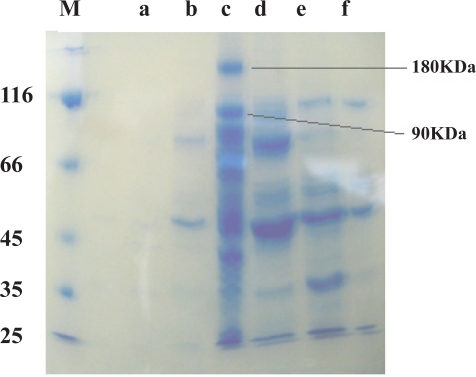
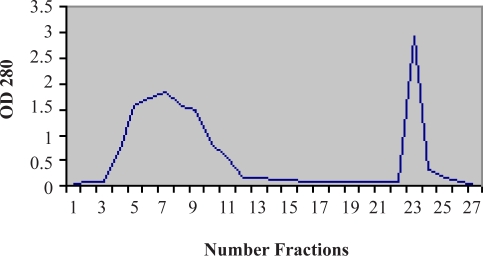
The 160 fractions obtained from ion exchange chromatography of the expressed bacterial cells were determined by a biophotometer in 280 nm (Fig 4&Fig 5). According to our findings the chromatography parameters were regularized as: resolution 2, selectivity 2.5, efficiency 4 and capacity 30%.
Figure 4.
Showing Hsp90β subunit purification chromatogram of fractions obtained from ion exchange chromatography of expressed construct
a, b: Loading & washing stages
c: Elution stage
d, e, f: Washing stage
Figure 5.
10% SDS-PAGE of obtained fractions which are the peaks signed in Fig4.It was showed 90kDa protein band in lane c.
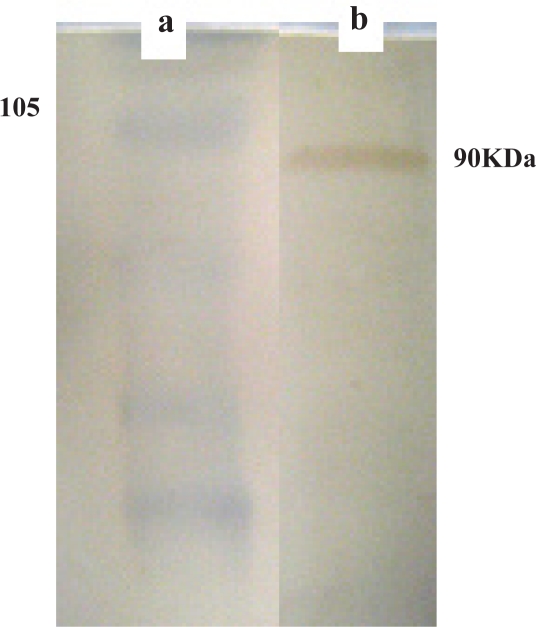
The Hsp90 protein required for preparation of a specific affinity chromatographic procedure was obtained by gel extraction of 90kDa band of fraction C (in Fig 4) and confirmed by western blotting using polyclonal antibody against Hsp90β subunit (Fig 9).
Figure 9.
Western Blotting of recombinant protein purified by βHsp90- specific sepharose 4B using polyclonal antibody
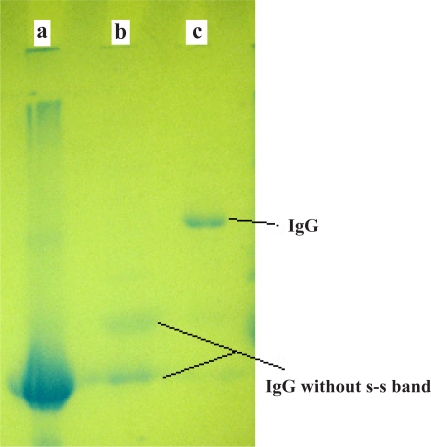
Figure 6 & Figure 7 show the result of IgG isolation by different stages of the protein A column chromatography and its electrophoresis with non-reducing gel. While in the native IgG sample (lane c), a single predominant band is observed, the reduced IgG sample by DTT in lane b shows two bands related to light and heavy chains of antibody. In order to obtain pure Hsp90 by simple affinity chromatography with recovery 50%, the interested protein was identified by double diffusion and western blotting tests (Figs. 8 and 9).
Figure 6.
Chromatogram of IgG purification from serum by protein A affinity chromatography
1- 3 –11: Loading
2- 12- 22: Washing
3- 23 – 25: Elution
4- 26–30: Washing
Figure 7.
10% SDS-PAGE of Ig G after Protein A affinity chromatography
Lane a: Total serum proteins
Lane b: Boiling with SDS sample buffer
Lane c: Purified IgG
Figure 8.
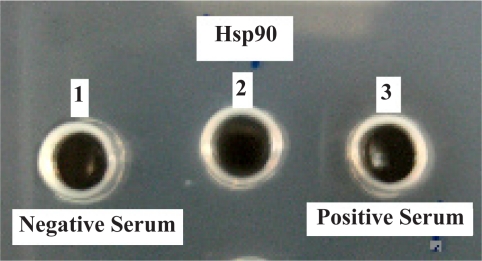
Immuno diffusion test using injected rabbit serum (+) and negative control (−)
1- Rabbit's serum without injection
2- Purified Hsp90 band by gel extraction
3- Immunized rabbit's serum
DISCUSSION
Hsps have a variety of important and functions in immunity (5) and stimulate a wide range of innate and adaptive immune responses. The peptide binding function allows chaperon proteins such as Hsp90, Hsp70, and gp96 to acquire antigenic proteins within the cells when administered outside of the cell to induce priming of CD8+ T lymphocytes in vivo18.
The main aim of the present study was to establish an efficient and easy-to-use method for purification of Hsp90. In immunoaffinity chromatography which can be considered as a subset of affinity chromatography, an immunoglobulin is used to bind analyte molecules. Because immunoglobulins show exquisite specificities towards antigens which are used in their production, immunoaffinity columns are ideal for purification of the targeted analytes (3). This study proposes that the specific IgG antibody against Hsp90 protein could be used for its purification by affinity chromatography; is highly selective for Hsp90 and has an appropriate affinity for this molecule. This strategy in which antibodies are immobilized in a single orientation generally involves immobilization of an intermediate protein such as protein A that specifically binds to the Fc region of an antibody and it can be coupled to a suitable commercially available matrix without affinity loss. In this study, the pGp1-2 expression vector was initially used for production of Hsp90. A heat-induced transition of Hsp90 was then necessary to exert the chaperone activity (18). This treatment which caused self-oligomerization of Hsp90 under substrate-free conditions indicates a close relationship between self-oligomerization and the substrate-binding properties. Many in vitro studies on the chaperone function of Hsp90 have been performed around 25°C and are triggered by the addition of denatured proteins in renatured buffers containing Hsp90 without other chaperones and co-chaperones (19).
CONCLUSIONS
In this study natural and oligomeric forms of the Hsp90β subunit were produced by the designed affinity chromatographic purification procedure without using any tag peptides or other materials such as IPTG leading to 50% recovery. In future chaperoning and adjuvant effects of Hsp90 in recombinant vaccines will be investigated.
ACKNOWLEDEMENTS
This study was supported by the Vice Chancellor for National Institute of Genetic Engineering and Biotechnology(grant number 355), Tehran, Iran and Shahid Beheshti University of Medical Sciences, Cellular and Molecular Biology Research Center.
REFERENCES
- 1.Barginear MF, Van PC, Rosen N, Modi S, Hudis CA, Budman DR. The heat shock protein 90 chaperone complex: an evolving therapeutic target. Curr Cancer Drug Tar. 2008;8:522–532. doi: 10.2174/156800908785699379. [DOI] [PubMed] [Google Scholar]
- 2.Smith DF, Whiteseli L, Katsanis E. Molecular Chaperones: Biology and Prospects for Pharmacological Intervention. Pharmacol Rev. 1998;4:493–514. [PubMed] [Google Scholar]
- 3.Minami M, Nakamura M, Emori Y, Minami Y. Both the N- and C- terminal chaperone sites of Hsp90 participate in protein refolding. Eur J Biochem. 2001;268:2520–2524. doi: 10.1046/j.1432-1327.2001.02145.x. [DOI] [PubMed] [Google Scholar]
- 4.Przepiorka D, Srivastava PK. Heat Shock protein-peptide complexes as immunotherapy for human cancer. Mol Med Today. 1998;4:478–484. doi: 10.1016/s1357-4310(98)01345-8. [DOI] [PubMed] [Google Scholar]
- 5.Basu S, Srivastava P. Fever-like temperature induces maturation of dendritic cells through induction of Hsp90. Int Immunol. 2003;15:1053–1061. doi: 10.1093/intimm/dxg104. [DOI] [PubMed] [Google Scholar]
- 6.Calderwood SK, Cicca DR. Heat shock proteins: stress proteins with janus-like properties in cancer. Int J Hyperther. 2008;24:31–39. doi: 10.1080/02656730701858305. [DOI] [PubMed] [Google Scholar]
- 7.Asea A, Calderwood S. Heat Shock Proteins: Potent Mediators of Inflammation and Immunity, The Netherlands. Springer. 2007;1:61–100. [Google Scholar]
- 8.Pashov A, Kenderov A, Kyurkchiev S, Kehayov I, Hristova S, Lacroix S, Giltiay N, Varamballi S, Kazatchkine MD, Kaveri SV. Auto antibodies to heat shock protein 90 in the human natural antibody repertoire. Int Immunol. 2002;14:453–461. doi: 10.1093/intimm/14.5.453. [DOI] [PubMed] [Google Scholar]
- 9.Purcell AW, Todd A, Kinoshita G, Lynch TA, Keech CL, Gething MJ, Gordon TP. Association of stress proteins with autoantigens: a possible mechanism for triggering autoimmunity? Clin Exp Immunol. 2003;132:193–200. doi: 10.1046/j.1365-2249.2003.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Innis MA, Gelfand DH, Sninsky JJ. PCR Strategies. London: Academic Press; 1995. pp. 58–65. [Google Scholar]
- 11.Kazemi B, Bandehpour M, Maghen L, Solgi G. Gene Cloning of 30 kDa Toxoplasma gondii Tachyzoites Surface Antigen (SAG1) Iran J Parasitol. 2007;2:1–8. [Google Scholar]
- 12.Sorensen H, Mortensen K. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol. 2005;115:113–128. doi: 10.1016/j.jbiotec.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotech. 1999;10:411–421. doi: 10.1016/s0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 14.Bandehpour M, Seyed N, Shadnoosh M, Pakzad P, Kazemi B. Using recombinant Chlamydia Major Outer Membrane Protein (MOMP) in ELISA diagnostic kit. Iran J Biotech. 2006;4:239–244. [Google Scholar]
- 15.Speicher KD, Kolbas O, Harper S, Speicher DW. Systematic analysis of peptide Recoveries from In-Gel Digestions for femtomole protein Identifications in Proteome studies. J Biomol Tech. 2000;11:74–86. [PMC free article] [PubMed] [Google Scholar]
- 16.Hay FC, Westwood OMR. Practical Immunology. USA: Blackwell Science; 2002. pp. 163–178. [Google Scholar]
- 17.Mikszta JA, DekkerIII JP, Harvayl NG, Dean CH, Brittingham JM, Huang J, Sullivan VJ, Dyas B, Roy CJ, Ulrich RG. Micro needle Based intradermaly delivery of the anthrax recombinant protective antigen vaccine. Infec Immun. 2005:6806–6810. doi: 10.1128/IAI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Kelly CG, Singh M, McGowan EG, Carrara A, Bergmeier LA, Lehner T. Stimulation of Th1-polarizing cytokines,C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of Heat Shock Protein 70. J Immunol. 2002;169:2422–2429. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- 19.Nemoto TK, Ono T, Tanaka K. Substrate-binding characteristics of proteins in the 90kDa heat shock protein family. Biochem J. 2001;354:663–670. doi: 10.1042/0264-6021:3540663. [DOI] [PMC free article] [PubMed] [Google Scholar]