Abstract
Background and the purpose of the study
Lamotrigine (LMG) undergoes extensive hepatic metabolism upon oral administration and its absorption is affected in the presence of food. This study was aimed to develop nanosuspension of LMG and investigate its formulation characteristics using L9 orthogonal array.
Methods
Nanosuspension was prepared using emulsification-solvent diffusion method. All the formulations were subjected to in-vitro evaluation and the statistically optimized one was used for stability, scanning electron microscopic and differential scanning calorimetric studies.
Results
Nanoparticles were spherical with little surface adsorbed drug. Formulation characteristics in terms of size, zeta potential, polydispersity index (PDI), entrapment efficiency (EE), drug content and in vitro drug release were consistent and within their acceptable range. All the batches provided a burst release profile during first 1 hr, followed by a controlled release extending up to 24 hrs. The values of n in Peppas model ranged between 0.2-0.4 for all the formulations indicative of Fickian release mechanism. The formulation remained reasonably stable up to 3 months. No interaction was observed among the drug and polymers.
Major conclusion
Results of in vitro drug release studies suggested that nanosuspension might be used as a sustained delivery vehicle for LMG. Statistical analysis revealed that size of the nanoparticles was most strongly affected by stabilizer type while EE was influenced by the drug-to-polymer ratio.
Keywords: Lamotrigine, L9 orthogonal array, Emulsification-solvent diffusion, Fickian diffusion
INTRODUCTION
Lamotrigine (LMG), an antiepileptic drug of Phenyltriazine class, is used for the treatment of partial seizures and those associated with the Lennox-Gastant syndrome. It is a basic drug (pKa 5.3) with an intrinsic solubility of 0.17 mg/ml and undergoes extensive hepatic metabolism upon oral administration (1). Also, its absorption is affected in the presence of food (2). Existing formulations of LMG provide immediate release with tmax ranging from 1.4 to 4.8 hrs and result into a release profile with various peaks and troughs. Therefore, it was proposed to develop the nanosuspension of LMG which would improve its solubility and provide the plasma concentrations within the therapeutic window over a longer period of time.
Nanosuspensions are sub-micron colloidal dispersions of pure drug particles in an outer liquid phase. They prolong the contact time of drug thereby enhancing its uptake via the GIT, provide the drug release which is unaffected by GI variations and have a better stability profile than liposomes. They also enhance saturation solubility of the drug as a result of improvement in dissolution rate (3–5).
Several methods have been proposed for the preparation of polymeric nanoparticles, e.g. solvent evaporation (6), nanoprecipitation (7) and salting-out (8). These methods suffer from certain potential disadvantages, such as the use of toxic solvents, low yield, high energy consumption and presence of bio-incompatible residual salts (9). Emulsification-solvent diffusion method is interesting for many reasons such as: the use of pharmaceutically acceptable organic solvents, high yields, good reproducibility and easy scale up (10). Eudragits have been used extensively for particulate systems such as microspheres (11), pseudolatex (12) and nanoparticles (11). Their uses in the present study stems from the assumption that they interact with the negatively charged mucosal surface owing to their polycationic nature (13) which would prolong the residence of formulation in gastro-intestinal tract and render the treatment more effective.
Various factors affecting the properties of nano-suspension using L9 orthogonal design have been reported (14). Thus, objective of the study was to develop and evaluate the controlled release nanosuspension of LMG by emulsification diffusion method and to study various process parameters affecting their characteristics by using experimental design approach.
MATERIAL AND METHODS
Material
Lamotrigine (LMG), poly vinyl alcohol (PVA) and poloxamer 188 were obtained as gift samples from Glenmark Pharmaceuticals Ltd, Mumbai, India. Eudragit RLPO (E-RLPO) and Eudragit RSPO (E-RSPO) were obtained from Lupin Research Park, Pune, India. Tween 80 was procured from Loba Chemie, Mumbai, India. All other chemicals were of analytical grade and used without any further purification.
Methods
Preparation of the nanosuspension
Nanosuspension was prepared by the emulsification-diffusion process (4). Triple distilled water (TDW) and ethyl acetate were mutually saturated for 10 min to achieve initial thermodynamic equilibrium (15). Weighed amount of polymer and LMG (as per the experimental design) were dissolved in 10 ml of water saturated solvent followed by its emulsification with 20 ml of 5% (w/v) stabilizer at 2000 rpm for 15 min. Then 80 ml of TDW was added to the emulsion with subsequent stirring for 15 min to induce diffusion of organic solvent into the continuous phase and leading to the formation of nanoparticles (10). Organic solvent was removed by evaporation for approximately 50 min under reduced pressure at 30°C. Each experimental run was performed in triplicate.
Characterizations of the prepared nanosuspension Fourier transform infrared (FT-IR) and Differential scanning calorimetric (DSC) studies
FT-IR spectra of LMG and its physical mixtures (1:1) with the polymers were recorded by grinding and dispersing the samples with micronised IR grade KBr powder. The mixture was dissolved in chloroform and casted on a sodium chloride disk, and subjected to FT-IR measurement over the range of 4000–400 cm− with a resolution of 4 cm−1 for 50 scans (Schimadzu, Model 8400, Japan).
DSC studies were performed for the pure drug, pure polymer and freeze dried sample (Decibel Instruments, India) of the optimized formulation. The sample (∼5 mg) was weighed on aluminium pan and heated to 300°C at a rate of 10°C/min and the thermogram was recorded (DU-PONT, Model 9900, USA). An empty aluminum pan was used as a reference (16).
Particle size, polydispersity index (PDI) and zeta potential (ζ)
Nanosuspensions were diluted with filtered (0.22 µm) ultrapure water and analyzed by photon correlation spectroscopy (PCS) with Master sizer (Malvern Instruments, UK) yielding the mean particle diameter of the suspension and polydispersity index (17). Values of ζ were assessed by determining the electrophoretic mobility of particles using the same instrument. The mobility, µ was converted to the ζ-potential by Smoluchowski relation (18).
Total drug content (TDC) and entrapment efficiency (EE)
An aliquot (0.5 ml) of the prepared nanosuspension was evaporated to dryness. The residue was dissolved in methanol and filtered with a 0.45 µm filter. Total drug content was determined by UV spectrophotometry at λmax of 309 nm using the formula:
For the analysis of free dissolved drug (FDD), 2 ml of nanosuspension was centrifuged at 15000 rpm at 4°C (Remi Instruments, Mumbai, India) for 1 hr and the supernatant was immediately analyzed spectrophotometrically (λmax 266 nm). Settled nanoparticles were washed three times with 0.1N HCl and analysis of combined washings provided surface adsorbed drug (SAD). Entrapment efficiency was determined from TDC and FDD using the formula:
In-vitro drug release studies
Dialysis bag diffusion technique was used to study in-vitro release of drug from the prepared nanosuspension (19). The formulation (2ml) was then placed in the dialysis bag (Sigma Aldrich, Molecular Weight cut off 12000 Da), hermetically sealed and immersed into a 100 ml beaker containing 50 ml of the release media maintained at 37±0.5°C and stirred at 500 rpm (Decibel Instruments, India). Aliquots of 5 ml were with drawn at pre-determined time intervals (½, 1, 2, 3, 4, 5, 6, 7, 8 and 24 hrs) and immediately restored with the same volume of fresh media maintained at the same temperature. The study was carried out by buffer change method using acidic buffer (0.1 N HCl) of pH 1.2 for the first 2 hrs, citrate buffer (pH 4.5) for next 2 hrs and phosphate buffer (PB, pH 7.4) for the rest of the study period, i.e. 20 hrs. Since LMG has limited solubility in PB of pH 7.4 under the conditions of in-vitro dissolution studies; 1% v/v Tween 80 was used to maintain the sink condition. The drug was analyzed spectrophotometrically at 266 nm for acidic buffer and at 309 nm for citrate and phosphate buffers. Data from in-vitro drug release studies were fitted to various models to study the drug release mechanism. Table 1
Table 1.
Control variables with their levels used in experimental design
| Variables | Level 1 (−1) | Level 2 (0) | Level 3 (+1) |
|---|---|---|---|
| Amount of polymer (per 50 ml) | 200 mg | 400 mg | 800 mg |
| Amount of drug (per 50 ml) | 12.5 mg | 25 mg | 50 mg |
| Stabilizer type | PVA | PLX | Tween 80 |
| Polymer type | E-RLPO | E- RLPO: E-RSPO (1:1) | E-RSPO |
PVA-Poly vinyl alcohol, PLX-Poloxamer 188, E-RLPO-Eudragit RLPO, E-RSPO-Eudragit RSPO
In addition, the optimized formulation was subjected to the drug release studies in citrate buffer (pH 4.5) for initial 4 hrs and in PB of pH 7.4 with 1% Tween 80 for the rest of 20 hrs to assess its release properties in altered pH conditions due to the presence of food in the stomach and as a result to establish its superiority over the conventional formulations.
Scanning electron microscopic (SEM) and stability studies
Scanning electron micrographs (Hitachi S3400N, Japan) of the statistically optimized formulation were taken to study the size and morphology of the prepared nanoparticles (18). For stability studies, formulation was stored in hermetically closed glass vials and kept at 40±2°C/75±5%RH for 3 months. It was evaluated for particle size, entrapment efficiency and in-vitro drug release studies.
Statistical optimization and analysis
Experimental variables (amount of polymer, amount of drug, types of the stabilizer and polymer) were identified during preformulation studies and investigated at three pre-decided levels (−1, 0 and +1) using a fractional factorial design in the form of L9 orthogonal array. Classical approach of varying one variable at a time requires a total of 81 34 experiments. However, present design simplified the experimental effort and reduced the number of experiments to nine. Experimental runs were performed in randomized sequence and signal-to-noise ratio (SNR) was calculated using the performance characteristics “smaller-the-better” or “larger-the-better”, whichever appropriate (14, 20, 21). ANOVA was performed for each batch. P values of 0.05 were considered significant. The formulation prepared under statistically optimized experimental conditions was utilized for DSC, SEM and stability studies.
RESULTS AND DISCUSSION
FT-IR and DSC studies
Fig. 1 shows the FT-IR spectra of pure LMG and its 1:1 mixtures with E-RLPO and E-RSPO. Characteristic peaks of LMG were obtained at 3445 cm−1, 2353 cm−1, 1620 cm−1 and in the range of 1300–1500 cm−1 which remained unaffected in the presence of polymers. Neither disappearance nor suppression of the peaks was observed, thus ruling out the possibilities of drug-polymer interaction. Furthermore, FT-IR spectra of the formulation also showed peaks in the region of 1300–1500 cm−1 which confirmed loading of drug into the formulation with no interactions with the polymer (data not shown). As it might be evidenced from DSC studies (Fig. 2), pure LMG showed a sharp endothermic peak at 217.8°C with a melting enthalpy of 116.6 J/g while that of E-RSPO showed a relatively flat thermal profile indicative of the amorphous nature of the polymer. A weak peak could be detected at 71.0°C for E-RSPO with a melting enthalpy of −5.4 J/g.
Figure 1.
FT-IR spectra: a) LMG, b) LMG+E-RSPO c) LMG+E-RLPO
Figure 2.
DSC thermograms: a) pure drug, b) E-RSPO nanoparticles loaded with LMG, c) E-RSPO
None of the peaks of LMG could be detected in the thermogram of drug loaded nanoparticles thus neglecting the possibilities of the presence of crystalline drug in the formulation. A peak, detected at 82.6°C, confirmed molecular dispersion of LMG in the formulation which is characterized by a single Tg that shifts between those of pure drug and polymer as a function of drug to polymer ratio in the mixture (22, 23).
Particle size, polydispersity index (PDI) and zeta potential (ζ)
Particle size ranged from 55 nm (Batch LNP3) to 478 nm (Batch LNP8) and seemed to be affected by relative viscosity of the polymeric dispersion in the presence of stabilizers which followed the trend: PVA>Poloxamer>Tween (observed visually). Batches with Tween as stabilizer (Batches LNP3, LNP5 and LNP7) possessed the least particle size while those containing PVA (Batches LNP1, LNP6 and LNP8) had the largest particle size (Table 2). PVA contains a number of OH groups, which might have formed hydrogen bonds with the solvent resulting in an increased viscosity of the dispersion. Consequently, the energy applied during homogenization might have become insufficient to overcome the resistive viscous forces, leading to comparatively larger droplets. Besides, PVA forms a connected network with the polymer at the interface and remains associated despite repeated washings, which may be another reason for the formation of larger sized nanoparticles (9). On the other hand, interactions between lipophilic portions of LMG with that of Tween might be substantial for the formation of smaller droplets (5).
Table 2.
Particle size, zeta potential, PDI, drug content and entrapment efficiency for various batches
| Batch code | Size (nm)(Mean±SD), n=3 | Zeta potential(mV) (Mean±SD), n=3 | PDI (Mean±SD),n=3 | %TDC(Mean±SD), n=3 | % EE (Mean±SD),n=3 |
|---|---|---|---|---|---|
| LNP 1 | 323.5±2.12 | 21.97±2.59 | 0.189±0.02 | 95.23±0.32 | 71.86±0.58 |
| LNP 2 | 184±1.41 | 13.82±2.008 | 0.553±0.05 | 96.22±0.54 | 60.68±0.61 |
| LNP 3 | 54.5±4.94 | 0.60±0.008 | 0.351±0.02 | 98.57±0.45 | 52.79±0.44 |
| LNP 4 | 327.5±3.53 | 11.43±0.86 | 0.662±0.04 | 96.17±0.34 | 80.80±0.35 |
| LNP 5 | 60.5±2.12 | 0.66±0.02 | 0.403±0.03 | 97.58±0.44 | 71.11±1.45 |
| LNP 6 | 293.5±10.60 | 16.98±1.69 | 0.195±0.07 | 98.85±0.58 | 64.56±1.004 |
| LNP 7 | 69.0±1.41 | 0.79±0.08 | 0.387±0.025 | 98.01±0.25 | 83.72±0.24 |
| LNP 8 | 477.5±3.53 | 24.92±0.87 | 0.203±0.054 | 97.94±0.466 | 85.77±0.77 |
| LNP 9 | 219.5±2.13 | 14.68±0.26 | 0.540±0.014 | 98.02±0.554 | 73.39±0.2 |
The difference in values of PDI which ranged between 0.189 and 0.662 could be attributed to the efficiency of stabilizers, which cover the organic/ aqueous interface of the emulsion nanodroplets and prevent them form coalescing to each other. Batches with PVA as stabilizer provided better PDI values. Presence of sufficient polymer chains of PVA might have provided comparatively better coverage and therefore, stabilization to the emulsion droplets allowing a homogeneous NP distribution (10). Values of ζ were found to be in the range of +0.60 to + 24.92 indicative of stable formulations (3). Its significantly wider range indicates that it was affected by the variables considered in the study (Table 2). Total drug content for all batches was found to be greater than 95%. Entrapment efficiency varied from 52.79 to 85.77%, depending upon the polymer to drug ratio (Table 2).
In-vitro drug release studies
All batches were similar in providing more than 98% drug release at the end of 24 hrs; indicative of structural homogeneity of the polymeric matrix and uniform distribution of drug content (24). However, they provided a burst release during first 1 hr due to a simultaneous release of surface bound drug (being more than 18%). It differed significantly among different batches (P<0.05, one way ANOVA). Burst phase was, however, followed by hydration and swelling of the nano-matrix which eventually led to a controlled release profile lasting up to 24 hrs. Hydration brings about an increment in the diffusional path length of molecules and consequently the rate of their diffusion becomes lower (25). Therefore, gaining of controlled release profile and its maintenance could be assumed to be dependent upon the relative hydration rate of the polymer and integrity of the hydrated matrix. Therefore, superiority of one formulation over the other could be established on the basis of avoidance of burst release, achievement of a controlled release profile and its maintenance in a time dependent manner.
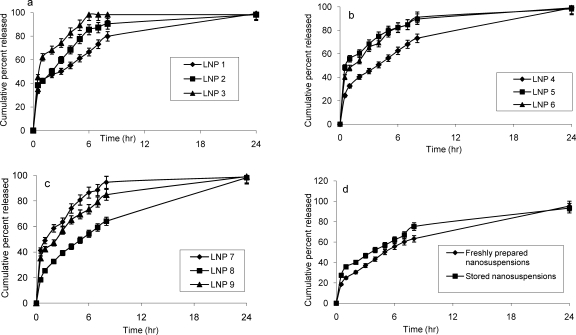
The release rate was found to be mainly affected by: polymer to drug ratio (P: D), stabilizer type and particle size (Fig. 3, a-c). Formulations containing E-RLPO (Batches LNP1, LNP5 and LNP9) possessed similar P: D (8: 1) and differed only with respect to stabilizer type. However, burst effect provided by them followed the order LNP1<LNP9<LNP5. Since other three variables (amount of drug, amount of polymer and polymer type) were common for each of the above formulations, the only factor responsible could be the stabilizer type, i.e. Tween. This idea was strengthened from the finding that other formulations containing Tween (Batches LNP3 and LNP7) also provided significantly higher burst effect (P<0.05, one way ANOVA). Due to surfactant activity of Tween, it might have caused fluidization of the hydrated matrix thus enhancing its rate of erosion and exposing the core of nanoparticles to the solvent front. Consequently, all the formulations containing Tween showed an exceptionally higher burst effect.
Figure 3.
In-vitro release profiles of the prepared LMG nanosuspensions; Figure 4 (d) provides comparative in-vitro release profiles of the freshly prepared and stored batch of optimized nanosuspension (Bars represent standard deviation).
Formulations containing 1:1 mixture of E-RLPO and E-RSPO (Batches LNP2, LNP6 and LNP7) provided burst effect in the order of LNP2<LNP6<LNP7. The difference between LNP2 and LNP6 was not significant (P<0.05, one way ANOVA) which may be due to their similar P: D (8: 1) values. LNP7 showed a significantly higher burst effect (CPR=41% in 0.5 hr) in spite of having higher P: D (64: 1) due to comparatively lower particle size (69 nm) and presence of Tween which might have outweighed the effect of high P: D. A decrease in the mean particle size in nanoparticles leads to an increase in the release rate which could be explained on the basis of surface area relationship (26, 27).
Formulations containing E-RSPO (Batches LNP3, LNP4 and LNP8) followed the order of LNP8<LNP4<LNP3 in showing burst effect; the difference was significant for each possible pair (P<0.05, one way ANOVA). LNP3 having Tween as stabilizer showed exceptionally higher burst effect and 83% of drug was released within 4 hrs. Its lowest P: D (4: 1) and particle size (53 nm) might also have contributed to this effect. LNP4 and LNP8 had similar P: D (32: 1) but the latter exhibited the least burst effect amongst all the batches thus emphasizing the superiority of PVA over poloxamer as stabilizer.
In-vitro drug release performed in citrate buffer pH 4.5 did not exhibit any significant difference in terms of rate and extent (P>0.05, one way ANOVA; data not shown), thus signifying that drug release from the formulation was independent of pH.
Dissolution profile modeling
In-vitro drug release data were fitted to various models and regression coefficient value (r2) was calculated. Higuchi equation showed a better fit (r2>0.9) than the first-order and zero order equations. The values of n, as calculated by Peppas equation (28), ranged between 0.2–0.4 (<0.5) for all the formulations, which is indicative of Fickian type of release mechanism. The results are in agreement to the one reported for ciprofloxacin-Eudragit nanoparticles (29).
Analysis of results and statistical optimization of the formulation
Formulation could be evaluated on the basis of either of the following parameters: particle size, zeta potential, polydispersity index, entrapment efficiency and release properties. As compared to the other parameters, particle size and EE showed considerable variations; therefore these parameters were selected as response parameters and their signal-to-noise ratio (SNR) for each experimental run was determined (Table 3). “Higher the better” hypothesis was considered for %EE since its high value assures that maximum quantity of drug is restrained within the matrix. For particle size, “lower the better” hypothesis was adopted with the assumption that better tissue penetration and cellular uptake could be achieved with the NPs of sufficiently low particle size (30).
Table 3.
SN ratio for the response parameters at different levels
| Factors | Signal-to-noise ratio | |||||||
|---|---|---|---|---|---|---|---|---|
| Particle size | Entrapment efficiency | |||||||
| −1 | 1 | + 1 | R value | −1 | 1 | +1 | R Value | |
| Amount of polymer | −43.41 | −45.10 | −45.73 | 2.32 | 35.75 | 37.13 | 38.15 | 2.41 |
| Amount of drug | −45.76 | −44.84 | −43.64 | 2.12 | 37.91 | 37.12 | 35.99 | 1.92 |
| Stabilizer type | −51.14 | −47.48 | −35.72 | 15.32 | 37.33 | 37.14 | 36.65 | 1.68 |
| Polymer type | −44.22 | −43.81 | −46.21 | 2.41 | 37.16 | 36.77 | 37.19 | 1.39 |
It was concluded that the least particle size could be attained at the factor setting; A1B3C3D2 and stabilizer type was its most powerful determinant. Highest EE could be obtained at A3B1C1D1 signifying that both of these responses can not have their desired values at the same varaible setting. ANOVA results along with R value suggested that stabilizer type was highly significant in determining particle size with a P value of 0.006 at 95% confidance level, while polymer type was insignificant at the same level (31). Thus, other three factors can be arbitrarily assigned level settings without having signiicant effect on the response. Factors A and B, with P values of 0.008 and 0.018 respectively at 95% confidence level, were found to have their highest effects on EE (Table 4). Therefore, level settings C3D2 and A3B1 are of significant importance for the size and EE, respectively. Size of the nanoparticles was considered to be a relatively more important response parameter and therefore it was selected to prepare the stastically optimized formulation. Formulation, prepared in triplicate, was subjected to stability, DSC and SEM studies in addition to other evaluations (data not given).
Table 4.
ANOVA table for the response parameters
| Factors | DoF | SS | V | %contibution | F |
|---|---|---|---|---|---|
| A) Particle size | |||||
| Amount of polymer | (2) | (7004) | (3502.0) | 4.1005 | Pooled |
| Amount of drug | (2) | (5237) | (2618.5) | 3.166 | Pooled |
| Stabilizer type | 2 | 141139 | 71119.5 | 81.98 | 22.88 |
| Polymer type | 2 | 18528 | 9264.1 | 10.84 | 3.03 |
| Pooled error | (4) | (12241) | (6121.5) | ||
| Total | 8 | 170808 | 100 | ||
| B) Entrapment efficiency | |||||
| Amount of polymer | 2 | 553.134 | 276.567 | 57.71 | 21.29 |
| Amount of drug | 2 | 350.652 | 175.326 | 36.59 | 12.86 |
| Stabilizer type | (2) | (35.405) | (17.713) | 3.69 | Pooled |
| Polymer type | (2) | (19.122) | (9.(61) | 1.99 | Pooled |
| Pooled error | (4) | (54.(27) | (27.264) | ||
| Total | 8 | 958.314 | 100 | ||
DoF; degree of freedom, SS; sum of squares, V; variance
SEM and stability studies
SEM microphotographs showed the presence of definite and regular nanoparticles, mostly spherical in shape and with no sign of aggregation (Figure 4). Furthurmore, they showed a homogeneous matrix with little evidence of crystals on the surface. Figure 4 b confirmed the size range of nanoparticles in the range of 214–218 nm. Upon storage, particle size increased from 480 to 683 nm and EE reached to 82.31%. The crystal growth may be due to Ostwald ripening and can be avoided by a careful choice of surfactant (18). The decrease in EE may be attributed to the movement of drug from the matrix to the surface under the influence of temperature. However, electrophoretic behaviour of the formulation did not change significantly (data not shown). Stored formulation showed a slightly higher burst release as compared to the freshly prepared batch. The release was, however, controlled thereafter for the whole period of the study (Fig. 3 d).
CONCLUSION
LMG loaded eudragit nanosuspensions were successfully prepared using emulsification-solvent diffusion method. Properties of prepared nanosuspensions in terms of size, zeta potential, PDI, EE, drug content and in-vitro drug release were consistent and within their acceptable ranges. As evidenced form statistical analysis, size of the nanoparticles was most strongly affected by stabilizer type while EE was influenced by the drug-to-polymer ratio. Release rate seemed to be governed by rate of diffusion of drug from polymeric matrix. The formulation remained reasonably stable up to 3 months under stressed storage conditions. In-vivo studies, proposed in future, will establish its potential as an alternative to the existing conventional formulations.
ACKNOWLEDGEMENTS
School of Material Science, I.T-B.H.U. is acknowledged for providing DSC facility. Authors thank University Grants Commission, New Delhi for their financial support to carry out this research work.
REFERENCES
- 1.Mashru R, Sutariya V, Sankalia M, Sankalia J. Transbuccal delivery of lamotrigine across porcine buccal mucosa: in vitro determination of routes of buccal transport. J Pharm Sci. 2005;8:54–62. [PubMed] [Google Scholar]
- 2.Sharma C, Dubey R, Kumar H, Saha N. Food reduces the bioavailability of lamotrigine. Indian J Med Res. 2005;121:659–664. [PubMed] [Google Scholar]
- 3.Muller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv Drug Del Rev. 2001;47:3–19. doi: 10.1016/s0169-409x(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 4.Esmaeili F, Atyabi F, Dinarvand R. Preparation and characterization of estradiol-loaded PLGA nanoparticles using homogenization-solvent diffusion method. DARU. 2008;16:196–202. [Google Scholar]
- 5.Kocbek P, Baumgartner S, Kristl J. Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int J Pharm. 2006;312:179–186. doi: 10.1016/j.ijpharm.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Song C, Labhasetwar V, Guzman L, Topol E, Levy RJ. Dexamethasone nanoparticles for intraarterial localisation in retinosis in rats. Proc Intern Symp Control Rel Bioact Mater. 1995;22:444–445. [Google Scholar]
- 7.Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Rel. 1999;57:171–185. doi: 10.1016/s0168-3659(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 8.Allemann E, Leroux JC, Gurny R, Doelker E. In vitro extended release properties of drug-loaded poly(DL-lactic acid) nanoparticles produced by a salting out procedure. Pharm Res. 1993;10:1732–1737. doi: 10.1023/a:1018970030327. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Cui W, Bei J, Wang S. Preparation of poly (lactide-co-glycolide-co-caprolactone) nanoparticles and their degradation behaviour in aqueous solution. Polymer Degrad Stab. 2006;91:1929–1936. [Google Scholar]
- 10.Rodriguez SG, Allemann E, Fessi H, Doelker E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res. 2004;21:1428–1439. doi: 10.1023/b:pham.0000036917.75634.be. [DOI] [PubMed] [Google Scholar]
- 11.Jaeghere FD, Allemann E, Doelker E, Gurny R, Cerny R, Galli B, Steulet AF, Muller I, Schutz H. pH-dependent dissolving nano- and microparticles for improved peroral delivery of a highly lipophilic compound in dogs. AAPS Pharm Sci Tech. 2001;3 doi: 10.1208/ps030108. article 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintanar-Guerrero D, Allemann E, Fessi H, Doelker E. Pseudolatex preparation using a novel emulsion–diffusion process involving direct displacement of partially water-miscible solvents by distillation. Int J Pharm. 1999;188:155–164. doi: 10.1016/s0378-5173(99)00216-1. [DOI] [PubMed] [Google Scholar]
- 13.Pignatello R, Ricupero N, Bucolo C, Maugeri F, Maltese A, Puglisi G. Preparation and characterization of eudragit retard nanosuspensions for the ocular delivery of cloricromene. AAPS Pharm Sci Tech. 2006;7:E192–E198. doi: 10.1208/pt070127. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Chen B, Zhang Z, Yao S. Focused microwave-assisted solvent extraction and HPLC determination of effective constituents in Eucommia ulmodies Oliv. (E. ulmodies) Talanta. 2004;63:659–665. doi: 10.1016/j.talanta.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Trotta M, Debernardi F, Caputo O. Preparation of solid lipid nanoparticles by a solvent emulsification–diffusion technique. Int J Pharm. 2003;257:153–160. doi: 10.1016/s0378-5173(03)00135-2. [DOI] [PubMed] [Google Scholar]
- 16.Murtaza G, Ahmad A, Waheed AA, Naeem AM. Salbutamol sulphate-ethylcellulose microparticles: formulation and in-vitro evaluation with emphasis on mathematical approaches. DARU. 2009;17:209–216. [Google Scholar]
- 17.Maincent P, Verger ML, Fluckiger L, Kim Y, Hoffman M. Preparation and characterization of nanoparticles containing an antihypertensive agent. Eur J Pharm Biopharm. 1998;46:137–143. doi: 10.1016/s0939-6411(98)00015-0. [DOI] [PubMed] [Google Scholar]
- 18.Teeranachaideekul V, Junyaprasert VB, Souto EB, Muller RH. Development of ascorbyl palmitate nanocrystals applying the nanosuspension technology. Int J Pharm. 2008;354:227–234. doi: 10.1016/j.ijpharm.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 19.Verger ML, Fluckiger L, Kim Y, Hoffman M, Maincent P. Preparation and characterization of nanoparticles containing an antihypertensive agent. Eur J Pharm Biopharm. 1998;46:137–143. doi: 10.1016/s0939-6411(98)00015-0. [DOI] [PubMed] [Google Scholar]
- 20.Tiwari S, Singh S, Rawat M, Tilak R, Mishra B. L9 orthogonal design assisted formulation and evaluation of chitosan-based buccoadhesive films of miconazole nitrate. Curr Drug Del. 2009;6:305–316. doi: 10.2174/156720109788680921. [DOI] [PubMed] [Google Scholar]
- 21.Zang C, Friswell MI, Mottershead JE. A review of robust optimal design and its application in dynamics. Comp Struct. 2005;83:315–326. [Google Scholar]
- 22.Shmeis RA, Wang Z, Krill SL. A mechanistic investigation of an amorphous pharmaceutical and its solid dispersions. Part I. A comparative analysis by thermally stimulated depolarization current and differential scanning calorimetry. Pharm Res. 2004;21:2025–2030. doi: 10.1023/b:pham.0000048193.94922.09. [DOI] [PubMed] [Google Scholar]
- 23.Vasanthavada M, Tong WQ, Kislalioglu MS. Phase behaviour of amorphous molecular dispersions. II. Role of hydrogen bonding in solid solubility and phase separation kinetics. Pharm Res. 2005;22:440–448. doi: 10.1007/s11095-004-1882-y. [DOI] [PubMed] [Google Scholar]
- 24.Adibkia K, Shadbad MRS, Nokhodchi A, Javadzedeh A, Jalali MB, Barar J, Mohammadi G, Omidi Y. Piroxicam nanoparticles for ocular delivery: physicochemical characterization and implementation in endotoxin-induced uveitis. J Drug Target. 2007;15:407–416. doi: 10.1080/10611860701453125. [DOI] [PubMed] [Google Scholar]
- 25.Wong CF, Yuen KH, Peh KK. Formulation and evaluation of controlled release eudragit buccal patches. Int J Pharm. 1999;178:11–22. doi: 10.1016/s0378-5173(98)00342-1. [DOI] [PubMed] [Google Scholar]
- 26.Pongpaibul Y, Price JC, Whitworth CW. Preparation and evaluation of controlled release indomethacin microspheres. Drug Dev Ind Pharm. 1984;10:1597–1616. [Google Scholar]
- 27.Lemos-Senna E, Wouessidjewe D, Lesieur S, Duchene D. Preparation of amphiphilic cyclodextrin nanospheres using the emulsification solvent evaporation method. Influence of the surfactant on preparation and hydrophobic drug loading. Int J Pharm. 1998;170:119–128. [Google Scholar]
- 28.Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 29.Dillen K, Vandervoort J, Van den MG, Ludwig A. Evaluation of ciprofloxacin-loaded Eudragit RS100 or RL100/PLGA nanoparticles. Int J Pharm. 2006;314:72–82. doi: 10.1016/j.ijpharm.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 30.Le WK, Park JY, Yang EH, Suh H, Ki SH, Chung DS, Choi K, Yang CW, Park JS. Investigation of the factors influencing the release rates of cyclosporin A-loaded micro- and nanoparticles prepared by highpressure homogenizer. J Control Rel. 2002;84:115–123. doi: 10.1016/s0168-3659(02)00239-0. [DOI] [PubMed] [Google Scholar]
- 31.Zeng W, Chen HZ. Air pressure pulsation solid state fermentation of feruloyl esterase by Aspergillus niger. Biores Tech. 2009;100:1371–1375. doi: 10.1016/j.biortech.2008.08.032. [DOI] [PubMed] [Google Scholar]