Abstract
Background and the purpose of the study
MEN1 is an important tumor suppressor gene that encodes a nuclear protein called menin. Recent data suggest that interactions between menin and other proteins have important roles in control of the cell cycle and apoptosis. In addition, estrogen receptor (ER), an important prognostic factor is differentially expressed in breast cancer cells. In this study the MEN1 gene and protein expression in MCF7, T47D and MDA-MB-468 breast cancer cell lines with different ER status following exposure to adriamycin (ADR) was investigated.
Materials and methods
Cytotoxicity of ADR on these cell lines was determined using MTT assay. The mRNA and protein levels were analyzed in tested cell lines using RT-PCR and immunocytochemistry (ICC) assays, respectively.
Results
ADR cytotoxicity was highest on MDA-MB-468 and lowest on MCF7 cells. MEN1 mRNA showed significant decrease after ADR exposure only in the MDA-MB-468 cell line. Menin protein expression was higher in MDA-MB-468 and lower in MCF7 cells.
Conclusion
Differential molecular responses to adriamycin were observed in cancer cell lines. Molecular data also suggest that MEN1 as a new biomarker can be used in combination with current biomarkers for prediction of response to chemotherapy.
Keywords: Breast cancer, MEN1, Menin, Adriamycin, RT-PCR, Immunocytochemistry
INTRODUCTION
Breast cancer, one of the most common malignancies affecting women today (1), is associated with different types of genetic alterations such as mutations in oncogenes and tumor suppressor genes. MEN1, classified as a gate keeper tumor suppressor gene is responsible for multiple endocrine neoplasia type1 (2) and is widely observed in non-endocrine as well as endocrine and exocrine tissues (3, 4). Menin, the protein product of the MEN1 gene is a 67-kDa nuclear protein (5). The nuclear localization of menin suggests that it may have functions such as telomerase activity, transcriptional regulation, DNA replication or cell cycle control (6, 7). These important functions of menin may make it potentially a good candidate biomarker to predict the responses of cancer cells to chemotherapy. Menin interacts with a diverse group of transcription factors and chromatin modifying proteins including Jun, Smad3, mSin3A, histone deacetylases and vitamin D (8–10). These reports suggest a role for menin in the regulation of gene expression at the level of transcription and chromatin modification (11). Menin has also been shown to interact with FancD2, a protein that participates in DNA repair, as well as RPA2, a protein required for DNA replication, recombination and repair (12, 13). Menin also directly binds to DNA which is important for its function in regulating cell proliferation (14). Furthermore, it has been reported that complementation of menin-null cells with menin increases the activation of caspase 8 in response to TNF-a treatment, suggesting a role in promoting apoptosis as another critical function of menin (15). It is well-known that most of the chemotherapeutic drugs induce DNA damage that leads to cell death via apoptosis. Therefore, it can be crucial to elaborate relationship of the interaction of menin expression in different cancer cells and anticancer activity of drugs. On the other hand, menin is a transcriptional co-activator of the nuclear receptors for estrogen and vitamin D and can directly interact with the estrogen receptor (ER) in a hormone dependent manner (10, 16). Estrogen and its receptor (ER) play important roles in the genesis and malignant progression of breast cancer. ERa regulates the transcription of various genes as a transcription factor. It binds to estrogen response elements (ERE) upstream of the target genes (17). Differential expression of ER in breast cancer cells is an important biomarker in cancer therapy and hence, it is interesting to know the possible role of ER and menin in responses of different breast cancer cells to chemotherapy.
Therefore, the aim of the present study was to investigate the expression pattern of MEN1 tumor suppressor gene and menin protein as a biomarker in MDA-MB-468, T47D and MCF7 breast cancer cell lines with different ER status to predict the response to drugs such as adriamycin, a well-known chemotherapeutic drug.
MATERIAL AND METHODS
Cell lines and culture conditions
The human breast cancer cell lines (MCF7, T47D and MDA-MB-468) were obtained from National Cell Bank of IRAN (Pasteur Institute). Cells were maintained in RPMI 1640 culture medium (Gibco, UK) supplemented with 10% fetal bovine serum (Gibco, UK) and 100 U ml−1 of penicillin and 100 ng ml−1 of streptomycin at 37°C in 5% humidified CO2 incubator.
Determination of cytotoxicity of different concentrations or concentrations of adriamycin on MCF7, T47D and MDA-MB-468 breast cancer cell lines
The cells were seeded in 96-well plates at 1×104 cells/well in RPMI 1640 culture medium and incubated at 37°C in 5% CO2 incubator for 48 hrs. The cells were then exposed to adriamycin (EBEWE Pharma, Austria) at different concentrations (100 nM, 250 nM, 500 nM and 1000 nM) for 48 hrs. The medium was changed every 48 hrs with corresponding assay medium and the anti-proliferative effect of adriamycin was evaluated using MTT assay (18).
Determination of the time dependency anti-proliferative activity of adriamycin on MCF7, T47D and MDA-MB-468 breast cancer cells
For this purpose cells were exposed to culture medium containing 500 nM of drug for MCF7 and 250 nM for T47D and MDA-MB-468 cell lines. Then MTT method (18) was daily used to determine the growth curve of each cell line within 5 days of drug exposure.
RNA isolation
The cells (MCF7, T47D and MDA-MB-468) were seeded in T-25 flasks in RPMI 1640 culture medium and incubated in a humidified CO2 incubator (5% CO2, 37°C). After 48 hrs culture medium was changed and adriamycin [MCF7 (500nM), T47D & MDA-MB-468 (250nM)] was added to the corresponding flasks. The medium was changed every 48 hrs with corresponding assay medium. After 48 hrs total RNA was isolated using TriPure isolation reagent (Roche, Germany) according to the previously described method (19).
RT-PCR
The cDNA from each sample was synthesized and subjected to polymerase chain reaction (PCR) according to the previously described method (19). To amplify sequence of MEN1, specific primers were designed by Perl Primer software followed by BLAST (sense,5′-GCT GGC TGT ACC TGA AAG GA -3′; antisense,5′-CTT GTG GTA GAG GGT GAG TG -3′). As an internal control, the P-actin specific primers were also designed by Perl Primer software followed by BLAST (sense,5′-TGA CGGGGT CAC CCA CAC TGT-3′; antisense,5′-CTA GAA GCA TTT GCG GTG GAC-3′). The PCR setting for MEN1 and P-actin was 30 cycles at 95°C for 30s, 57°C for 30s and 72°C for 30s. The PCR products were visualized using 1.2% agarose gel electrophoresis and staining with ethidium bromide. In negative control template was replaced by DEPC water. Experiment was repeated in 3 separate time and results were visualized and photographed using Bio Doc It (UVP Co, USA) to be analyzed for densitometry by Lab Works 4.5 software.
Immunocytochemical analysis of menin expression
The cells (MCF7, T47D and MDA-MB-468) were seeded in 8-well chamber slides (Lab Teck, USA) in RPMI 1640 culture medium and incubated in a humidified CO2 incubator (5% CO2, 37°C). After 48 hrs culture medium was changed and adriamycin [MCF7 (500 nM), T47D & MDA-MB 468 (250 nM)] was added for 48 hrs to wells of each chamber slide while the medium of the control wells remained RPMI 1640. The cells were then subjected to immunocytochemical analysis as previously described (20) to determine the menin expression using menin primary antibody (Abcam, USA). Finally immunostained cells were studied under light microscope and photographed. A section in which incubation with the primary antibody was omitted used as negative control.
Statistical analyses
All cellular and molecular experiments were repeated 2–3 times after optimization and data were presented as mean±SE of independent experiments that were analyzed using one way ANOVA followed by dunnett post test. Mean differences with P<0.05 were considered statistically significant.
RESULTS
Cytotoxic effects of different concentrations of Adriamycin on MCF7, T47D and MDA-MB-468 breast cancer cell lines
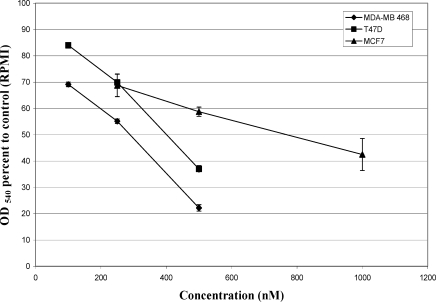
Different patterns of cytotoxicity of adriamycin (ADR) exposure for 48 hrs were observed in tested cell lines using MTT assay. ADR showed significant dose-dependent anti-proliferative effect on MCF7, T47D and MDA-MB-468 cells (Fig. 1).
Figure 1.
Cytotoxic effects of different concentrations of adriamycin on breast cancer cell lines. Cells were seeded in 96-well plates and exposed for 48hrs to adriamycin to determine its cytotoxicity using MTT method. Data are mean±SE of the average of 4 wells in three independent experiments.
Time-course cytotoxic effects of adriamycin on MCF7, T47D and MDA-MB-468 breast cancer cell lines
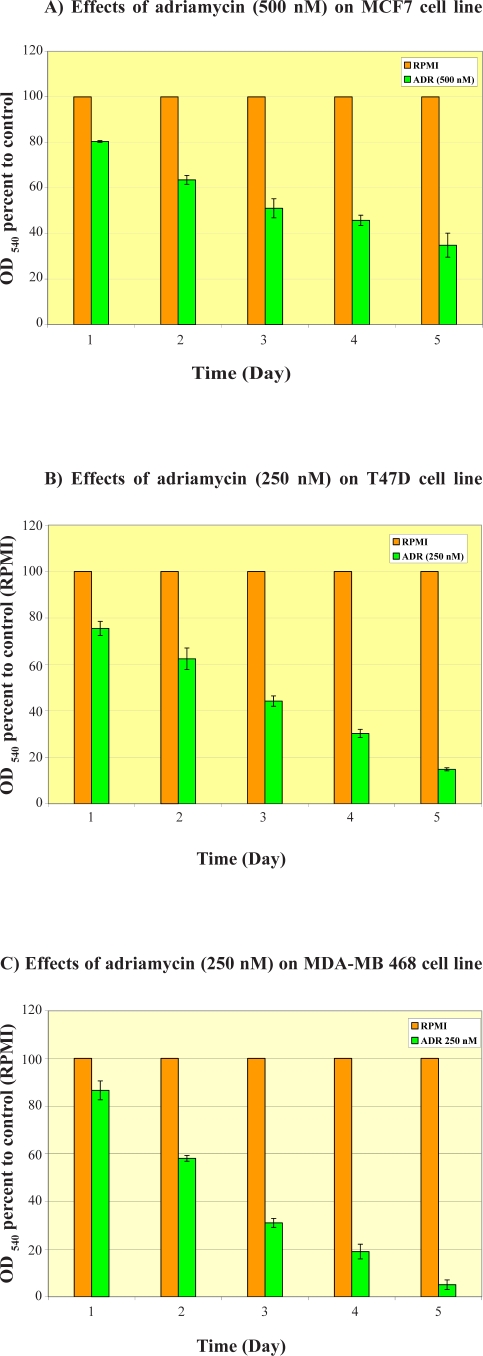
Adriamycin showed time-dependent anti-proliferative effects on MCF7, T47D and MDA-MB-468 cells (Fig. 2). The cytotoxicity was highest on MDA-MB-468 cells after 5 days exposure to 250 nM of ADR than T47D cells in MTT assay. The MCF7 cells was less sensitive to cytotoxic effects of ADR at all time-points in comparison to other two cell lines.
Figure 2.
Time-course cytotoxic effects of adriamycin on breast cancer cell lines. Cells were seeded in 96-well plates and exposed for 5 days to adriamycin to determine its cytotoxicity using MTT method. Data are mean±SE of the average of 4 wells in three independent experiments for MCF7 (A), T47D (B), and MDA-MB-468 (C) cell lines.
Effect of adriamycin on the mRNA level of MEN1
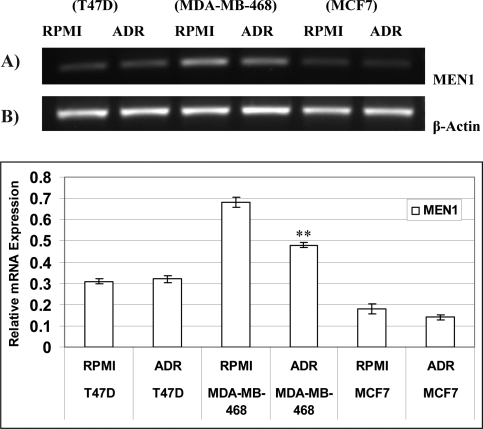
RT-PCR analyses of MEN1 mRNA expression showed slight decrease in MDA-MB-468 and MCF7 cell lines after exposure to ADR (Fig. 3). The expression of MEN1 mRNA was highest in MDA-MB-468 and lowest in MCF7 cell line in comparison with untreated samples (Fig. 3). MEN1 mRNA expression was significantly reduced after ADR treatment only in MDA-MB-468 cells.
Figure 3.
Effect of adriamycin on the mRNA levels of MEN1 in T47D, MDA-MB-468 and MCF7 cell lines. Expression level of MEN1 mRNA was determined using RT-PCR that repeated 3 times after optimization (A). Densitometric analysis of 3 separate experiments using Lab Works 4.5 software is shown as mean ±SE of relative intensity of each band normalized to β-Actin (B). ** indicates significant difference compared to RPMI with p<0.001.
Immunostaining of breast cancer cell lines with menin antibody
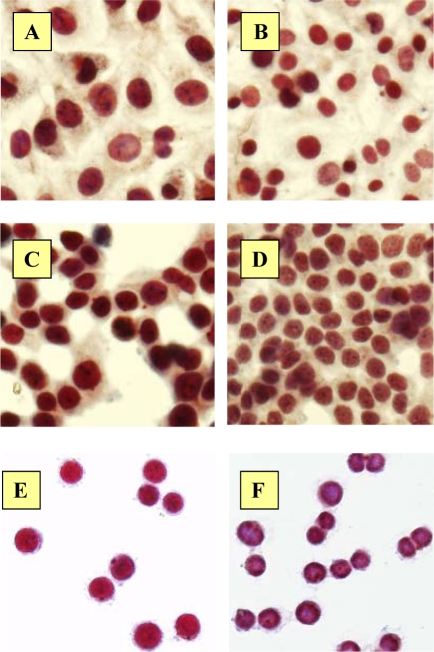
Menin expression showed an increase after ADR exposure in all cell lines which qualitatively was higher in MDA-MB-468 and T47D and lower in MCF7 cell lines in comparison to immunostaining pattern of RPMI control under the light microscope (Fig. 4).
Figure 4.
Immunostaining of MCF7, T47D and MDA-MB-468 cells with menin antibody. ADR treated MCF7 (A), T47D (C) and MDA-MB-468 (E) and untreated MCF7 (B), T47D (D) and MDA-MB-468 (F) cells showed different pattern and intensity of menin expression visualized by labvision detection system using AEC chromogen. (Magnification 400x)
DISCUSSION
ADR is a topoisomerase IIα poison. Topoisomerase IIα is a nuclear enzyme that transiently breaks and re-joins the phosphodiester backbone of both strands of the double helix. Therefore, it is essential for DNA replication, chromosome segregation, and maintenance of chromosome structure. ADR forms a stable complex with DNA and topoisomerase IIα resulting in inhibition of the normal function of the enzyme. The complexed enzyme is unable to religate DNA and as a result DNA strand breaks and DNA damage would be increased (21).
In this study, the MEN1 mRNA and protein expressions were analyzed in different breast cancer cells following exposure to ADR. MEN1 mRNA level decreased significantly after ADR treatment only in MDA-MB-468 that is ER negative but not in ER positive MCF7 and T47D cell lines. The difference between MEN1 mRNA level in MCF7 that is lower than T47D cells could be due to other genetic differences between these two cell lines such as lower ER and higher progesterone receptor (PR) expression and also mutation in p53 gene in the T47D cells in comparison to the MCF7 cells. The molecular differences between cell lines under study further emphasizes on inverse relationship between MEN1 and expression level of ER.
It has been already reported that menin has multifunctions such as transcriptional regulation, repression of cell proliferation, and promotion of apoptosis (22). Analysis of DNA synthesis in cultured cells also revealed that overexpression of menin inhibited DNA synthesis (23). In this study, menin expression in all cell lines increased relatively after exposure to ADR. Similarly, gamma irradiation has been reported to increase menin in the nuclear matrix (11). Similarly, it has also been shown that menin responds to UV induced DNA damage by localizing to the chromatin (23). Therefore, increased in menin expression in MDA-MB-468, MCF7 and T47D cell lines after exposure to ADR could be explained by its role in repairing DNA damage caused by adriamycin and induction of apoptosis. Interestingly, MEN1 mRNA expression showed to be highest in ER-negative MDA-MB-468 cells and lowest in the highly ER-positive MCF7 cell line indicating an inverse relationship between MEN1 and ER expression in these cell lines.
ER mRNA was re-expressed in cancer cells in a time and dose dependent manner, with up to five fold increase in ER expression. However, the ER CpG island remained methylated with ER mRNA levels induced to only 1 to 10% of those which were observed in MCF7 and T47D ER+ cell lines (24). It has been reported that menin interacts with ER and increases the activity of ER in breast cancer that is important as a predictive factor in resistance to hormone therapy with tamoxifen (25).
Another possible mechanism underlying the inverse relationship between MEN1 and ER expression in breast cancer cells could be recruitment of HDAC by menin which is associated with decrease in acetylation of histones H3 and H4, and thus reduction of ER expression (26).
Studying the expression of MEN1, a putative tumor suppressor gene, is important as a biomarker to predict the drug response of cancer cells at both mRNA and protein levels following exposure to adriamycin, a well known anticancer drug. The expression pattern of MEN1, especially at protein level after treatment with ADR is consistent with its role in the induction and regulation of apoptosis.
In this study, the expression of MEN1 gene was analyzed for the first time in breast cancer cell lines and it was found that MEN1 is expressed differentially in breast cancer cell lines with different ER status and a negative correlation between MEN1 and ER expression was observed.
Finding of this investigation suggest that the clinical spectrum of multiple endocrine neoplasia type 1 also might include breast cancer, although more studies are required to elucidate the exact role of the MEN1 gene in this disease. In addition, these data suggest the requirement for considering new biomarkers that can be used in combination with current ones for early diagnose of disease and prediction of response to chemotherapy.
ACKNOWLEDGEMENTS
Authors would like to thank office of vice-chancellor for research of Tehran University of Medical Sciences and Mashad University of Medical Sciences financial for support of this research project.
REFERENCES
- 1.Jemal A., Murray T., Samuels A. Cancer statistics. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Chandrasekharappa S.C., Guru S.C., Manickam P., Olufemi S.E., Collins F.S., Emmert-Buck M.R., Debelenko L.V., Zhuang Z., Lubensky I.A., Liotta L.A., Crabtree J.S., Wang Y., Roe B.A., Weisemann J., Boguski M.S., Agarwal S.K., Kester M.B., Kim Y.S., Heppner C., Dong Q., Spiegel A.M., Lee Burns A., Marx S.J. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 3.Ikeo Y., Sakurai A., Suzuki R., Zhang M., Koizumi S., Takeuchi Y., Yumita W., Nakayama J., Hashizume K. Proliferation –Associated Expression of the MEN1 Gene as Revealed by In Situ Hybridization: Possible Role of the Menin as a Negative Regulator of Cell Proliferation Under DNA Damage. Lab Invest. 2000;80(6):797–804. doi: 10.1038/labinvest.3780084. [DOI] [PubMed] [Google Scholar]
- 4.Cavallari I., D'agostino D.M., Ferro T., Rosato A., Barzon L., Pasquali C., Fogar P., Theodoropoulou M., Esposito G., Boscaro M., Pagotto U., Tebaldi E., Fallo F., Chieco-Bianchi L., Ciminale V. In Situ Analysis of Human Menin in Normal and Neoplastic Pancreatic Tissues: Evidence for Differential Expression in Exocrine and Endocrine Cells. J Clin Endocrin Metabol. 2003;88:3893–3901. doi: 10.1210/jc.2002-021840. [DOI] [PubMed] [Google Scholar]
- 5.Guru S.C., Goldsmith P.K., Lee Burns A., Marx S.J., Spiegel A.M., Collins F.S., Chandrasekharappa S.C. Menin, the product of the MEN1 gene, is a nuclear protein. PNAS USA. 1998;95:1630–1634. doi: 10.1073/pnas.95.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manabu H, Satoru K, Xianxin H, Hidetoshi T, Miki N, Masahiro T, Junko S, Yoshiko M, Mitsuhiro N, Tomomi I, Yasunari M, Masaki I. Role of menin in the regulation of telomerase activity in normal and cancer cells. Int J Oncol. 2008;33:333–340. [PubMed] [Google Scholar]
- 7.Hussein N., Casse H., Fontanière S., Morera A., Asensio M.J., Bakeli S., Lu J.L., Coste I., Di Clemente N., Bertolino P., Zhang C.X. Reconstituted expression of menin in Men1-deficient mouse Leydig tumour cells induces cell cycle arrest and apoptosis. Eur J Cancer. 2007;43:402–414. doi: 10.1016/j.ejca.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 8.Balogh K., Racz K., Patocs A., Hunyady L. Menin and its interacting proteins:elucidation of menin function. Trends Endocrin Metabol. 2006;17:357–364. doi: 10.1016/j.tem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Kim H., Lee J. E., Cho E. J., Liu J. O., Youn H. D. Menin, a tumor suppressor, represses JunD mediated transcriptional activity by association with an mSin3A histone deacetylase complex. Cancer Res. 2003;63:6135–6139. [PubMed] [Google Scholar]
- 10.Koen D, Radhika V, Rick N, Roel B, Gerlof V, Jacqueline W, Cornelis L, Alain K, Marc T. Regulation of vitamin D receptor function in MEN1-related parathyroid Adenomas. Mol Cell Endocrinol. 2009;313:1–8. doi: 10.1016/j.mce.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Yuq ing Y, Xianxin H. In search of tumor suppressing functions of menin. Mol Cell Endocrinol. 2007;266:34–41. doi: 10.1016/j.mce.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilaria C, Micol S-B, Francesca R, Annalisa M, Paola F, Daniela B, Manuela DV, Sergio P, James GH, Luigi C-B, Giovanni E, Vincenzo C, Donna MA. Decreased Expression and Promoter Methylation of the Menin Tumor Suppressor in Pancreatic Ductal Adenocarcinoma. Genes Chromosomes Cancer. 2009;48:383–396. doi: 10.1002/gcc.20650. [DOI] [PubMed] [Google Scholar]
- 13.Sukhodolets K., Hickman A., Agarwal S. K., Sukhodolets M., Obungu V., Novotny E., Crabtree J., Chandrasekharappa S. C., Collins F. S., Spiegel A. M., Burns A. L., Marx S. J. The 32-kilodalton subunit of replication protein A interacts with menin, the product of the MEN1 tumor suppressor gene. Mol Cell Biol. 2003;23:493–509. doi: 10.1128/MCB.23.2.493-509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La P., Silva A.C., Hou Zh., Wang H., Schnepp R.W., Yan N., Shi Y., Hua X. Direct Binding of DNA by Tumor Suppressor Menin. J Biol Chem. 2004;279:49045–49054. doi: 10.1074/jbc.M409358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnepp R.W., Mao H., Sykes S.M., Zong W.X., Silva A., La P., Hua X. Menin induces apoptosis in murine embryonic fibroblasts. J Biol Chem. 2004;279:10685–10691. doi: 10.1074/jbc.M308073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreijerink K.M.A., Mulder K.W., Winkler G.S., Hoppener J.W.M., Lips C.J.M., Timmers H.T.M. Menin links estrogen receptor activation to histone H3K4 trimethylation. Cancer Res. 2006;66:4929–4935. doi: 10.1158/0008-5472.CAN-05-4461. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi S-I., Eguchi H., Tanimoto K., Yoshida T., Omoto Y., Inoue A., Yosida N., Yamaguchi Y. The expression and function of estrogen receptor ( and ( in human breast cancer and its clinical application. Endocrine-Related Cancer. 2003;10:193–202. doi: 10.1677/erc.0.0100193. [DOI] [PubMed] [Google Scholar]
- 18.Abdolmohammadi MH, Fouladdel Sh, Shafiee A, Amin Gh, Ghaffari SM, Azizi E. Anticancer effects and cell cycle analysis on human breast cancer T47D cells treated with extracts of Astrodaucus persicus (Boiss.) Drude in comparison to Doxorubicin. DARU. 2008;16(2):112–118. [Google Scholar]
- 19.Kaabinejadian S., Fouladdel Sh., Ramezani M, Azizi E. Molecular analysis of Bcl-2 and cyclin D1 expression in differentially expressing estrogen receptor breast cancer MCF7, T47D and MDAMB-468 cell lines treated with adriamycin. DARU. 2008;16(3):182–188. [Google Scholar]
- 20.Azizi E., Abdolmohammadi M. H., Fouladdel Sh., Shafiee A., Amin Gh., Ghaffari S. M. Evaluation of p53 and Bcl-2 genes and proteins expression in human breast cancer T47D cells treated with extracts of Astrodaucus persicus (Boiss.) Drude in comparison to Tamoxifen. DARU. 2009;17(3):181–186. [Google Scholar]
- 21.Gewirtz D.A. A critical evaluation of the mechanisms of action proposed for the Antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 22.Farley S.M., Chen G., Guo S, Wang M., A, J., Lee F., Lee F., Sawicki M. Menin Localizes to Chromatin Through an ATR-CHK1 Mediated Pathway After UV-Induced DNA Damage. J Surg Res. 2006;133:29–37. doi: 10.1016/j.jss.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Pinzone J.J., Stevenson H., Strobl J.S., Berg P.E. Molecular and Cellular Determinants of Estrogen Receptor α Expression. Mol Cell Biol. 2004;24:4605–4612. doi: 10.1128/MCB.24.11.4605-4612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scacheri P.C., Rozenblatt-Rosen O., Caplen N.J., Wolfsberg T.G., Umayam L., Lee J.C., Hughes C.M., Shanmugam K.S., Bhattacharjee A., Meyerson M., Collins F.S. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. PNAS USA. 2004;101:1892–1897. doi: 10.1073/pnas.0308698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imachi H, Murao K, Dobashi H, Bhuyan MM, Cao X, Kontani K, Niki S, Murazawa C, Nakajima H, Kohno N, Yamashita H, Iwase H, Hayashi SI, Ishida T, Yamauchi A. Menin, a product of the MENI gene, binds to estrogen receptor to enhance its activity in breast cancer cells: possibility of a novel predictive factor for tamoxifen resistance. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0581-0. Oct.22 (E.Pub.Ahead) [DOI] [PubMed] [Google Scholar]
- 26.Dreijerink K.M.A., Höppener J.W.M., Timmers H.T.M., Lips C.J.M. Mechanisms of Disease: multiple endocrine neoplasia type 1- relation to chromatin modifications and transcription regulation. Nat Clin Pract Endocrinol Metabol. 2006;2:562–570. doi: 10.1038/ncpendmet0292. [DOI] [PubMed] [Google Scholar]