Abstract
Background and the purpose of the study
Lercanidipine hydrochloride (LRDP) is used in the treatment of hypertension because of its selectivity and specificity on the smooth vascular cells. The pharmacokinetic parameters make LRDP a suitable candidate for transdermal delivery. The purpose of the study was to select a suitable formulation for the development of transdermal drug-delivery system (TDDS) of LRDP and to determine the effect of penetration enhancer, limonene on drug permeation
Methods
The matrix type TDDS of LRDP were prepared by solvent evaporation technique. Formulations A1, A2, A3, A4, A5 and A6 were composed of Eudragit RL100 (ERL) and hydroxypropyl methyl cellulose (HPMC) in 1.5:8.5, 3:7, 4:6, 6:4, 7:3 and 8.5:1.5 ratios respectively. All the six formulations carried 10 mg of LRDP/patch area, 8% v/w of d-limonene as a penetration enhancer, 20% v/w of propylene glycol as plasticizer in methanol and dichloromethane as solvent system. The prepared TDDS were evaluated for physicochemical characteristics, in-vitro release, ex-vivo permeation and skin irritation. The ex-vivo permeation studies were carried out across excised rat skin using Franz diffusion cell.
Results
All the formulations exhibited satisfactory physicochemical characteristics. Cumulative percentage of the drug released in 24 hrs from the six formulations were 82.0%, 74.9%, 63.2%, 63.5%, 59.8% and 53.5% respectively. Corresponding values for the cumulative amounts of the drug permeated across the rat skin for the above matrix films were 2644.5, 2347.2, 2249.5, 1933.4, 2021.5 and 1663.4 µg/cm2 respectively. By fitting the data into zero order, first order and Higuchi model, it was concluded that drug release from matrix films followed Higuchi model and the mechanism of the drug release was diffusion mediated. The patches were seemingly free of potentially hazardous skin irritation.
Conclusions
The patches composed of ERL, HPMC (1.5:8.5) with 8% v/w limonene as penetration enhancer may be selected for the development of TDDS of LRDP for potential therapeutic use by using a suitable adhesive layer and backing membrane.
Keywords: Lercanidipine hydrochloride, Transdermal patch, d-limonene, Eudragit RL 100, Hydroxypropyl methyl cellulose
INTRODUCTION
It has been shown that transdermal route of administration is not subjected to the hepatic first pass effect which result in the required systemic bioavailability of the drug (1). However, the success of a transdermal drug delivery system (TDDS) depends on the ability of the drug to penetrate the skin in sufficient quantities to maintain therapeutic levels. Several methods have been reported in the literature to enhance the drug penetration across biological membranes (2). For many therapeutic agents, the desired effect may not be possible without the use of penetration enhancers. An ideal enhancer should be pharmacologically inactive, nonirritant, and should not damage the skin irreversibly. Many of the chemical enhancers such as dimethyl sulfoxide, surfactants, alcohols, and urea and its derivatives have been screened as penetration enhancers. The adverse effects of some of these enhancers restrict their use widely. Currently, there has been an upsurge in the use of naturally occurring chemicals such as terpenes which are isolated from natural essential oils and are safe and non-irritating penetration enhancers (3). d-limonene, a cyclic terpene is free from toxic effects and has been used as a penetration enhancer in the transdermal delivery of several drugs (4, 5). The use of Eudragit RL100 (ERL) and hydroxypropyl methyl cellulose (HPMC) in preparation of matrix patches has been reported (6).
Lercanidipine hydrochloride (LRDP) is used in the treatment of hypertension because of its selectivity and specificity on the smooth vascular cells. The drug is administered orally at a dose of 10–20 mg daily as its hydrochloride salt and reduces the diastolic blood pressure significantly (7). After oral administration, LRDP is completely and erratically absorbed from the gastrointestinal tract (8). The absolute bioavailability is reduced to approximately 10% because of its extensive first pass metabolism to inactive metabolites (7). Mean half-lives of the drug has been reported 2.8 and 4.4 hrs in human after single dose of 10 and 20 mg of LRDP, respectively (8). These pharmacokinetic parameters make LRDP a suitable candidate for transdermal delivery.
In the present investigation, an attempt was made to deliver LRDP transdermally via patches to overcome drawback of poor oral bioavailability and erratic oral absorption. Survey of literature and patent databases did not reveal any transdermal dosage form of LRDP for the purpose of improving bioavailability. Hence, the objective of present investigation was to formulate transdermal polymeric films with ERL and HPMC containing LRDP using d-limonene as permeation enhancer and to evaluate them by ex vivo permeation studies.
MATERIAL AND METHODS
Material
Lercanidipine hydrochloride and Eudragit RL 100 were gifts samples from Sun Pharmaceuticals (Baroda, India), and Aurobindo Pharmaceuticals (Hyderabad, India) respectively. d-Limonene was purchased from Himedia, Mumbai, India. Liquid mercury, hydroxypropyl methyl cellulose 15 cps (HPMC), propylene glycol (PG), polyethyleneglycol 400 (PEG 400) were purchased from SD Fine Chemicals, Mumbai, India.
METHODS
Preparation of rat abdominal skin
Albino rats weighing 150–200 gm were selected for permeation studies. The animals were sacrificed using anesthetic ether. The hair of the test animals was carefully trimmed short with a pair of scissors and the full thickness skin was removed from the abdominal region. The epidermis was prepared surgically by heat separation technique (9), which involved soaking of the entire abdominal skin in water at 60°C for 45 sec, followed by careful removal of the epidermis. The epidermis was washed with water and used for ex vivo permeability studies.
Effect of d-limonene on permeation of LRDP
Franz diffusion cell with a surface area of 2.64 cm2 was used for ex vivo permeation studies. The rat skin was mounted between the compartments of diffusion cell with stratum corneum facing the donor compartment. LRDP solution (5 mg in 3 ml of phosphate buffered saline (PBS) of pH 7.4 containing PEG 400) was placed in the donor compartment containing different concentrations of d-limonene (0, 4, 8, 12% v/v). The 0% d-limonene served as control and PEG 400 was used to solubilize LRDP. The receiver compartment contained 13 ml of 40% v/v PEG 400 in PBS of pH 7.4 and the contents were stirred using magnetic stirrer. The whole assembly was kept at 37±0.5°C. Samples of 3 ml were collected at preset time points up to 24 hrs and replenished with 40% v/v PEG 400 in PBS pH 7.4. The samples were filtered through 0.45 µ syringe filter (Sartorius AG, Goettingen, Germany) and drug content in the samples was measured using UV/visible spectrophotometer (Shimadzu Pharmspec1700, Shimadzu Inst. Japan) at 354 nm. The cumulative amount of the permeated drug was calculated.
Analysis of permeation data and Statistics
The drug concentration in the permeates was corrected for sampling effects according to the equation described by Hayton and Chen (10).
Where is the corrected concentration of the nth sample, C n is the measured concentration LRPD in the'n' th sample, C n−1 is the measured concentration LRDP in the (n−1)th sample, V T is the total volume of the reciever fluid and V S is the volume of the sample drawn.
As described by Barry (11), the steady state flux (Jss) and permeability coefficient (Kp) are defined by:
where, A is the effective diffusion area; Cs, the concentration in the saturated solution and (dQ/dt)ss is the steady state. slope.
The penetration enhancing effect of d-limonene was calculated in terms of enhancement ratio (ER) by using the following equation (12).
The cumulative amount permeated and flux values obtained were tested for significant differences using a one-way analysis of variance (ANOVA) or unpaired t test.
Preparation of transdermal films
The matrix type transdermal patches containing LRDP were prepared using different ratios of ERL and HPMC (Table 1). The polymers were weighed in requisite ratios by keeping the total polymer weight at 1.0 gm and allowed to swell for 2 hrs in solvent mixture (3:2 ratio of dichloromethane, methanol). The drug solution was added to the polymeric solution while stirring. Propylene glycol was incorporated as plasticizer and d-limonene as penetration enhancer. The solution was poured into a glass ring of about 26 cm2, placed on the surface of liquid mercury and kept in a petriplate. The solvent was allowed to evaporate for 24 hrs. Aluminum foil was used as backing film. The polymer was found to be self adhesive due to the presence of Eudragit polymer along with plasticizer. The patches were cut to give required area and used for evaluation.
Table 1.
Composition and Physicochemical Properties of Lercanidipine hydrochloride Transdermal Patches
| Cod | Drug (mg) | Polymer | Weight (mg) | Thickness (µ) | Folding Endurance | Drug content (mg) |
|---|---|---|---|---|---|---|
| LRDP | ERL: HPMC | |||||
| A1 | 100 | 1.5:8.5 | 131.5±5.27 | 326±6.53 | 30.3±3.51 | 9.9±0.59 |
| A2 | 100 | 3:7 | 135.2±2.82 | 301±7.10 | 31.3±1.52 | 10.3±0.35 |
| A3 | 100 | 4:6 | 124.7±4.56 | 301±3.34 | 26.7±3.51 | 10.5±0.39 |
| A4 | 100 | 6:4 | 117.5±6.53 | 275±6.36 | 48.3±4.50 | 10.1±0.12 |
| A5 | 100 | 7:3 | 119.0±4.08 | 264±2.26 | 46.0±4.58 | 10.1±0.28 |
| A6 | 100 | 8.5:1.5 | 125.3±2.98 | 250±4.12 | 52.3±2.51 | 10.5±1.06 |
Note: Each patch (2.64 cm2) contained 10 mg of lercanidipine hydrochloride.
20% v/w of propylene glycol to the total polymer weight, incorporated as plasticizer.
8% v/w of d-limonene to the total polymer weight was used as penetration enhancer.
All values are expressed as mean±SD (n=3). ERL indicates Eudragit RL 100; HPMC, Hydroxypropyl Methyl Cellulose.
Evaluation of physicochemical properties
Thickness and weight variation
The thickness of patches was assessed at 6 different points using digital micrometer (Mitutoyo, Japan) and for each formulation, three randomly selected patches were used. For weight variation test, 3 films (each 2.64 cm2) from each batch were weighed individually and the average weight was calculated.
Folding endurance
The folding endurance was measured manually as the reported method (13). Briefly, a strip of the film (4×3 cm) was cut evenly and repeatedly folded at the same place till it broke.
Flatness
Longitudinal strips were cut from the prepared patch, the length of each strip was measured and then the variation in the length due to the non-uniformity in flatness was measured. Flatness was calculated by measuring constriction of strips, and 0% constriction was considered to be 100% flatness (14).
Determination of drug content
Patch (2.64 cm2) from each formulation was taken, cut into small pieces and was allowed to dissolve in a 100 ml solution containing 15 ml of methanol and 85 ml of 40% v/v PEG 400 in PBS pH 7.4. The solution was filtered, diluted suitably and the absorbance of the solution was measured using UV/visible spectrophotometer at a wavelength of 354 nm against reference solution prepared with placebo films.
Percentage of moisture content
The films were weighed individually and kept in a desiccator containing activated silica at room temperature for 24 hrs. The individual films were weighed repeatedly until a constant weight was achieved. The percentage of moisture content was calculated as the difference between initial and final weight with respect to the final weight (15).
Percentage of moisture uptake
The films were weighed accurately and placed in a desiccator containing 200 ml of saturated solution of potassium chloride (84% relative humidity) at room temperature. After 3 days, the films were taken out and weighed. The percentage of moisture uptake was calculated as the difference between final and initial weight with respect to initial weight (13).
In vitro release studies
The in vitro release study was carried out using Franz diffusion cell. The drug containing film with a support of backing membrane was sandwiched in a dialysis membrane with molecular weight cut off
between 12000 to 14000 (Himedia, Mumbai, India) and was further placed between compartments of diffusion cell. The dialysis membrane had been soaked for 24 hrs in 40% v/v PEG 400 in PBS of pH 7.4. The donor compartment was open at the top and exposed to atmosphere. The donor and receptor compartments held together using a clamp and receptor compartment was provided with sampling port. The receptor compartments contained 13 ml of 40% v/v PEG 400 in PBS of pH 7.4 and the contents were stirred at a speed of 400 rpm. The whole assembly was kept on a magnetic stirrer and study was conducted at a temperature of 37±0.5°C. The samples of 3 ml were collected at preset time points up to 24 hrs and replenished with fresh medium. The samples were filtered using syringe filter (Sartorius 0.45µ) and drug content in the samples was estimated using UV/ visible spectrophotometer at 354 nm. Cumulative percentage of the released drug was calculated and plotted against time.
Ex-vivo permeation studies
Franz diffusion cell with a surface area of 2.64 cm2 was used for ex-vivo permeation studies. Excised rat skin was mounted between the compartments of the diffusion cell with stratum corneum facing the donor compartment. The stratum corneum side of the skin was kept in intimate contact with the transdermal patch under the test. The receiver compartment contained 13 ml of 40% v/v PEG 400 in PBS of pH 7.4, stirred with a magnetic stirrer at a speed of 400 rpm. The whole assembly was kept on a magnetic stirrer and study was conducted at 37±0.5°C. The amount of the permeated drug was determined by removing 3 ml at preset time points up to 24 hrs and replenishing with an equal volume of fresh medium. The samples were filtered using syringe filter (Sartorius 0.45µ) and the absorbance was measured at 354 nm spectrophotometrically. The cumulative amount of drug permeated was calculated and plotted against time.
Primary skin irritancy studies
The study was conducted on the basis of the approval of institutional animal ethical committee. Albino rabbits of either sex, each weighing 1.5 to 2.0 kg were used in this study (n=3 in each group). They were housed in cages in the animal house under controlled temperature and light conditions. They were fed a standard laboratory diet and had access to water ad libitum. The dorsal surface of the rabbits was cleared and the hair was removed by shaving. The skin was cleared with rectified spirit. The control patch (without any drug, group I) and an experimental patch (A1, group II) were applied to the shaved skin of rabbits and secured using USP adhesive tape (Johnson & Johnson limited, Mumbai). A 0.8% (v/v) aqueous solution of formaldehyde was applied as a standard irritant (group III). Its effect was compared with the test. The animals were observed for any sign of erythema and oedema for a period of 7 days and scored as reported by Draize et al. (16).
RESULTS AND DISCUSSION
Effect of d-limonene on permeation of LRDP
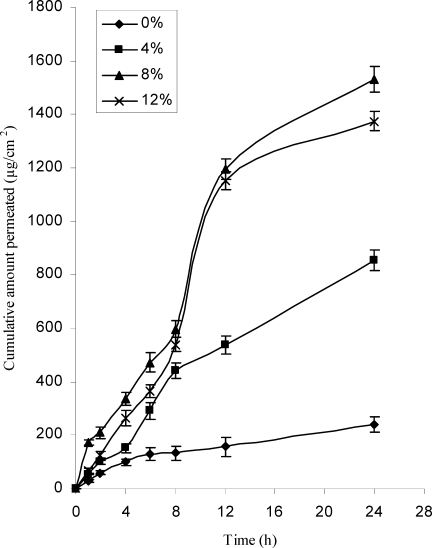
The effect of concentration of d-limonene on cumulative permeation through rat skin is shown in Figure 1. Solution containing 8 and 12% v/v of d-limonene showed similar flux values (64.4±1.88 and 58.0±1.38 µg/cm2/hr) and permeability coefficients (3.4±0.09 and 3.1±0.07 cm hr−1×10−2). The flux values obtained with 8 and 12% v/v of d-limonene were significantly different (p<0.05) to lowest values obtained with 4% d-limonene (34.4±1.47 µg/cm2/hr) and control (9.7±1.08 µg/cm2/hr). The permeability coefficient obtained with 8 and 12% d-limonene were 6.2 and 5.5 times higher than that observed with control. The permeation of LRDP was not affected by increasing d-limonene concentration from 8 to 12% v/v; hence in the preparation of patches, d-limonene at concentration of 8% v/v was incorporated.
Figure 1.
Effect of concentration of d-limonene on cumulative permeation, mean±SD (n=3).
The effectiveness of hydrocarbon limonene has also been demonstrated for other lipophilic drugs such as ketoprofen and valsartan (17, 18). The great enhancement by limonene suggests that there were possibly multiple mechanisms that could have resulted in a more permeable pathway for LRDP. They include an increased solubility of LRDP within skin, partial extraction of stratum corneum (SC) lipids (19), phase separation within the SC lipid lamellae (20).
Physicochemical characterization of patches
The results of the physicochemical characterization of the patches are shown in Table 1. The weights and thicknesses were found in the range of 117.5–135.2 mg and 250–326 µ respectively. As the proportion of HPMC decreased, the thickness was also decreased. Good uniformity of drug content among the batches was observed for all formulations and ranged from 9.9 to 10.5 mg. The results indicate that the process which was employed to prepare patches in this study was capable to produce patches with uniform drug content and minimal patch variability. The flatness study showed that all formulations had the same strip length before and after their cuts, indicating 100% flatness. As a result there was no constriction and all patches had a smooth, flat surface; and that smooth surface could be maintained when the patch was applied to the skin. Folding endurance test results indicated that the patches did not break and maintained their integrity with general skin folding when applied. The thinner the film was, more flexible it was.
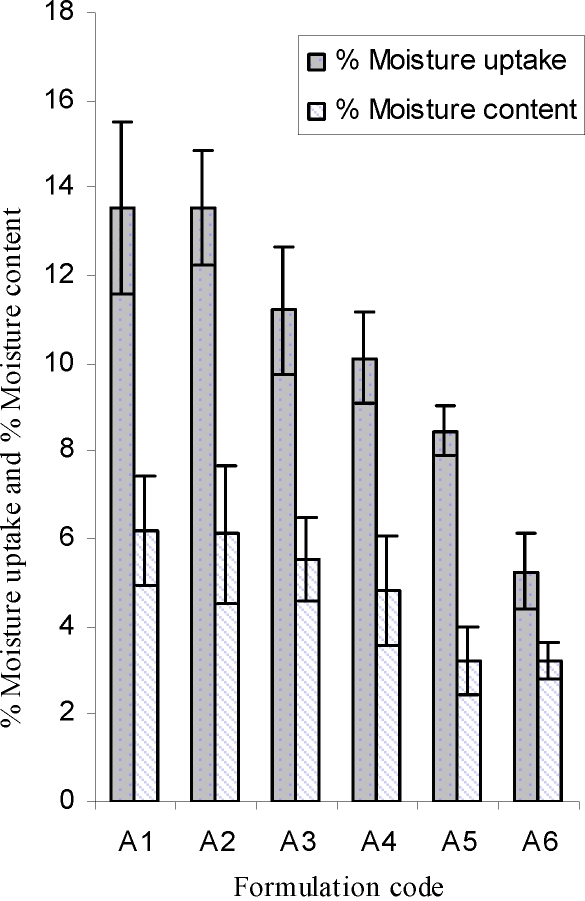
The results of % moisture uptake and % moisture content studies are shown in Figure 2. The results revealed that the increase in the concentration of hydrophilic polymer was directly proportional to the increase in moisture content and moisture uptake of the patches. The% moisture content in the patches ranged from 3.2 to 6.2. The % moisture uptake in the formulations were in the range of 5.3 to 13.5. The integrity of formulations was not changed on moisture uptake. The low moisture content in the formulations resulted in stability of patches and not giving a completely dried and brittle film (21).
Figure 2.
Moisture uptake and moisture content of lercanidipine patches, mean±S.D (n=3).
In-vitro release studies
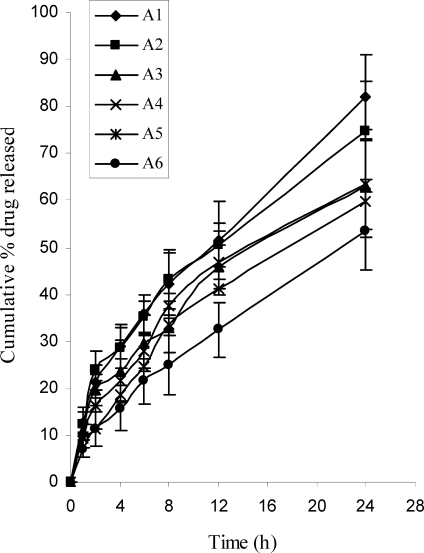
The results of in vitro drug release studies from transdermal patches are depicted in Figure 3. Formulation A1 exhibited greatest (82) percentage of drug release, which was significantly (P<0.05) different from the lowest value obtained by formulation A6 (53.5%). In the present study, it was observed that as the concentration of hydrophilic polymer (HPMC) increased in the formulations, the drug release rate increased substantially. Addition of hydrophilic component to an insoluble film former tends to enhance the release rates.
Figure 3.
In vitro release profile of LRDP from transdermal patches, mean±SD (n=3).
Data of the in vitro release was fit into different equations and kinetic models to explain the release kinetics of LRDP from transdermal patch. The kinetic models (22) used were zero-order equation, first-order equation, Higuchi and Korsemeyer-Peppas models. The cumulative amount of the drug released from the patches, when plotted against square-root of time, the release profiles of drug seemed to follow Higuchi model as it was evidenced by correlation coefficients (r2=0.98 to 0.99) better than zero order (r2=0.95 to 0.98) and first order (r2=0.52 to 0.57). The data was further treated as per the following equation
where, Mt/M8 is the fractional release of the drug, Mt is the amount released at time t, M8 is the total amount of drug contained in the transdermal patch, t is the release time, K is a kinetic constant, and n is the diffusional release exponent indicative of the operating release mechanism. The n values obtained (0.527 to 0.626) by this equation indicated that the drug release was by non-fickian model.
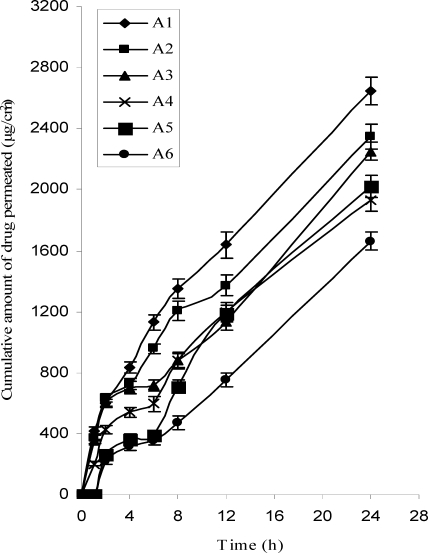
Ex-vivo permeation studies
The results of ex-vivo drug permeation studies from transdermal patches are shown in Table 2 and Figure 4. The formulation A1 exhibited the greatest (2644.5 µg/cm2) cumulative amount of drug permeation, which was significantly (p<0.05) different compared to the lowest value observed with the formulation A6 (1663.4) during 24 hrs. The flux obtained with formulation A1 was found to be maximum (106.5 µg/cm2/hr). When the permeability coefficients of different formulations were compared, A1 was found to have highest value (2.8×10−2 cm hr−1). When the cumulative amount of the drug permeated per square centimeter of patches through the rat abdominal skin was plotted against time, it was found that permeation profiles of drug follow Higuchi's equation as it was evidenced by correlation coefficients (r2=0.93 to 0.99) better than first order (r2=0.54 to 0.68) and zero order kinetics (r2=0.91 to 0.98). As the proportion of HPMC increased in all formulations, permeation was increased. As described by Rao and Diwan, initial rapid dissolution of the hydrophilic polymer occurs when the patch is in contact with the hydrated skin, resulting in the accumulation of high amounts of drug in the skin surface and thus leading to saturation of the skin with drug molecules at all times (23).
Table 2.
In vitro Drug Release, Ex vivo Skin Permeation, Transdermal Flux and Permeability Coefficient of Lercanidipine hydrochloride Transdermal Patches
| Formulation Code | Q24a (%) | Q24b (µg/cm2) | Jssc (µg/cm2/hr>) | Kpd (cm hr-1#x0xB4; 10-2) |
|---|---|---|---|---|
| A1 | 82.0±8.93 | 2644.5±91.73 | 106.5±3.61 | 2.76±0.093 |
| A2 | 74.9±10.42 | 2347.2±83.74 | 94.2±3.18 | 2.44±0.082 |
| A3 | 63.2±9.46 | 2249.5±60.25 | 92.5±2.30 | 2.40±0.059 |
| A4 | 63.5±11.43 | 1933.4±79.42 | 82.1±3.17 | 2.05±0.082 |
| A5 | 59.8±5.34 | 2021.5±68.40 | 872±2.15 | 2.19±0.071 |
| A6 | 53.5±8.46 | 1663.4±58.37 | 72.1±2.25 | 1.81±0.058 |
Q24a Cumulative% of the drug that released, results are the mean±SD of triplicate observations.
Q24b Cumulative amount (µg) of the drug that permeated per cm2, results are mean±SD of triplicate observations.
Jssc Transdermal flux, values represent mean±SD (n=3).
Kpd Permeability Coefficient, values represent mean±SD (n=3).
Figure 4.
Ex vivo permeation profile of LRDP from transdermal patches through rat abdominal skin, mean±S.D (n=3)
Primary skin irritancy studies
The primary skin irritancy study of the transdermal patches, placebo patch and patch A1 showed a skin irritation score (erythema and edema) of less than 2 (Table 3). According to Draize et al., (18) compounds producing scores of 2 or less are considered non-irritant. Hence, the transdermal patches were free of skin irritation.
Table 3.
Skin Irritation Scorns Following Transdsrmal Patch Administration
| Rabbit No. | Group I (without any drug) | Group II (A1) | Group III (Formalin) | |||
|---|---|---|---|---|---|---|
| Erythemaa | Edemab | Erythema | Edema | Erythema | Edema | |
| 1 | 0 | 1 | 0 | 0 | 3 | 2 |
| 2 | 1 | 0 | 0 | 1 | 3 | 1 |
| 3 | 0 | 1 | 1 | 1 | 3 | 2 |
| Average ±S.D | 0.34±0.58* | 0.67±0.58* | 0.34±0.58* | 0.67±0.58* | 3±0 | 1.67±0.58 |
p<0.05, significant compared with formalin.
: Erythema scale: 0, none; 1, slight; 2, well defined; 3, moderate; and 4, scar formation.
: Edema scale: 0, none; 1, slight; 2, well defined; 3, moderate; and 4, severe.
CONCLUSIONS
Based on the results of this study, it may be concluded that polymers selected were better suited for the development of TDDS of LRDP and the formulation A1 may be used for further pharmacokinetic and pharmacodynamic studies in humans or animals.
ACKNOWLEDGEMENTS
The authors are grateful to the management of the institute, Sultan-Ul-Uloom Educational Society, Banjarahills, Hyderabad for providing the facilities. The authors acknowledge M/s Aurobindo Pharmaceuticals, Hyderabad, India and Sun Pharmaceuticals (Baroda, India) for gift samples of Eudragit RL100 and lercanidipine hydrochloride respectively.
REFERENCES
- 1.Chien YW. Novel Drug Delivery Systems. NewYork: Marcel Dekker; 1992. p. 301. [Google Scholar]
- 2.Petkar KC, Kuchekar BS. In-vitro percutaneous absorption of losartan potassium in human skin and prediction of human skin permeability. DARU. 2007;15(2):53–60. [Google Scholar]
- 3.Williams AC, Barry BW. Prediction of Percutaneous Penetration. In: Scott RC, Guy RH, Hadgraft J, editors. Vol. 2. London: IBC Technical Services; 1990. pp. 224–230. [Google Scholar]
- 4.Krishnaiah YSR, Satyanarayana V, Bhaskar P. Influence of limonene on the bioavailability of nicardipine hydrochloride from membrane-moderated transdermal therapeutic systems in human volunteers. Int J Pharm. 2002;247:91–102. doi: 10.1016/s0378-5173(02)00401-5. [DOI] [PubMed] [Google Scholar]
- 5.Dnyanesh NT, Vavia PR. Acrylate-based transdermal therapeutic system of nitrendipine. Drug Dev Ind Pharm. 2003;29(1):71–78. doi: 10.1081/ddc-120016685. [DOI] [PubMed] [Google Scholar]
- 6.Ramesh G, Vamshi Vishnu Y, Kishan V, Madhusudan Rao Y. Development of carvedilol transdermal patches: evaluation of physicochemical, ex vivo and mechanical properties. PDA J Pharm Sci Technol. 2008;62(6):391–401. [PubMed] [Google Scholar]
- 7.Bang ML, Chapman MT, Goa LK. Lercanidipine a review of its efficacy in the management of hypertension. Drugs. 2003;63:449–2472. doi: 10.2165/00003495-200363220-00013. [DOI] [PubMed] [Google Scholar]
- 8.Barchielli M, Dolfini E, Farina P. Clinical pharmacokinetics of lercanidipine. J Cardiovasc Pharmacol. 1997;29:S1–S15. [Google Scholar]
- 9.Kaidi Z, Singh J. In vitro percutaneous absorption enhancement of propranolol hydrochloride through porcine epidermis by terpenes/ethanol. J Control Rel. 1999;62:359–366. doi: 10.1016/s0168-3659(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 10.Hayton WL, Chen T. Correction of perfusate concentration for sample removal. J Pharm Sci. 1982;71:820–821. doi: 10.1002/jps.2600710726. [DOI] [PubMed] [Google Scholar]
- 11.Barry BW. Penetration enhancers: Dermatological formulations. New York: Marcel Dekker; 1983. pp. 160–172. [Google Scholar]
- 12.Williams AC, Barry BW. Terpenes and the lipid-protein partitioning theory of skin penetration enhancement. Pharm Res. 1991;8:17–24. doi: 10.1023/a:1015813803205. [DOI] [PubMed] [Google Scholar]
- 13.Devi K, Saisivum S, Maria GR, Deepti PU. Design and evaluation of matrix diffusion controlled transdermal patches of verapamil hydrochloride. Drug Dev Ind Pharm. 2003;5:495–503. doi: 10.1081/ddc-120018638. [DOI] [PubMed] [Google Scholar]
- 14.Arora P, Mukherjee B. Design, development, physicochemical and in vitro and in vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J Pharm Sci. 2002;91:2076–2089. doi: 10.1002/jps.10200. [DOI] [PubMed] [Google Scholar]
- 15.Gupta R, Mukherjee B. Development and in vitro evaluation of diltiazem hydrochloride transdermal patches based on povidone-ethyl cellulose matrices. Drug Dev Ind Pharm. 2003;29:1–7. doi: 10.1081/ddc-120016678. [DOI] [PubMed] [Google Scholar]
- 16.Draize JH, Woodword G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–379. [Google Scholar]
- 17.Rhee YS, Choi JG, Park ES, Chi SC. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228(1-2):161–70. doi: 10.1016/s0378-5173(01)00827-4. [DOI] [PubMed] [Google Scholar]
- 18.Rizwan M, Aqil M, Ahad A, Sultana Y, Ali MM. Transdermal delivery of valsartan: I. Effect of various terpenes. Drug Dev Ind Pharm. 2008;34(6):618–26. doi: 10.1080/03639040701833740. [DOI] [PubMed] [Google Scholar]
- 19.Krishnaiah YSR, Satyanarayana V, Bhaskar P. Effect of limonene on the in vitro permeation of nicardipine hydrochloride across the excised rat abdominal skin. Pharmazie. 2002;57:842–847. [PubMed] [Google Scholar]
- 20.Moghimi HR, Williams AC, Barry BW. A lamellar matrix model for stratum corneum intercellular lipids. V. Effects of terpene penetration enhancers on the structure and thermal behaviour of the matrix. Int J Pharm. 1997;146:41–54. [Google Scholar]
- 21.Mutalic S, Udupa N. Glibenclamide transdermal patches: physicochemical, pharmacodynamic, and pharmacokinetic evaluations. J Pharm Sci. 2004;93:1577–1594. doi: 10.1002/jps.20058. [DOI] [PubMed] [Google Scholar]
- 22.Costa P, Lobo SJM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 23.Rao PR, Diwan PV. Formulation and in vitro evaluation of polymeric films of diltiazem hydrochloride and indomethacin for transdermal administration. Drug Dev Ind Phar. 1998;24:327–336. doi: 10.3109/03639049809085627. [DOI] [PubMed] [Google Scholar]