Abstract
Background and the Purpose of the study
Central Angiotensin Converting Enzyme (ACE) has an important role on cerebral microcirculation and metabolism. However, its role in terms of protecting the brain from ischemic/reperfusion (I/R) injury are debatable. This study evaluated the role of ACE, using enalapril as ACE inhibitor, in protection of the brain from I/R injury during transient focal cerebral ischemia (TFCI) in normotensive rat.
Method
Male Sprague Dawley rats (280–320g) randomly assigned to control ischemic and enalapril pre-treated ischemic groups. Enalapril was injected intraperitoneally 1 h before middle cerebral artery occlusion (MCAO) at the dose of 0.03 or 0.1 mg/kg. Cerebral ischemia was induced by 60 min MCAO followed by 24 hrs reperfusion. After evaluation of neurological deficit scores (NDS) the animal was sacrificed for assessment of cerebral infarction and edema.
Results
TFCI induced cerebral infarctions (283±18 mm3), brain edema (4.1±0.4%) and swelling (9.8±1.5%) with NDS of 3.11±0.36. Non-hypotensive dose of enalapril (0.03 mg/kg) improved NDS (1.37±0.26), reduced cerebral infarction (45%), brain edema (54%) and swelling of the lesioned hemispheres (34%) significantly. However, hypotensive dose of enalapril (0.1 mg/kg) could improve neurological activity (1.67±0.31) and failed to reduce cerebral infarction (276±39 mm3) and swelling (10.4±1.4%).
Conclusion
In the rat model of transient focal cerebral ischemia, inhibition of angiotensin converting enzyme with non-hypotensive doses of enalapril has the benefit of improving neurological activity, reducing cerebral infarction, brain swelling and edema of acute ischemic stroke. Therefore, it is reasonable to conclude that central renin-angiotensin system may participate in ischemic/reperfusion injury of the cerebral cortex.
Keywords: Cerebral ischemia, Lesion volume, Edema, ACE inhibition, Enalapril
INTRODUCTION
The critical role of renin–angiotensin system (RAS) in cardiovascular and fluid hemostasis is well established and some evidences exist about the role of angiotensin-converting enzyme (ACE) activity and angiotensin II in ischemic neuronal injury (1). It is suggested that inhibition of the RAS might be effective not only in reducing the incidence of stroke but also attenuating neuronal injury after stroke (2). Recent clinical studies revealed that ACE inhibitors, which are used as anti-hypertensive drugs, reduce the risk and the severity of secondary attacks of the stroke (3). Long term treatment with ACE inhibitors has shown to prevent the occurrence of stroke in spontaneously hypertensive or salt loaded-Dahl salt-sensitive rats (4, 5). Moreover, ACE inhibitors are reported to improve neurological recovery from cerebral ischemia/reperfusion (I/R) injury and reduce the mortality rate in spontaneously hypertensive rats (6). Pathological remodeling of cerebral vessels that occur during chronic hypertension is reported to interfere with the outcome of ACE inhibition and other neuroprotective agents (5). Therefore, this study was designed to induce cerebral I/R injury in normotensive rats to alleviate the interaction of hypertension in protective action of ACE inhibitors.Experimental-induced transient focal cerebral ischemia is widely used to examine the functional impairments resemble to those that have been observed in human stroke (7). Previously the beneficial effects of non-hypotensive dose of enalapril, an ACE inhibitor, in improving neurological activity and reducing cerebral infarction volume in normotensive rats exposed to 60 min middle cerebral artery occlusion (MCAO) was reported (8). Brain edema is a destructive phenomenon that worsens cerebral I/R injury. In the present study previous experiments were repeated and the effects of enalapril on the intensity of tissue swelling and brain edema which was induced during 60 min MCAO and 24 hrs reperfusion was evaluated.
MATERIAL AND METHODS
Male normotensive Sprague Dawley rats (280–320 g) were obtained from central animal house facility of Shiraz Medical Sciences University (Shiraz, Iran). All protocols of the study were approved by the institutional animal ethics committee of Shiraz Medical Sciences University which follows the NIH guidelines for care and use of animals. Animals were housed at room temperature of 22−24°C, humidity of 40–60% and light period of 07.00–19.00 controlled environments. The animals were then randomly divided into 4 groups.
Animals were food fasted overnight but had access to water. Anesthesia conducted by intraperitoneal (IP) injection of 400mg/kg chloral hydrate. The trachea was cannulated from the mouth with a polyethylene tube to prevent possible asphyxia, artificially ventilate, or to oxygenate the animal in the case of hypoxia as described previously (8, 9). However, the animals were breathing spontaneously during surgery and the experiment. Core and cranial temperatures were recorded continuously by two probes, one inserted into the rectum and the other in the vicinity of left temporalis muscle, and the temperature maintained at 37±1°C with a heating pad and a heating lamp.
Experimental protocol
Group 1 (sham, n=16), rats underwent the surgery at the neck region and received a single IP injection of the vehicle (1ml/kg normal saline) without being exposed to MCAO.
Group 2 (control ischemic; n=15), rats received a single IP injection of the vehicle 1 h before MCAO. Afterwards, surgery performed at the neck region, the same as sham group. After resting for 30 min brain ischemia achieved by 60 min MCAO followed by 24 h reperfusion.
Group 3 (enalapril pre-treated ischemic rats with 0.03 mg/kg; n=14), rats received a single IP injection of 0.03 mg/kg of enalapril (Sigma Chemicals, UK) 1 h prior to MCAO. All procedures which were performed on rats of group 2 were also repeated in this group.
Group 4 (enalapril pre-treated ischemic rats with 0.1 mg/kg; n=6), rats of this group received a single IP injection of 0.1 mg/kg of enalapril 1 h before induction of MCAO. Other procedures were similar to groups 2 and 3. Due to low survival rate the rats of this group that survived after 24 hrs reperfusion were only used for the evaluations of cerebral infarction volumes and brain swelling and were not used for the assessment of brain edema.
Induction of transient focal cerebral ischemia
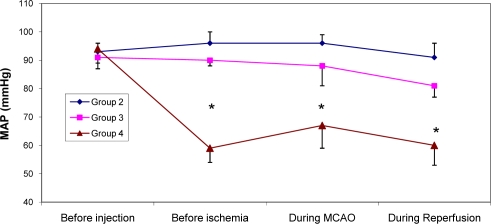
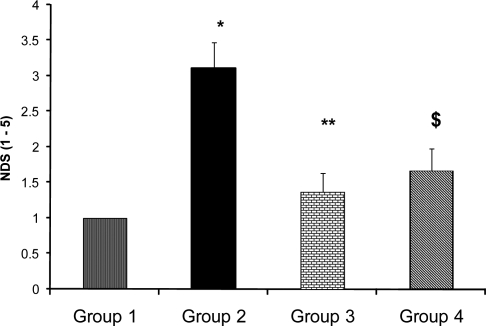
Sixty min MCAO and 24 hrs reperfusion of the right cerebral hemisphere carried out by intraluminal filament method described by Longa et al (10) and modified by Vakili et al (9). One hr after termination of ischemia all incisions which were made during surgery sutured and after recovery the animal returned to a warm cage for recuperation and housed during reperfusion period. Few rats of each group were randomly selected and their posterior tail artery cannulated to continuously record mean arterial blood pressure (MAP) during the experiment. (Fig. 1)Behavioral tests were performed by a blinded observer 24 hrs after surgery in the sham group, or 24 hrs after MCAO in the ischemic groups that survived the ischemic trauma. As described previously, five-point scale grading neurological deficit score (NDS) test was carried out to evaluate the neurological outcome (8). After evaluation of NDS (Fig. 2) the rats of each group were randomly assigned to assess, 1) cerebral infarction volumes and 2) edema formations during I/R injury. Overall, approximately 35% of the animals of groups 2, 20% of group 3 and 54% of group 4 died during reperfusion period and their data could not be collected for the evaluations of NDS, cerebral infarction volumes or edema.
Figure 1.
Mean arterial blood pressure (MAP) in control ischemic (group 2; n=5) and ischemic rats pre-treated with enalapril at doses of 0.03 mg/kg (group 3; n=4) or 0.1 mg/kg (group 4; n=5) at 1 h before MCAO (pre-ischemia), 10 min after MCAO and 30 min after MCA reopening (Reperfusion).
Values are mean±SEM.
*=values are significantly different from groups 2 and 3 at P<0.05,)
Figure 2.
Neurological deficit score (NDS) in sham (group 1), control ischemic (group 2) and enalapril pre-treated rats with 0.03mg/kg (group 3) and 0.1mg/kg (group 4) subjected to 60 min MCA occlusion and 24 hrs reperfusion. Values are mean±SEM.
*=Significantly different from group 1 (P<0.05).
**=Significantly different from group 2 (p<0.05).
$= Significantly different from groups 1 and 2 (p<0.05)
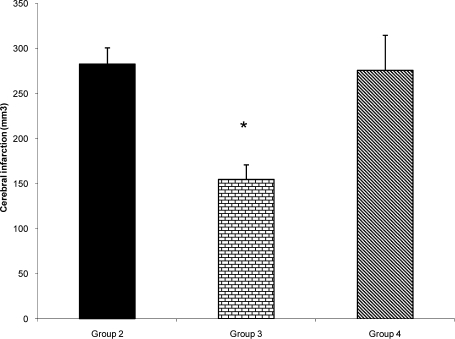
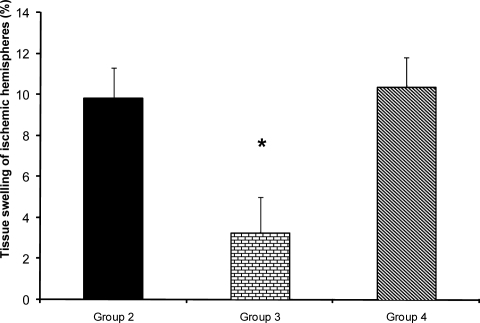
After NDS the animal was slaughtered under deep anesthesia, the brain was removed, cleaned and solidified by immersion in 4°C cold saline kept in the refrigerator for 5–10 min. Frontal sections of 2-mm thick slices were prepared using a brain matrix and stained with triphenyltetrazolium chloride (TTC) described previously (8, 9). In this staining technique, nonischemic brain region was colored red by converting TTC to a deep red formazan compound and ischemic areas remained white due to failure of conversion (Fig. 3). After staining, the slice images were digitized, using a Cannon camera, and cerebral infarction volume (Fig. 4) areas were determined with computer based NIH image analyzer software (8, 9). The tissue swelling induced in the lesioned hemispheres of TTC staining method was evaluated (Fig. 5) using the equation 1 described by Swanson et al (11).
| 1 |
were LHV and NHVare Lesioned and Non-lesioned Hemisphere Volume.
Figure 3.
Photographs illustrating the coronal sections of rat brain slices stained with TTC 24 hrs after neck surgery in sham (group 1) or after 60 min occlusion of middle cerebral artery and 24 hrs reperfusion in control ischemic (group 2), and enalapril pre-treated ischemic rats (0.03 or 0.1 mg/kg, groups 3 and 4) as described in the text. MCA occlusion induced different magnitudes of infarctions in the right hemispheres without affecting the left sides. Non-ischemic areas are colored deep red, whereas, ischemic areas are white. Note the similarity of ischemic areas of brain slices of groups 2 and 4. There is a marked decrease in the ischemic areas of group 3.
Figure 4.
Cerebral infarct volume of ischemic (right) hemispheres in control (Group 2; n=9), and enalapril pre-treated ischemic rats with 0.03mg/kg (Group 3; n=8) or 0.1mg/kg (group 4; n=5) subjected to 60 min MCA occlusion and 24 hrs reperfusion. Animals received treatments 1 h before MCA occlusion. Values are mean±SEM
*=significantly different from groups 2 and (P<0.05)
Figure 5.
Percent tissue swelling of lesioned (right) hemispheres after 60 min MCA occlusion and 24 hrs reperfusion in control ischemic (group 2) and enalapril pre-treated animals (0.03mg/kg; Group 3) or (0.1mg/kg; Group 4).
*=significantly different from groups 2 and 4 at p<0.05).
The more accurate way of determining the brain edema formation (Figs. 6 and 7) during I/R injury is to use wet/dry method described by Goth and colleagues (12). The percentage of brain water content and edema formation for each hemisphere was calculated by the measurement of the wet weight (WW) and dry weight (DW) of ipsilateral lesioned (LH) and contralateral non-lesioned (NH) hemispheres (Equations 2 and 3).
| 2 |
| 3 |
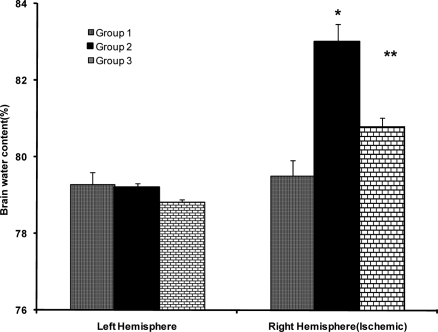
Figure 6.
Brain water content (WC) of right and left hemispheres of sham 24 hrs after operation (group 1), and control ischemic (group 2) and 0.03 mg/kg enalapril pre-treated ischemic groups (group 3) after 60 min occlusion of right MCA and 24 hrs reperfusion.
Values are mean±SEM
*=significantly different from ipsilateral hemispheres of groups 1 and 2 (P<0.05)
$=Significantly different from ipsilateral hemispheres of group 1 (p<0.05).
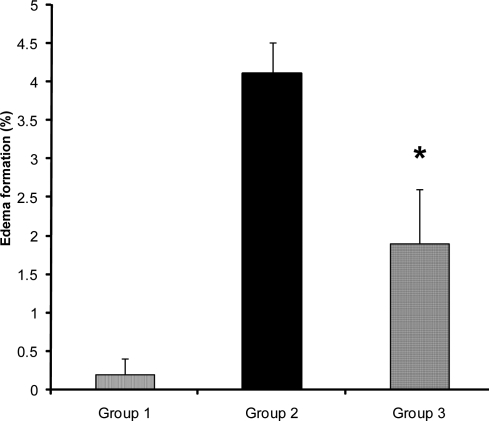
Figure 7.
Edema (%) of lesioned (right) hemispheres in control (group 2) and 0.03mg/kg enalapril pre-treated ischemic rats (group 3) subjected to 60 min MCAO and 24 hrs reperfusion.Values are mean±SEM
*=Significantly different from group 2 (P<0.05)
Statistical analyses
Values are expressed as mean±SEM. One-way ANOVA with post-hoc of Duncan's test was used to compare the mean values of MAP. Holms-Sidack method was also used for pairwise comparisons of other parameters. Statistical significance was accepted at P<0.05.
RESULTS AND DISCUSSION
In this study the beneficial effects of enalapril, an ACE inhibitor on the neurological activity, cortical infarction and brain edema of normotensive rats exposed to 60 min middle cerebral artery occlusion were investigated. On the basis of reports, the lowering arterial blood pressure reduces the risk of stroke in patients with the history of stroke, (13) or during ischemia in experimental animals (14). In chronic hypertensive rats reduction of MAP is an important parameter that interferes with ischemic brain injury (15). On the basis of reports enalapril protect the brain from ischemic injury mainly by lowering arterial blood pressure (4, 14). To examine the protective actions of ACE inhibition in ischemic stroke without involvement of arterial hypotension the instruction of previous investigators about the dose of enalapril was followed and non-hypotensive dose of enalapril (0.03 mg/kg) was chosen for protection against acute stroke in normotensive rats (8, 15). The MAP of control ischemic rats of group 2 and 0.03 mg/kg enalapril pre-treated ischemic rats of group 3 during ischemia and reperfusion period were all in normal physiological range and quantitatively there were no statistical differences between them (Fig. 1). This similarity indicated that occlusion and re-opening of MCA as well as IP injection of 0.03 mg/kg of enalapril (group 3) did not alter arterial blood pressure. However, results indicated that inhibition of ACE activity per se could improve neurological activity (Fig. 2) with a concomitant reduction of brain infarction (Figs. 3 and 4). This finding has the support of the pervious reports in that the protective effects of ACE inhibitors are independent of blood pressure reduction in chronic hypertensive rats (16). Some of these results are also in accordance with our previous report, in normotensive rats (8), and the reports of Ravati et al, in hypertensive rats (15).
Asano et al, have indicated that ACE inhibitors reduce cerebrovascular tone and potentiate hyperperfusion phase of brain ischemia (17). Consequently cerebral vasodilatory action of ACE inhibitors help collateral vessels to have a better blood flow during ischemia and reperfusion phase to nourish the penumbra of ischemic region (4, 17). However in this study the persistence of severe arterial hypotension, which is observed during ischemia and reperfusion phase of group 4, (Fig. 1), might has reduced collateral circulations drastically and suppressed the protective actions of enalapril (Figs. 3 and 4). In agreement with these results, previous reports showed that acute reduction of arterial blood pressure after stroke worsens neurological outcome, whereas long-term blood pressure control may reduce the incidence of stroke and improves neurological activity (6, 14). This statement also has the support of other reports in that, in acute experimental ischemic stroke, the most likely mechanisms responsible for the benefits of reduced arterial blood pressure is the improvement of blood flow during reperfusion period and the reduction of inflammatory damages that may occur afterward (14).
Brain edema is an overriding complication of ischemic stroke that elevates intracranial pressure and degrades clinical outcomes (18). To examine the influence of ACE inhibition in brain edema formation two independent techniques were used, and the magnitude of edema formation during I/R injury were evaluated. The direct measurement of absolute WC of ipsilateral lesioned hemispheres in comparison with their own contralateral non-lesioned hemispheres, revealed that 60 min ischemia significantly elevated their WC. However, no differences were observed in the WC of non-lesioned hemispheres of all groups, in comparison to both hemispheres of sham-operated rats, indicated that drug treatment and vascular manipulation of lesioned hemispheres did not affect their WC (Fig. 6). Therefore, the elevated WC of lesioned hemispheres showed that brain edema which occurred during I/R injury was prevented by 0.03 mg/kg enalapril (Fig. 7). These data quantitatively are comparable with the values reported by other reports (12, 19). The values of edema formation in ischemic hemispheres indicate that the trend of edema formation is qualitatively well correlated with the tissue swelling (Fig. 7), and the magnitude of brain swelling is also well correlated with cerebral infarct size of ischemic rats. Therefore, in agreement with other reports (20), it is believed that inhibition of ACE during ischemia, which is not accompanied with severe hypotension, not only reduces cerebral infarction but it protects the lesioned brain from the side effects of edema formation.
CONCLUSION
The results of this study indicate that pre-treatment of normotensive rats with non-hypotensive dose of enalapril prevents the deterioration effects of ACE activity in cerebral ischemia/reperfusion injury. However, high dose of enalapril associated with severe arterial hypotension can not protect the brain from such injuries.
ACKNOWLEDGMENT
The authors cordially appreciate the help of Dr Shokrollah Sabetghadam Jahromi of Toronto University, Canada, and Dr Abolhasan Ahmadiani of Shahid Beheshti University, Tehran, Iran, for providing us the drugs. This work was financially supported (grant No 83-2375) by Vice Chancellor for Research & Medicinal & Natural Products Chemistry Research Center of Shiraz University of Medical Sciences, Shiraz, Iran.
REFERENCES
- 1.Unger T., Badoer E., Ganten D., Lang R.E., Retting R. Brain Angiotensin., pathways and pharmacology. Circulation. 1993;77:40–54. [PubMed] [Google Scholar]
- 2.Culman J., Blume A., Gohlke P., Unger T. The renin-angiotensin system in the brain., possible therapeutic implications for AT (1)-receptor blockers. J Hum Hypertens. 2002;3:S64–S70. doi: 10.1038/sj.jhh.1001442. [DOI] [PubMed] [Google Scholar]
- 3.Brdon J., Kaiser S., Hagemann F., Zhao Y., Culman J., Gohlke P. Comparison between early and delayed systemic treatment with candesartan of rats after ischaemic stroke. J hypertens. 2007;25:187–196. doi: 10.1097/01.hjh.0000254376.80864.d3. [DOI] [PubMed] [Google Scholar]
- 4.Ito T., Yamakawa H., Bregonzio C., Terrón J.A., Falcón-Neri A., Saavedra J.M. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke. 2002;33:2297–303. doi: 10.1161/01.str.0000027274.03779.f3. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J., Pavel J., Macova M., Yu Z.X., Imboden H., Ge L., Nishioku T., Dou J., Delgiacco E., Saavedra J.M. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke. 2006;37:1271–1276. doi: 10.1161/01.STR.0000217404.64352.d7. [DOI] [PubMed] [Google Scholar]
- 6.Lee R.M., Wang H., Smeda J.S. Perindopril treatment in the prevention of stroke in experimental animals. J Hypertens Suppl. 1996;14:S29–S33. [PubMed] [Google Scholar]
- 7.Yamori Y., Horie R., Handa H., Sato M., Fukase M. Pathogenetic similarity of stroke–prone spontaneously hypertensive rats and humans. Stroke. 1976;7:46–53. doi: 10.1161/01.str.7.1.46. [DOI] [PubMed] [Google Scholar]
- 8.Panahpour H., Nekooeian A.A., Dehghani G.A. Inhibition of angiotensin-converting enzyme reduces 8. cerebral infarction size in experimental-induced focal cerebral ischemia in the rat. Iran J Med Sci. 2007;32:12–17. [Google Scholar]
- 9.Vakili A., Nekooeian A.A., Dehghani G.A. Aminoguanidine reduces infarct volume and improves 9. neurological dysfunction in transient model of focal cerebral ischemia in rat. DARU. 2006;14:31–36. [Google Scholar]
- 10.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 11.Swanson R.A., Morton MT., Tsao-Wu G., Savalos R.A., Davidson C., Sharp F.R. Semi automated method for measuring brain LV. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 12.Gotoh O., Asano T., Koide T., Takakura K. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: The time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke. 1985;16:101–109. doi: 10.1161/01.str.16.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell P.M., Buchan A., Johnston S.C. Recent advances in management of transient ischaemic attacks and minor ischaemic strokes. Lancet Neurol. 2006;5:323–331. doi: 10.1016/S1474-4422(06)70408-2. [DOI] [PubMed] [Google Scholar]
- 14.Elewa HF., Kozak A., Johnson MH., Ergul A., Fagan S.C. Blood pressure lowering after experimental cerebral ischemia provides neurovascular protection. Hypertension. 2007;25:743–745. doi: 10.1097/HJH.0b013e3280149708. [DOI] [PubMed] [Google Scholar]
- 15.Ravati A., Junker V., Kouklei M., Ahlemeyer B., Culmsee C., Krieglstein J. Enalapril and moexipril protect from free radical-induced neuronal damage in vitro and reduce ischemic brain injury in mice and rats. Eur J Pharmacol. 1999;373:21–33. doi: 10.1016/s0014-2999(99)00211-3. [DOI] [PubMed] [Google Scholar]
- 16.Gohlke P., Linz W., Schölkens B., Van Even P., Martorana P., Unger T. Vascular and cardiac protection 16. by ramipril in spontaneously hypertensive rats, prevention versus regression study. Br J Clin Pract Suppl. 1996;84:1–10. [PubMed] [Google Scholar]
- 17.Asano T., Gotoh O., Koide T., Takakura K. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. II, Alteration of the eicosanoid synthesis profile of brain microvessels. Stroke. 1985;16:101–109. doi: 10.1161/01.str.16.1.110. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg G.A. Ischemic brain edema. Prog. Cardiovasc Dis. 1999;42:209–216. doi: 10.1016/s0033-0620(99)70003-4. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y., Koizumi C., Marumo T., Omura T., Yoshida S. Serum S100B indicates brain edema formation and predicts long-term neurological outcomes in rat transient middle cerebral artery occlusion model. Brain Res. 2007;1137:140–145. doi: 10.1016/j.brainres.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Nag S., Kilty D.W. Cerebrovascular changes in chronic hypertension, Protective effects of enalapril in rats. Stroke. 1997;28:1028–1034. doi: 10.1161/01.str.28.5.1028. [DOI] [PubMed] [Google Scholar]