Abstract
Background and the purpose of the study
The species Hymenocrater calycinus, belongs to the plant family Lamiaceae and grows wildly in the north-east of Iran. Previously, the antimicrobial activity of the plant extracts was reported. In the present study, the bioactivity-guided fractionation of the methanol extract of H. calycinus and the combination effects of the isolated compound with cell wall active agents against S. aureus and E. coli was investigated.
Methods
Column and thin layer chromatographic methods were used for isolation and purification and spectroscopic data (MS, 1H- and 13C-NMR, HMQC, HMBC and 1H-1H COSY) were employed for identification of the compound isolated from the extract. A disk diffusion method was used to determine the antibacterial activity of the isolated compound against S. aureus and E. coli in comparison with 7 different antibiotics.
Results
The isolated compound 1 was identified as 3-(3, 4- dihydroxyphenyl) lactic acid 2-O-quinic acid. Compound 1 (500 µg/disc) enhanced antibacterial effect of ampicillin, ciprofloxacin, vancomycin and cefepime against S. aureus and activated the effects of ampicillin and vancomycin against E. coli.
Conclusion
Results showed that the compound 1 was not active against both tested strains at any concentration below 1 mg/disk, and as a result the enhancing effect of the compound could be due its association with antibiotics.
Keywords: Hymenocrater calycinus; Lamiaceae; 3-(3, 4- dihydroxyphenyl) lactic acid 2-O- quinic acid; Antibacterial effect
INTRODUCTION
Infectious diseases are the main cause of approximately one-half of all death in tropical countries (1). During the past decade life-threatening fungal and bacterial infections have increased dramatically in immunocompromised patients (2). Among the microorganisms, first Esherichia coli and second Staphylococcus aureus are pathogens responsible for most primary and secondary skin infections. S. aureus is also colonized in 30% of burn wounds (3). In recent years, the resistance of these microorganisms to other antimicrobial agents have increased significantly (4).
Plants have provided a good source of novel anti-infective compounds (5, 6). The first generation of the plant drugs were usually simple botanicals which were used in crude form and the second generation were the pure and active isolated molecules (1).
In the search for potent herbal antimicrobial compound, the activity of an extract of Hymenocrater calycinus (Lamiaceae), which is growing wildly in the north east of Iran and some parts of Turkmania as an endemic plant and is called Gol-e-Arvaneh in Persian language, was investigated (7, 8). The synonym of this plant is Hymenocrater macrophyllum Bunge (8) and there are several reports on the biological and phytochemical properties of this genus in the literatures (9, 10). Antibacterial and antifungal activity of the methanol extract of H. sessilifolius, by agar well diffusion and disk diffusion assays has also been reported previously (10).
It has been found that the efficacy of antimicrobial agents against different pathogens, including S. aureus, P. aeruginosa, and E. coli can be improved by combining with crude plant extracts (eg. Salvia officinalis) (11).
This paper reports isolation and identification of an active compound from the methanol extract of H. calycinus, which is used traditionally in infectious diseases (10), by the bioactivity-guided fractionation and its antibacterial synergy with cell wall active antibiotics against S. aureus and E. coli.
MATERIAL AND METHODS
General experimental procedures
1H- and 13C-NMR spectra were measured on a Brucker Avance 500 DRX (500 MHz for 1H and 125 MHz for 13C) spectrometer with tetramethylsilane as an internal standard and chemical shifts are given in δ (ppm). EI-MS data were recorded on Agilent Technology (HP TM) instrument with 5973 Network Mass Selective Detector (MS model). The FT-IR spectra were recorded on a Nicolet 550 instrument. Silica gel 60F254 pre-coated plates (Merck) were used for TLC. The components were detected by spraying with anisaldehyde-H2SO4 reagent followed by heating.
Plant collection
Aerial parts of Hymenocrater calycinus (Boiss.) Benth., were collected from Firuzkuh near to Delichaee village, during flowering stage in July 2005. Two Herbarium specimens were identified by Dr. Ali Reza Naghinejad from the Faculty of Sciences, University of Tehran. One specimen was deposited at Central Herbarium of Tehran University and another one housed at Herbarium of Medicinal Plants Research Center, Tehran University of Medical Sciences.
Extraction and isolation procedure
The flowered aerial parts of H. calycinus (400 g) was cut into small pieces and percolated with methanol at room temperature for 72 hrs. Percolation was repeated two more times. The MeOH extract (7.4 g) was successively subjected to reverse phase silica gel column chromatography with MeOH: H2O (4:6, 7:3 and 1:0) to give five fractions (A-E). Fraction A (3.3 g) was further fractionated by a silica gel CC with AcOEt: MeOH (6:4) to yield five fractions (A1- A5). Compound 1 (35 mg) was obtained from A3 (0.5 g) using sephadex LH20 CC with MeOH.
Evaluation of the combination effects of compound 1 with different antibiotics
A disk diffusion method, based on National Committee for Clinical Laboratory Standards (12), was used to determine the activity of different antibiotics and compound 1 against tested strains (S. aureus, ATCC 29737 and E. coli, ATCC 8739) on Müeller-Hinton agar (Merck, Germany) plates. The standard antibiotics disks were supplied from Mast Co., UK. To determine the combination effect, each standard disc paper (ampicillin, ciprofloxacin, vancomycin, cephalexine, penicillin G, cefixime and cefepime) was impregnated with the tested compound (500 µg). A single colony of the test strains were grown overnight in Müeller -Hinton broth medium on a rotary shaker (200 rpm) at 35°C. The inocula were prepared by diluting the overnight cultures with 0.9% NaCl to a 0.5 McFarland standard and were applied to the plates along with the standard and prepared disks containing of tested compound (500µg/disc). Similar experiments were carried out with isolated compound alone. After incubation at 37°C for 24 hrs, the zones of inhibition were measured. Mean surface area of the inhibition zone (mm2) was calculated for each tested antibiotic from the mean diameter. The percentage of increase in the inhibition zone for different antibiotics were calculated as (b2-a2)/a2× 00 where a and b are the inhibition zones A (antibiotic only) and B (antibiotic plus compound 1), respectively. All experiments were carried out in triplicates and standard deviations were negligible.
RESULTS AND DISCUSSION
In this research, dried aerial parts of H. calycinus, was percolated with methanol to give 7.4 g of the extract (18.5% based on the dried weight of the plant sample). The extract has shown antibacterial and antifungal activity (13) and in this study one of its active components was isolated by the reverse phase silica gel and sephadex column chromatography. The isolated compound showed a pink spot with anisaldehyde-H2SO4 reagent on thin layer chromatogram On the basis of physicochemical and spectral data its structure was established as 3-(3, 4- dihydroxyphenyl) lactic acid 2-O-quinic acid:
Pale yellow amorphous crystal; m. p. 160-161 °C; [α]25 −9.0° (c=0.003, MeOH); IR (CHCl3) νmax 3332, 2925, 2229, 1731, 1605, 1445, 1228, 1073, 749 cm−1; EI-MS (70eV) m/z (%): 123 100, 110 42, 198 18, 152 11; 1H and 13C-NMR spectra are shown in Table 1.
Table 1.
The NMR data of the compound 1 in DMSO-d6.
| Carbon No. | 13C- NMR (ppm) | 1H- NMR (ppm) | HMBC | H-HCOSY |
|---|---|---|---|---|
| 1 | 132.2 | H-5, H-7a, H-7b, H-8 | ||
| 2 | 117.8 | 6.65 (d, J= 1.6 Hz, 1H) | H-6, H-7a, H-7b | |
| 3 | 145.5 | H-5 | ||
| 4 | 144.0 | H-2, H-6 | ||
| 5 | 116.0 | 6.57 (d, J= 8.0 Hz, 1H) | H-6 | |
| 6 | 120.7 | 6.44 (dd, J= 8.0, 1.6 Hz, 1H) | H-2, H-7a, H-7b | H-5 |
| 7 | 41.4 | 2.36 (dd, J=13.8, 8.7 Hz, 1H) 2.84 (dd, J=13.8, 2.8 Hz, 1H) | H-2, H-6, H-8 | H-7b, H-8 H-7a, H-8 |
| 8 | 73.5 | 3.64 (dd, J= 8.7, 2.9 Hz, 1H) | H-7a | H-7a, H-7b |
| 9 | 177.7 | H-7a, H-7b, H-8 | ||
| 1′ | 74.5 | H-2′b, H-6′a, H-6′b | ||
| 2′ | 40.7 | 1.51 (dd, J=12.1, 3.8 Hz, 1H) 1.61 (dd, J=12.1, 11.2 Hz, 1H) | H-2′b, H-3′ H-2′a, H-3′ | |
| 3′ | 67.2 | 3.93 (m, 1H) | H-2′b | H-2′a, H-2′b, H-4′ |
| 4′ | 74.2 | 3.41 (t, 1H) | H-5′, H-3′ | H-3′, H-5′ |
| 5′ | 69.8 | 3.57 (m, 1H) | H-8, H-4′ | H-4′, H-6′a, H-6′b |
| 6′ | 37.5 | 1.45 (dd, J=13.8, 3.5 Hz, 1H) 1.72 (dd, J=13.8, 4.4 Hz, 1H) | H-2′b | H-5′, H-6′b H-5′, H-6′a |
| 7′ | 180.3 | H-2′b, H-6′a, H-6′b |
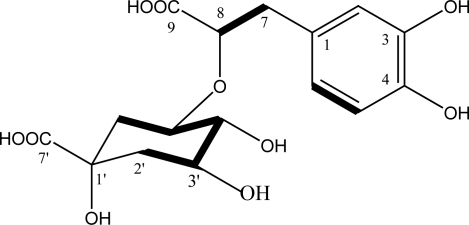
Fig 1.
Chemical structure of the isolated compound 1 from H.calycinus and H-H COSY correlations (bold bands).
The 1H-NMR spectrum of compound 1 exhibited aromatic ABX –type signals at 6.44 (dd, J= 8.0, 1.6 Hz, 1H), 6.57 (d, J= 8.0 Hz, 1H) and 6.65 (d, J= 1.6 Hz, 1H), assignable to H-6, 5 and 2, respectively. Aliphatic ABX-type signals at 2.36 (dd, J= 13.8, 8.7 Hz, 1H), 2.84 (dd, J= 13.8, 2.8 Hz, 1H) and 3.64 (dd, J= 8.7, 2.9 Hz, 1H) are assignable to H-7, H-7′ and H-8 of 3, 4- dihydroxyphenyl- lactic acid part of the compound 1 respectively. The 13C NMR data revealed the presence of a quinic acid moiety characterized with two methylenes (δ 37.5 and 40.7), three oxymethines (δ 67.2, 69.8, and 74.2), one quaternary carbon (δ 74.5), and one carboxyl group (δ 180.3), as well as a 3, 4- dihydroxyphenyl- lactic acid moiety characterized by 6 aromatic carbon (δ 116.0-145.5, C-1 to C-6), one methylene at δ 41.4(C-7), one oxygenated CH at δ 73.0 (C-8), and one carboxylic acid at δ 177.7 (C-9).
The OH group of the position of 5’ of quinic acid unit is connected (via etheric linkage) to the C-8 of the 3, 4- dihydroxyphenyl- lactic acid based on the HMBC correlations (see Table 1). The molecular formula, C16H20O10, revealed the structure of compound 1 as 3-(3, 4- dihydroxyphenyl) lactic acid 2-O-quinic acid.
Fig 2.
HMBC correlations of compound 1 (H →C).
While, many esters of quinic acid have been identified as bioactive natural compounds (14), the compound of this study is considered to be a new phenylpropanoid derivative of quinic acid which has not been reported previously. Antibacterial assays indicated that the compound 1 did not show any activity at the tested concentrations (125, 250 and 500 µg/disc), however, this compound (500µg/disc) enhanced the activity of several antibiotics against S. aureus and E. coli (Table 2 and Table 3). Ampicillin, ciprofloxacin, vancomycin and cefepime caused 48%, 22%, 36% and 38% increase in the inhibition zone of surface area of the compound 1 against S. aureus respectively. Also, ampicillin and vancomycin represented 26% and 36% fold increase in the surface area of inhibition zone of compound 1 against E. coli.
Table 2.
Zone of inhibition (mm) of different antibiotics against S. aureus (in the absence and presence of compound 1 at concentration of 500 (g/disk).
| Antibiotics (µg/disk) | Inhibition zone against S. aureus | Increase in the inhibition zone (%) | |
|---|---|---|---|
| Antibiotic (A) | Antibiotic+compound 1 (B) | ||
| Ampicillin 30 | 23 | 28 | |
| Cefixime 5 | 16 | 16 | 0 |
| Ciprofloxacin 5 | 19 | 21 | 22 |
| Vancomycin 30 | 12 | 14 | 36 |
| Penicillin G (10UT) | 24 | 24 | 0 |
| Cefepime 30 | 17 | 20 | 38 |
| Cephalexine 30 | 21 | 21 | 0 |
Table 3.
Zone of inhibition (mm) of different antibiotics against E. coli (in the absence and presence of compound 1 at concentration of 500 (g/disk).
| Antibiotics (µg/disk) | Inhibition zone against E. coli | Increase in the inhibition zone (%) | |
|---|---|---|---|
| Antibiotic (A) | Antibiotic+compound 1 (B) | ||
| Ampicillin 30 | 16 | 18 | 26 |
| Cefixime 5 | 0 | 0 | 0 |
| Ciprofloxacin 5 | 0 | 0 | 0 |
| Vancomycin 30 | 18 | 21 | 36 |
| Penicillin G (10UT) | 0 | 0 | 0 |
| Cefepime 30 | 31 | 31 | 0 |
| Cephalexine 30 | 22 | 22 | 0 |
ACKNOWLEDGEMENTS
This research was supported by Tehran University of Medical Sciences and Health Services grant (No. 4775).
REFERENCES
- 1.Iwu MM, Cuncan AR, Okunji CO. New antimicrobials of plant origin. In: Janick J, editor. Perspectives on new crops and new uses. Alexandria: ASHS Press; 1999. [Google Scholar]
- 2.Trevejo RT, Barr MC, Robinson RA. Important emerging bacterial zoonotic infections affecting the immunocomprised. Vet Res. 2005;36:493–506. doi: 10.1051/vetres:2005011. [DOI] [PubMed] [Google Scholar]
- 3.Mahboubi M, Mohammadi-Yeganeh S, Bokaee S, Dehdashti H, Feizabadi M. Antimicrobial activity of essential oil from Oleveria decumbens and its synergy with vancomycin against Staphylococcus aureus . Herba Polon. 2007;53:69–76. [Google Scholar]
- 4.Voss A, Milativic D, Wallrauch-Schwarz C, Rosdahl VT, Braveny I. Methicillin resistant S. aureus in Europe. Eur J Clin Microb Infect Dis. 2004;13:50–55. doi: 10.1007/BF02026127. [DOI] [PubMed] [Google Scholar]
- 5.Khan A, Rahman M, Islam MS. Antibacterial, antifungal and cytotoxic activities of 3,5-diacetyltambulin isolated from Amorphophallus ampanulatus Blume ex. Decne. Daru. 2008;16:239–244. doi: 10.4103/0253-7613.40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin GR, Dehmoobed Sharifabadi A, Salehi Surmaghi MH, Yasa N, Aynechi Y, Emami M, Shidfar MR, Amin M, Moghadami M, Kordbacheh P, Zeini F. Screening of Iranian plants for antifungal activity: Part 1. Daru. 2002;10:38–48. [Google Scholar]
- 7.Rechinger KH. Hymenocrater Fisch & CA Mey. In: Rechinger KH, editor. Flora Iranica, Labiatae, No 150. Graz (Austria): Akademische Druck-u Verlagsanstalt; 1982. pp. 239–250. [Google Scholar]
- 8.Mozaffarian V. A Dictionary of Iranian Plant Names. Farhang Moaser Publication, Tehran. 1996:282–283. [Google Scholar]
- 9.Firouznia A, Rustaiyan A, Nadimi M, Masoudi S, Bigdeli M. Composition of the Essential Oil of Hymenocrater calycinus (Boiss.) Benth. from Iran. J Essent Oil Res. 2005;7:527–529. [Google Scholar]
- 10.Zaidi MA, Crow SAJR. Biollogically active traditional medicinal herbs from Balochistan, Pakistan. J Ethnopharm. 2005;96:331–334. doi: 10.1016/j.jep.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Horiuchi K, Shiota S, Kuroda T, Hatano T, Yoshida T, Tsuchiya T. Potentiation of antimicrobial activity of aminoglycosides by carnosol from Salvia officinalis . Biol Pharm Bull. 2007;30:287–290. doi: 10.1248/bpb.30.287. [DOI] [PubMed] [Google Scholar]
- 12.Momen-Roknabadi N, Gohari AR, Monsef-Esfehani HR, Attar F, Hajiaghaee R, Saeidnia S, Jamalifar H, Kamalinia G, Shahverdi AR. Antifungal and antibacterial activities of Pentanema divaricatum and its active constituent. Z Naturforsch. 2008;63c:649–52. doi: 10.1515/znc-2008-9-1006. [DOI] [PubMed] [Google Scholar]
- 13.Gohari AR, Saeidnia S, Shahverdi AR, Yassa N, Malmir M, Mollazade K, Naghinejad AR. Phytochemistry and antimicrobial compounds of Hymenocrater calycinus . EurAsia J BioSci. 2009;3:64–68. [Google Scholar]
- 14.Zidorn C, Peterson BO, Udovicic V, Larsen TO, Duus JO, Rollinger JM, Omgania KH, Elmerer EP, Stuppner H. Podospermic acid, 1,3,5-tri-O- (7,8-dihydrocaffeoyl) quinic acid from Podospermum laciniatum (Asteraceae) Tetrahed lett. 2005;46:1291–1294. [Google Scholar]