Abstract
Background and the purpose of the study
Bifidobacterial strains are excessively sensitive to acidic conditions and this can affect their living ability in the stomach and fermented foods, and as a result, restrict their use as live probiotic cultures. The aim of the present study was to obtain bifidobacterial isolates with augmented tolerance to simulated gastrointestinal condition using cross-protection method.
Methods
Individual bifidobacterial strains were treated in acidic environment and also in media containing bile salts and NaCl. Viability of the acid and acid-bile-NaCl tolerant isolates was further examined in simulated gastric and small intestine by subsequent incubation of the probiotic bacteria in the corresponding media for 120 min. Antipathogenic activities of the adapted isolates were compared with those of the original strains.
Results and major conclusion
The acid and acid-bile-NaCl adapted isolates showed improved viabilities significantly (p<0.05) in simulated gastric fluid compared to their parent strains. The levels of reduction in bacterial count (Log cfu/ml) of the acid and acid-bile-NaCl adapted isolates obtained in simulated gastric fluid ranged from 0.64–3.06 and 0.36–2.43 logarithmic units after 120 min of incubation. There was no significant difference between the viability of the acid-bile-NaCl-tolerant isolates and the original strains in simulated small intestinal condition except for Bifidobacterium adolescentis (p<0.05). The presence of 15 ml of supernatants of acid-bile-NaCl-adapted isolates and also those of the initial Bifidobacterium strains inhibited pathogenic bacterial growth for 24 hrs. Probiotic bacteria with improved ability to survive in harsh gastrointestinal environment could be obtained by subsequent treatment of the strains in acid, bile salts and NaCl environments.
Keywords: Probiotic, Bifidobacterium isolates, Simulated gastrointestinal fluid, Stress adaptation, Cross protection mechanisms
INTRODUCTION
Probiotics are defined as ‘live microbial foods which are associated with beneficial health effects on the host when ingested in appropriate amount (1). According to this definition, the viability and metabolic activity of probiotic bacteria should be maintained from production to consumption. It is generally recommended that probiotic products should contain at least 107of live microorganisms per gram or per milliliter in order to exert their beneficial health effects (2, 3).
The harsh conditions of the gastrointestinal tract including the acidic environment of the stomach and the bile salts secreted in the duodenum are important impediments to the viability of ingested bacteria (4, 5). Therefore production of bifidobacteria with augmented adaptation to stress conditions is required in order to enhance their probiotic characteristics (4, 6).
Methods based on stress adaptation and cross protection are currently of researcher's interests (2, 4). Most of the approaches to induce tolerance to the lethal stress circumstances include stress adaptation and cross-protection mechanisms based on exposures of bifidobacterial cells to sub-lethal stress-treatments of starvation, heat, bile, salts or acidic pH (2, 4).
The aim of the present study was to obtain bifidobacterial isolates with improved probiotic properties and augmented resistance to simulated gastrointestinal condition using cross-protection method.
MATERIAL AND METHODS
Bacterial strains and culture conditions
The bacterial strains used in this study were Bifidobacterium angulatum PTCC (Persian Type Culture Collection-Iran) 1366, Bifidobacterium animalis PTCC 1631, Bifidobacterium bifidum PTCC 1644 and Bifidobacterium adolescentis PTCC 1536. Strains were stored at −70 °C and routinely sub-cultured on MRS broth (Merck GMBH, Germany) enriched with 0.05% L-cysteine (Merck GMBH, Germany) (MRSBC), at 37°C (7). All incubations were performed in anaerobic jars (H2/CO2/N2; 10:5:85, Anoxomat WS8000, Mart_ Microbiology, Lichtenvoorde and the Netherlands). Bacterial enumeration was conducted by plate counting of the cultures on MRSC agar (8).
Evaluation of sensitivity of Bifidobacterium strains to artificial gastrointestinal conditions
The ability of the Bifidobacterium strains to tolerate gastrointestinal transit was conducted according to the previously reported method (9). Quantities of individual strains (108–109 cells ml−1) were incubated in sterile saline solution (0.5% w / v NaCl), containing 3 g/liter pepsin (Merck GMBH, Germany) (pH=2) at 37°C. Transit tolerance of the strains was evaluated by harvesting aliquots at different times (0, 90, and 120 min) and further plating on MRSC agar. After exposure to simulated gastric condition, cells were harvested by centrifugation (6,000 rpm, 15 min) and washed in sterile saline solution (10). The bacterial cells were then exposed to pancreatine (1g/liter; Merck GMBH, Germany) and bile salts (0.5% w/v ox gall; Merck GMBH, Germany) in sterile saline solution (0.5% w / v NaCl; Merck GMBH, Germany) (pH=8). Aliquots were taken after 0, 90 and 120 min and the tolerance to small intestinal transit was determined by counting the viable cells appearance on MRSC agar enriched with L-cysteine hydrochloride (0·05% w/v) (11).
Adaptation methods
Acid adaptation of Bifidobacterium strains
Fresh MRSBC media (pH=2) were inoculated with 1% of PBS-washed overnight cultures of the individual bifidobacterial strains. Acid-adapted isolates were recovered by plating them on MRSC agar at neutral pH after incubation at 37 °C for 16 hrs and further storage of the plates in anaerobic condition for 3 to 4 days (4).
Bile and Sodium Chloride adaptation of Bifidobacterium Strains
Cells of overnight cultures of acid-adapted isolates were harvested by centrifugation (6000 rpm, 15 min), washed with phosphate-buffered saline (PBS), and diluted in fresh MRSBC supplemented with bile salts Ox-gall; 3.0% w / v and NaCl 10.0% w / v. Cultures were incubated at 37 °C for 24 hrs, and then the acid-bile-NaCl-adapted isolates were recovered by plating them on MRSC agar. (4).
Evaluation of tolerance of acid and acid-bile-NaCl adapted isolates to simulated gastrointestinal conditions
Survival of the acid-bile-NaCl-adapted Bifidobacterium isolates in stress environment simulating stomach and intestine were assessed while acid-adapted isolates were only challenged in simulated gastric condition. Cell suspensions of individual strains (108–109 cells ml−1) were incubated in simulated gastric and small intestinal fluid as previously described. Aliquots were harvested at different times (0, 90, and 120 min) and bacterial counts were estimated by plating on MRSC agar media.
Pathogenic bacteria and culture conditions
The pathogenic bacteria used in this study were purchased from PTCC (Persian Type Culture Collection, Iran) and included; Eschershia coli ATCC 8739, Staphylococcus aureus ATCC 6538 and Pseudomonas aueroginosa ATCC 9027 (12). Fresh vegetative cultures of the individual bacteria were prepared by inoculating bacteria in Caso Agar Medium (Merck GMBH, Germany) and further incubation at 37°C for 24 hrs.
Phenotypic characterization of the acid-bile-NaCl-adapted Bifidobacterium isolates
Phenotypic of the acid-bile-NaCl-adapted isolates were compared with the original strains using conventional morphological as well as biochemical tests. A cocktail of biochemical tests were conducted on the acid-bile-NaCl-adapted Bifidobacterium isolates and the results were compared with those of the initial strains as well as those in the Bergey's manual of determinative bacteriology.
Comparison of anti-pathogenic properties of Bifidobacterium strains before and after treatment
Overnight cultures of acid-bile-NaCl-adapted Bifidobacterium isolates and also those of the initial strains were centrifuged (6,000 rpm, 15 min) and supernatants were filter sterilized. Aliquots of 5 and 15 ml of supernatants of the individual bifidobacterial cultures were added to flasks containing 100 ml of Muller Hinton Broth and also 1ml of cultures of the pathogenic bacteria. Control flasks did not contain bifidobacterial supernatants. Flasks were incubated aerobically at 37°C for 24 hrs and the kinetics of growth of pathogenic bacteria were determined by taking samples in 2 hrs intervals and subsequent enumeration of bacteria using the conventional pour plate technique.
Statistical analyses
The results are average of triplicate analyzes. Statistical analysis of the data was carried out using SPSS version 11.5. Statistical significance of the difference in survival rates of the adapted isolates and the initial bifidobacterium strains in simulated gastrointestinal conditions were analyzed by t-test (paired, one tailed). The results were considered statistically different at p<0.05.
RESULTS AND DISCUSSION
There is an increasing interest in the use of Bifidobacterium probiotic strains in functional foods and pharmaceutical products. Initial experiments conducted by testing the sensitivity of the bifidobacterial strains (Table 1) showed the lack of Bifidobacterium intrinsic resistance to acidic condition such as simulated human gastric condition. The ability of the individual bifidobacteria to survive in the simulated gastrointestinal transit varied among strains. The viability of all of the untreated tested strains decreased significantly (p<0.05) in simulated gastric juice. The range of loss in viability varied from 0.7 to 4.6 logarithmic units after an incubation time of 90 min, which was the average contact time of the ingested foods with gastric materials. The reduction in bacterial counts after 120 min of incubation was however, 1.9–7.16 logarithmic units (Table 1). B. animalis and B. bifidum seemed to be the most resistant strains to the acidic pH showing reduction in the bacterial count of about 2 logarithmic units after 120 min of incubation in simulated gastric fluid. In contrast, B. adolescentis showed to be the most vulnerable strain to the simulated gastric fluid ending with about 7 logarithmic units reduction in its viability after 120 min of incubation. Previous studies conducted by other research groups also have shown that most of the bifidobactrial strains e.g. B. bifidum, B. animalis, B. infantis, B. breve, B. longum and B. adolescentis lacked the intrinsic resistance to simulated gastric conditions (pH 2.0 for 90 min) (2, 4, 9, 13). This could limit their application as probiotic oral supplement. Therefore, approaches based on stress adaptation and cross protection were applied to achieve improved adapted isolates. It has been demonstrated that exposure of B. breve to pH 5.2 protects cells against lethal pH values of 2.0–5.0 (14) and exposure of bacteria to stress factors (heat, bile salts, or acid pH) can protect them against further stress conditions (7).
Table 1.
Viability of initial Bifidobacterium strains, acid-adapted and acid-bile-NaCl- adapted isolates in gastric and intestinal simulated fluid. Isolates were treated by subsequent incubation in acid, bile and salt environment.
| Viable count (log cfu ml−1; mean±S.D.@) during simulated gastric transit tolerance | Viable count (log cfu ml−1; mean±S.D.) during small intestinal transit tolerance | |||||
|---|---|---|---|---|---|---|
| Bacterial strain | 0 min¥ | 90 min | 120 min | 0 min | 90 min | 120 min |
| B. angulatum | 9.18±0.04 | 6.63±0.12¤* | 4.86±0.58 ¤* | 8.35±0.13 | 8.09±0.15 | 7.55±0.22 |
| (9.15±0.20)# | (7.16±0.14)* | (6.63±0.14)* | ND | ND | ND | |
| [9.17±0.06]§ | [7.23±0.06] ¤ | [7.75 ±0.05] ¤ | [8.45±0.16] | [8.28±0.21] | [8.10±0.25] | |
| B. animalis | 8.75±0.13 | 8.05±0.25 * | 6.85±0.11 ¤* | 9.12±0.06 | 9.02±0.19 | 8.87±0.15 |
| (8.78±0.11) | (8.59 ±0.73)* | (8.14 ±0.69) * | ND | ND | ND | |
| [8.82±0.11] | [8.71±0.15] | [8.46±0.09] ¤ | [9.12±0.10] | [9.09±0.30] | [8.87±0.15] | |
| B. bifidum | 8.45±0.11 | 7.25±0.19 ¤ | 6.28±0.12 ¤* | 8.87±0.10 | 8.56±0.25 | 8.11±0.10 |
| (8.52±0.15) | (7.57±0.18) | (7.07 ±0.08) * | ND | ND | ND | |
| [8.48±0.22] | [7.88±0.11] ¤ | [7.47±0.39] ¤ | [8.75±0.41] | [8.68±0.11] | [8.53±0.16] | |
| B. adolescentis | 9.27±0.05 | 4.67±0.10 ¤* | 2.11±0.05 ¤* | 8.95±0.11 | 7.41±0.05 ¤ | 7.05±0.08 ¤ |
| (9.21±0.47) | (7.25±0.31)* | (6.15 ±0.11)* | ND | ND | ND | |
| [9.18±0.08] | [7.86±0.08] ¤ | [6.75±0.61] ¤ | [8.91±0.11] | [8.13±0.21] ¤ | [7.35±0.49] ¤ | |
@ logarithmic Colony Forming Units per milliliters; mean±standard deviation
Min
The figures quoted in parenthesis represent logarithm of survival of acid-adapted isolates.
The figures quoted in closed brackets represent logarithm of survival of acid-bile-NaCl-adapted isolates.
It shows a significant difference between survivals of acid-adapted isolates with initial strains (p<0.05).
It shows a significant difference between survivals of acid-bile-NaCl-adapted isolates with initial strains (p<0.05).
ND=Not Determined
The viability of the bifidobacterial strains were not affected in simulated small intestinal transit fluid and were considered intrinsically tolerant except for B. adolescentis (Table 1).The level of reduction in bacterial counts by individual bacteria obtained in simulated small intestine fluid ranged from 0.1 to 1.54 and 0.25 to 1.9 logarithmic units after 90 and 120 min of incubation time respectively. It has been reported that the majority of strains were intrinsically resistant with no reduction in viability after 3 hrs exposure to simulated pancreatic juice (9). In an independent study, using a dynamic model of the stomach and the small intestine bile apparently played an influential role on the survival of the Bifidobacterium. (15). Pre-treatment of B. breve, B. animalis and B. longum at pH values of 5.2, 3.5 and at 47° C showed an effect in protection of these bacteria against bile (4). Previous studies have demonstrated a relationship between tolerance to acidic pH, high bile salt concentrations and high temperatures among the Bifidobacterium strains (14, 16, 17).
The ability of the acid-bile-NaCl-adapted Bifidobacterium isolates to survive in the simulated gastrointestinal juice and also the ability of acid-adapted strains to tolerate simulated gastric condition have been depicted in Table 1. All the acid and acid-bile-NaCl adapted isolates showed better survival in artificial gastric juice compared to the original strains. The ability of acid and acid-bile-NaCl adapted isolates to survive in simulated gastric fluid was significantly different (p<0.05) compared with their original strains. The level of reduction in bacterial counts (Log cfu/ml) of the acid and acid-bile-NaCl adapted isolates obtained after 90 min incubation in simulated gastric fluid ranged from 0.19 to 1.99 and 0.11 to 1.94 respectively while the level of reduction in bacterial counts of the acid and acid-bile-NaCl adapted isolates obtained in simulated gastric fluid ranged from 0.64 to 3.06 and 0.36 to 2.43 logarithmic units after 120 min of incubation. The acid and acid-bile-NaCl adapted isolates showed improved viabilities of 0.79 to 4.04 and 1.19 to 4.64 logarithmic units in simulated gastric fluid after 120 min of incubation time respectively. However, there was no significant difference between the viability of the acid-bile-NaCl-adapted isolates and initial strains in simulated small intestinal condition except for B. adolescentis (p>0.05). Among the bacterial strains adopted in current study B. animalis was found to be the most resistant strain while B. adolescentis showed to be the most vulnerable to the gastrointestinal fluids.
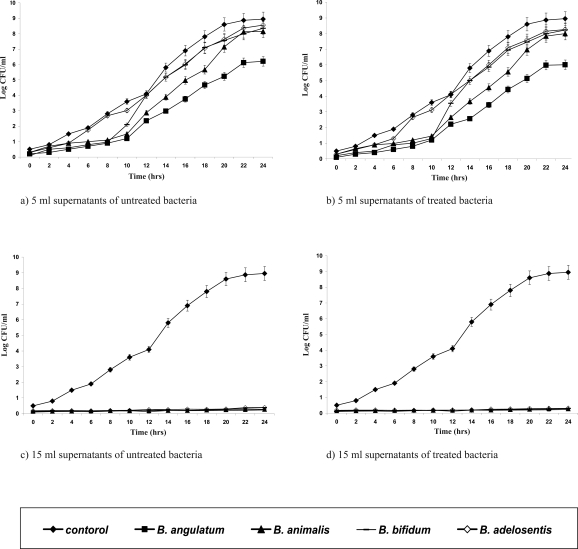
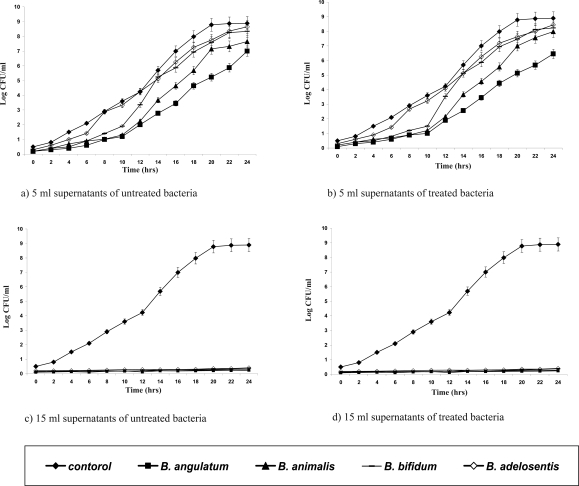
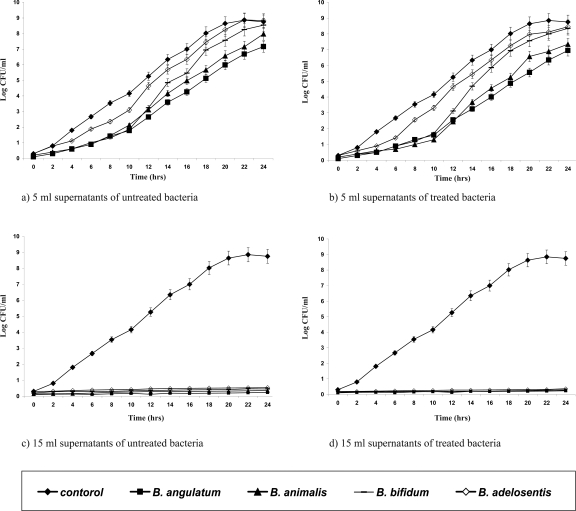
In vitro laboratory and animal studies have shown that bifidobacteria exert antagonistic activity against pathogens (18, 19). All the adapted isolates were shown to have kept their initial biochemical patterns (data not shown). Supernatants of the adapted strains were tested for their antipathogenic potentials against E. coli, S. aureus, Ps. aueroginosa (Figures 1–3). The Presence of 15 ml of supernatants of acid-bile-NaCl-adapted isolates and also those of the initial Bifidobacterium strains inhibited pathogenic bacterial growth for 24 hrs while those of the 5 ml of supernatants were not affected and could only expand the lag phase of the pathogens.
Figure 1.
Growth curves of Escherichia coli in the presence of different volumes (5, 15 ml) of supernatants of the acid-bile-NaCl-adapted (b & d) treated and initial (a & c) untreated strains.
Figure 2.
Growth curves of Pseudomonas aeruginosa in the presence of different volumes (5, 15 ml) of supernatants of the acid-bile-NaCl-adapted (b & d) treated and initial (a & c) untreated strains.
Figure 3.
Growth curves of Staphylococcus aureus in the presence of different volumes (5, 15 ml) of supernatants of the acid-bile- NaCl-adapted (b & d) treated and initial (a & c) untreated strains.
CONCLUSION
Bifidobacteria are key representatives of probiotic bacteria in the functional foods and pharmaceutical products. The lack of their intrinsic resistance to simulated human gastric is a barrier for their appropriate probiotic action. The improved properties of acid-bile-NaCl-adapted Bifidobacterium isolates produced by prolonged exposure to acid, bile and NaCl condition indicate that this strategy may be useful to enhance the stability and functional properties of probiotic strains. Both stress adaptation and cross protection methods were used to achieve adapted bifidobacterial isolates with improved tolerance to gastrointestinal transit. The adopted strains may be used as probiotics in production of functional foods.
ACKNOWLEDGEMENT
The authors wish to thank Pharmaceutical Sciences Research Center of the Tehran University of Medical Sciences for funding the current research work.
REFERENCES
- 1.Corcoran BM, Ross RP, Fitzgerald GF, Stanton C. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances. J Appl Microbiol. 2004;96:1024–1039. doi: 10.1111/j.1365-2672.2004.02219.x. [DOI] [PubMed] [Google Scholar]
- 2.Sanz Y. Ecological and functional implications of the acid-adaptation ability of Bifidobacterium: A way of selecting improved probiotic strains. Int Dairy J. 2007;17:1284–1289. [Google Scholar]
- 3.Gardiner GE, O'sullivan E, Kelly J, Auty MAE, Fitzgerald GF, Collins JK, Ross RP, Stanton C. Comparative Survival Rates of Human-Derived Probiotic Lactobacillus paracasei and L. salivarius Strains during Heat Treatment and Spray Drying. Appl Environ Microbiol. 2000;66(6):2605–2612. doi: 10.1128/aem.66.6.2605-2612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collado MC, Sanz Y. Induction of acid resistance in Bifidobacterium: a mechanism for improving desirable traits of potentially probiotic strains. J Appl Microbiol. 2007 doi: 10.1111/j.1365-2672.2007.03342.x. ISSN 1364-5072. [DOI] [PubMed] [Google Scholar]
- 5.Desmond C, Ross1 RP, O'Callaghan E, Fitzgerald G, Stanton C. Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia. J Appl Microbiol. 2002;93:1003–1011. doi: 10.1046/j.1365-2672.2002.01782.x. [DOI] [PubMed] [Google Scholar]
- 6.Doleyres Y, Lacroix C. Technologies with free and immobilized cells for probiotic bifidobacteria production and protection. Int Dairy J. 2005;15:973–988. [Google Scholar]
- 7.Sánchez B, Christine M, Vergès C, Collado MDC, Anglade P, Baraige F, Sanz Y, Reyes-Gavilán CGDL, Margolles A, Zagorec M. Low-pH Adaptation and the Acid Tolerance Response of Bifidobacterium longum Biotype longum. Appl Environ Microbiol. 2007;73(20):6450–6459. doi: 10.1128/AEM.00886-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saarela M, Rantala M, Hallamaa K, Nohynek L, Virkajärvi I, Mätto J. Stationary-phase acid and heat treatments for improvement of the viability of probiotic lactobacilli and bifidobacteria. J Appl Microbiol. 2004;96:1205–1214. doi: 10.1111/j.1365-2672.2004.02286.x. [DOI] [PubMed] [Google Scholar]
- 9.Charteris WP, Kelly PM, Morelli L, Collins JK. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol. 1998;84:759–768. doi: 10.1046/j.1365-2672.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- 10.Fazeli MR, Toliyat T, Samadi N, Hajjaran S, Jamalifar H. Viability of lactobacillus acidophilus in various vaginal tablet formulations. DARU. 2006;14(4):172–177. [Google Scholar]
- 11.Shahidi F, Mendosa AF, Boylstone T, Mohebbi M. A Perception to Survival of Bifidobacterium Spp. In Bioyoghurt, Simulated Gastric Juice and Bile Solution. World Appl Sci J. 2008;3(1):40–44. [Google Scholar]
- 12.Samadi N, Tarighi P, Fazeli MR, Mehrgan H. Evaluation of antimicrobial effectiveness of ophthalmic drops according to the pharmacopeial tests criteria. DARU. 2009;17(1):13–18. [Google Scholar]
- 13.Matsumoto M, Ohishi H, Benno Y. H+-ATPase activity in Bifidobacterium with special reference to acid tolerance. Int J Food Microbiol. 2004;93:109–113. doi: 10.1016/j.ijfoodmicro.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Park YS, Lee JY, Kim YS, Shin DH. Isolation and characterization of lactic acid bacteria from feces of newborn baby and from dongchimi. J Agric Food Chem. 2002;50:2531–2536. doi: 10.1021/jf011174i. [DOI] [PubMed] [Google Scholar]
- 15.Marteau P, Minekus M, Havenaar R, Huis In't veld JHJ. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine. Validation and the effect of bile. J Dairy Sci. 1997;80:1031–1037. doi: 10.3168/jds.S0022-0302(97)76027-2. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt G, Zink R. Basic features of the stress response in three species of bifidobacteria: B. longum, B. adolescentis, and B. breve . Int J Food Microbiol. 2000;55:41–45. doi: 10.1016/s0168-1605(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 17.Fozo EM, Kajfasz JK, Quivey RG. Low pH induced membrane fatty acid alterations in oral bacteria. FEMS Microbiol Lett. 2004;238:291–295. doi: 10.1016/j.femsle.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 18.Liévin V, Peiffer I, Hudault S, Rochat F, Brassart D, Neeser JR, L Servin A. Bifidobacterium strains from resident infant human gastrointestinal microflora exerts antimicrobial activity. Gut. 2000;47:646–652. doi: 10.1136/gut.47.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alain LS. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiology Reviews. 2004;28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]