Abstract
Background and the purpose of the study
Lamotrigine is a broad spectrum anticonvulsant drug widely used as mono- or adjunct- therapy in adults and children. The aim of this study was to develop controlled release liquid formulation of lamotrigine to improve bioavailability and compliance of pediatric and geriatric epileptic patients.
Methods
Multiple (w/o/w) emulsion was prepared using one step emulsification technique. It was evaluated for entrapment efficiency (EE), morphology, zeta potential (ZP), polydispersity index (PI), rheology, thermal property, in vitro drug release behavior and stability. In vivo studies in albino mice were carried out using maximal electroshock seizure (MES) test and strychnine induced seizure (SIS) pattern test and results were compared with marketed formulation.
Results
The EE of the formulations varied from 84.37% to 98.11%. The ZP and PI values of the prepared batches were in the range of +23.46 to +28.07 and 0.256 and 0.365, respectively. Microscopic observation clearly indicated the stability of the emulsions during the storage period. All batches exhibited controlled in vitro drug release up to 12 hrs. Batch C11 exhibited significantly longer duration of protection of seizure in mice against MES and exhibited comparable efficacy in SIS as compared to the marketed formulation.
Major Conclusion
Multiple emulsion of lamotrigine compared to the marketed tablet showed plasma drug concentration within therapeutic range for longer time and comparable efficacy.
Keywords: Multiple emulsion, Epilepsy, Pediatric and geriatric compliance
INTRODUCTION
Epilepsy is a common chronic neurological disorder characterized by recurrent unprovoked seizures. These seizures are transient signs and/or symptoms of abnormal, excessive or synchronous neuronal activity in the brain. About 50 million people worldwide suffer from epilepsy; of which almost 90% belong to developing countries. Although, the occurrence of epilepsy is uncertain, it is more likely to occur in young children or people above the age of 65 years. The prevalence of epilepsy has approximately an annual incidence rate of 40–70 per 100,000 in industrialized countries and 100–190 per 100,000 in resource-poor countries. Socioeconomically deprived people have been found to be at higher risk. In industrialized countries, the incidence rate has been found to be lower in children and higher among the elderly population (1).
Currently, about 20 medications have been approved by the Food and Drug Administration, for the treatment of epileptic seizures (2). Among these, lamotrigine is a broad spectrum anticonvulsant drug widely used as mono- or adjunct -therapy in adults and children. The drug is currently available as a tablet which is administered 2–3 times per day as divided doses of 25–600 mg (3). Although, tablets are very simple to manufacture and handle, oral liquid formulations with additional sustained release properties (4) are always preferred for pediatric, geriatric even dysphagic patients, due to their ease of administration and patient compliance. even at the time of epileptic attack.
In the present investigation, multiple emulsion was chosen as a liquid drug delivery system because of several advantages offered by this system over other conventional systems. Apart from easy swallowability, flexible dosing and improved bioavailability, it has capability of controlling the release of the active ingredients which improves patient compliance and also reduces the gastrointestinal side effects significantly by reducing the concentration of the drug exposed to the gastrointestinal mucosa (5, 6). The w/o/w multiple emulsion containing lamotrigine was developed and was evaluated for its physiochemical properties along with its anticonvulsant activity in mice.
MATERIAL AND METHODS
Lamotrigine was provided as a gift from Glenmark Pharmaceuticals Ltd., (India). Light liquid paraffin was purchased from Reidel Chemicals (India), Tween 80 and Span 80 (Loba Chemie Pvt. Ltd., India), glycerol (Qualigens Fine Chemicals India) and polyvinyl acetate and amaranth (Central Drug House India) were used in this study. Other chemicals of analytical grade were purchased locally and used as received.
Solubility studies
The solubility of the drug was determined in distilled water, acid buffer (pH 1.2), citrate buffer (pH 4.5) and phosphate buffer (pH 7.4). An excess amount of drug was added to 10 ml of each solvent in sealed glass vials and the resulting suspensions were stirred on a magnetic stirrer for 24 hrs at room temperature. Samples were then filtered, appropriately diluted and analyzed by UV spectrophotometry (Shimadzu, Model 7800, Kyoto, Japan) at 265.5 nm. Each solubility value was determined in triplicate.
Determination of effective hydrophilic lipophilic balance (EHLB)
The optimal ratio of hydrophilic and lipophilic surfactant was determined from the EHLB values which were calculated using the following formula:
| Equation 1 |
where A and B are the volume fraction of hydrophilic and lipophilic surfactants, respectively.
Fabrication of lamotrigine formulations
Four different formulations of lamotrigine were prepared, namely aqueous and oily drug suspension, simple emulsions (w/o and o/w) and multiple w/o/w emulsions, using liquid paraffin as the oil phase. The final volume of the formulations was 50 ml which contained 100 mg of lamotrigine (2 mg/ml). The compositions of all the batches prepared are given in table 1.
Table 1.
Formula for the preparation of aqueous and oily drug suspensions, simple (w/o and o/w) emulsion and different batches of drug loaded multiple emulsions
| Batch Code | Drug (%w/v) | Surfactant blend (%v/v) | Distilled water (%v/v) | Oil phase (%v/v) | PVA(%w/v) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tween 80 | Span 80 | LP | SFO | SBO | |||||
| Multiple w/o/w emulsion for formula optimization by single step emulsification | C1 | 2 | 4 | 6 | 53 | 35 | 0 | 0 | 0 |
| C2 | 2 | 4 | 6 | 63 | 25 | 0 | 0 | 0 | |
| C3 | 2 | 4 | 6 | 73 | 15 | 0 | 0 | 0 | |
| C4 | 2 | 6 | 4 | 53 | 35 | 0 | 0 | 0 | |
| C5 | 2 | 6 | 4 | 63 | 25 | 0 | 0 | 0 | |
| C6 | 2 | 6 | 4 | 73 | 15 | 0 | 0 | 0 | |
| aqueous drug suspension | C7 | 2 | 1 | 0 | 100 | 0 | 0 | 0 | 0 |
| oily drug suspension | C8 | 2 | 0 | 2 | 0 | 100 | 0 | 0 | 0 |
| simple wd/o emulsion | C9 | 2 | 0 | 8 | 40 | 60 | 0 | 0 | 0 |
| simple od/w emulsion | C10 | 2 | 4 | 0 | 60 | 40 | 0 | 0 | 0 |
| Multiple emulsion by two step emulsification (wd/o/w) | C11 | 2 | 4 | 6 | 73 | 15 | 0 | 0 | 0 |
| Marketed formulation | C12 | ||||||||
| Multiple emulsion with different PVA concentrations by one step emulsification | C13 | 2 | 4 | 6 | 72 | 15 | 0 | 0 | 1 |
| C14 | 2 | 4 | 6 | 70 | 15 | 0 | 0 | 3 | |
| C15 | 2 | 4 | 6 | 68 | 15 | 0 | 0 | 5 | |
| Multiple emulsion with different oil phase by one step emulsification | C16 | 2 | 4 | 6 | 73 | 0 | 15 | 0 | 0 |
| C17 | 2 | 4 | 6 | 73 | 0 | 0 | 15 | 0 | |
| C18 | 2 | 4 | 6 | 73 | 15 | 0 | 0 | 0 | |
| Multiple emulsion with drug (1:1) in both of water phases (wd/o/wd) by two step emulsification | C19 | 2 | 4 | 6 | 73 | 15 | 0 | 0 | 0 |
d- Indicates the presence of drug in that phase, LP, SFO and SBO indicate liquid paraffin, sunflower oil, soya bean oil, respectively.
Preparation of aqueous and oily drug suspension
For aqueous drug suspension, 100 mg of lamotrigine was added to 50 ml of 2% (v/v) aqueous solution of Tween 80 as surfactant. For oily drug suspension, 100 mg of drug was dispersed in 50 ml of liquid paraffin containing 2% (v/v) Span 80, under continuous stirring (Mechanical Agitator–702 R, Remi equipments, Mumbai, India).
Preparation of simple emulsions
Simple w/o emulsion was prepared by adding 20 ml of aqueous drug dispersion gradually into 30 ml of liquid paraffin containing 8% (v/v) Span 80 under continuous stirring. Simple o/w emulsion was prepared by gradual addition of 20 ml of oily drug dispersion into 30 ml of distilled water containing 4% (v/v) Tween 80 under stirring. Stirring was carried out at 2000 rpm for 5 min at room temperature, for each case.
Preparation of multiple w/o/w emulsions
Multiple w/o/w emulsions were prepared by both one step and two step emulsification methods (7).
Multiple w/o/w emulsion by one step emulsification method
In one step emulsification method (8), lamotrigine was dispersed in aqueous phase containing hydrophilic surfactant and the lipophilic surfactant was mixed with oil phase. The total amount of both surfactants was kept at 5.0% (v/v) and their ratios were adjusted to achieve the effective hydrophilic lipophilic (EHLB) balance of 8.5 and 10.5. The oil and aqueous phases were heated separately up to 70±5°C. The aqueous phase was then slowly added to the oil phase under stirring at 4000 rpm till it reached room temperature. The effects of various formulation variables like concentration of polyvinyl alcohol (1%, 3% and 5% w/v) in aqueous phase, use of different oil phases (liquid paraffin, sunflower oil, soya bean oil) on drug release profiles were evaluated.
Multiple w/o/w emulsion by two step emulsification method
First, 18 ml of aqueous drug dispersion was gradually introduced into 12 ml of liquid paraffin containing 8% (v/v) Span 80, under stirring at 2000 rpm. In the second step, the resulting simple emulsion was dispersed into 20 ml of distilled water containing 2% (v/v) Tween 80 by stirring at 1000 rpm. For the preparation of multiple emulsions the same quantity of drug was distributed equally in both aqueous phases, drug quantity added in first step was reduced to half and the remaining half was added to the aqueous solution of 2% (v/v) Tween 80 in the second step of emulsification. Two types of multiple emulsions i.e. wd/o/w and wd/o/wd were prepared by this technique to evaluate the effect of location of drug (d) in the emulsion on drug release.
Evaluation of multiple emulsions Stability studies of multiple emulsions for sedimentation
In order to study the extent of sedimentation, the batches were transferred into a tightly corked 50 ml graduated measuring cylinder and stored at room temperature without disturbance. The volume of sediment was noted after 90 days.
Morphological studies
The morphological characteristics (color, consistency and homogeneity) of multiple emulsions at the first day and 20 days after preparation were observed microscopically at 100X magnification using Coaxial Binocular Microscope (LBM-1200, Labkron Instruments, Haryana, India) with inbuilt halogen light source. The w/o/w type of multiple emulsions was examined by introducing amaranth (water soluble red dye) in the internal aqueous phase.
Entrapment efficiency
Entrapment efficiency (EE) of multiple emulsion was determined by centrifugation. The concentration of drug in the external aqueous phase separated on centrifugation was analyzed by UV spectrophotometer. The entrapment efficiency was calculated as follows:
| Equation 2 |
Where, C is the concentration of drug in the external aqueous phase expressed in percentage.
Polydispersity index and zeta potential
The polydispersity index (PDI) and the zeta potential of the multiple emulsions were estimated using Master sizer (Malvern Instruments Ltd., Worcestershire, UK) after their dilution with filtered (0.22 microns) ultrapure water.
Viscosity
Viscosity measurements of the multiple emulsions were carried out at room temperature on a Brookfield RVT viscometer (California, USA) at 20, 50 and 100 rpm using spindle number of S62 at torque 0.5 Nm.
Determination of thermal behavior by differential scanning calorimetry
Thermal behavior of multiple emulsion was studied using differential scanning calorimeter (DU-PONT, Model 9900, CA, USA) equipped with an intracooler and a refrigerated cooling system. Each sample was placed in an aluminium pan and was then crimped with an aluminium cover to provide hermetically sealed samples (1–2 mg). The heating rate was 10 °C/min. The DSC thermograph of pure drug was obtained at a temperature range of 25 °C to 300 °C and that of multiple emulsion was measured at a temperature range of 0 °C to −45 °C. Nitrogen was purged at 50 ml/min through cooling unit.
In vitro drug release studies
In vitro drug release study from aqueous and oily suspension, simple o/w emulsion, simple w/o emulsion and multiple w/o/w emulsion were carried out using diffusion cell technique (9). Test samples (10 ml) were placed in the diffusion cell of the donor compartment, separated with dialysis membrane from receptor compartment (250 ml beaker) containing 200 ml of the release medium. The release of lamotrigine from different batches was studied for a period of 12 hrs with 2 hrs in pH 1.2, followed by next 2 hrs in pH 4.5 and finally for 8 hrs in pH 7.4 buffers containing 2% (v/v) Tween 80. The diffusion medium was stirred at 100 rpm at 37±0.5 °C on a thermostatically controlled magnetic stirrer. Samples (5 ml) were withdrawn at different time intervals and analyzed spectrophotometrically and replaced with equivalent volume of the medium. The marketed formulation was available as dispersile tablet which was dispersed in 10 ml of water and the dispersion was placed in diffusion cell for drug release study. The release data for all batches were fitted to various models (Zero order, first order, Weibull, Higuchi, Peppas and Hixson-Crowel models) to determine the mechanism of drug release (10).
Determination of anticonvulsant activity in mice
The animal study protocol was duly approved by the Animal Ethical Committee, Banaras Hindu University. Albino mice (25±5 g), of either sex were randomly distributed into five different experimental groups. The mice were housed in groups of six (unless stated otherwise) in polypropylene cages at an ambient temperature of 25±10 °C and 45–55% relative humidity, with a 12:12 hrs light/dark cycle. Animals were provided with commercial food pellets with water ad libitum unless stated otherwise. Animals were acclimatized for at least one week before using them for experiments and exposed only once to an experiment. “Principles of laboratory animal care” (NIH publication number 85–23, revised 1985) guidelines were followed. Anticonvulsant activity in terms of abolition of hind limb tonic extensor, latency to first convulsion, percent lethality, percent protection against maximal electroshock and strychnine induced seizure at different time intervals were studied in albino mice. The study was conducted by oral administration of the samples to five groups of mice (n=6) with three groups receiving test formulation Batch C11 at three dose levels i.e. 18 (T1), 36 (T2) and 54 (T3) µmol/kg and the rest two groups receiving vehicle (control group) and the standard formulation (aqueous suspension of a dispersed tablet). The anticonvulsant activities of test samples were compared with that of the standard formulation and vehicle (control).
Maximal electroshock seizure (MES) test
This study was conducted after 60 min of dosing wherein the animals were stimulated by corneal electrodes for 0.2 sec using a Hugo Sachs stimulator (Type 207, Freiburg, FRG) with 50 mA and 60 Hz AC. The hind limb tonic extensor (HLTE) response 10–12 s after stimulation was taken as the positive end point (11). The percentage protection against seizure was also measured at time intervals of 1, 2, 4, 6 and 8 hrs. The percent protection against seizure provided by individual formulation was statistically compared (by applying Student's t-test and is shown in each table) with respect to corresponding control (vehicle) and marketed formulation (standard).
Strychnine induced seizure pattern test
In this test, strychnine (1–2 mg/kg, i.p.) in a volume of 0.01 ml/gm body weight was injected into the animals one hour post dosing and were observed for 10 min for onset and duration of tonic seizures. Abolition of the hind limb tonic extensor component was taken as the endpoint (11).
RESULTS AND DISCUSSION
Solubility studies
Lamotrigine showed pH dependent solubility with higher solubility at lower pH. The solubility of the drug was found to be very low in distilled water (0.201± 0.004 mg/ml) and pH 7.4 (0.204±0.001 mg/ml), and was high at pH 1.2 (2.235±0.146 mg/ml) and pH 4.5 (1.593±0.030 mg/ml). On addition of 2% (v/v) Tween 80 to water the solubility increased by approx. ten folds (2.560±0.031 mg/ml) and was found to be comparable to that of citrate buffer (pH of 4.5).
EHLB value studies
The volume fraction of the surfactant blends (v/v%) for each required HLB was obtained from equation 1. For EHLB of 8.5 and 10.5, Span 80 and Tween 80 were required to be mixed at 60:40 and 41.78:58.22 ratio, respectively.
Evaluation of multiple emulsion Microscopic examination
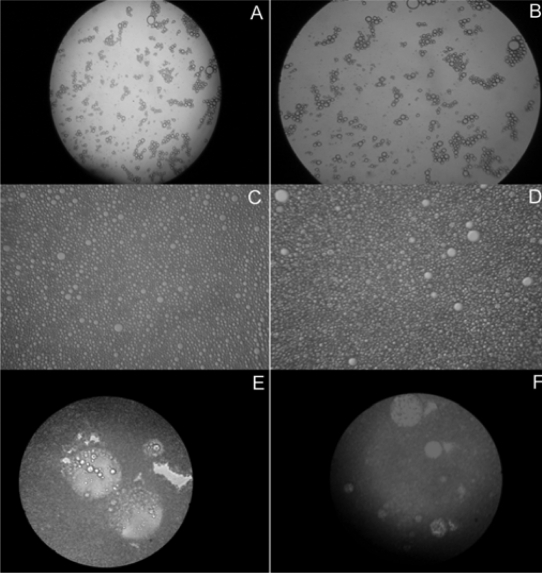
The photomicrographs of fresh (first day) and 4 weeks old samples of o/w and w/o emulsions are presented in figure 1 (A, B, C, and D). The micrographs of figure 1A (fresh) and 1B (4 weeks) of simple o/w emulsion show almost similar dispersed oil droplets. Similarly, photomicrographs of figure 1C (fresh) and 1D (4 weeks) of simple w/o emulsion showed dispersed aqueous droplets throughout the continuous oil medium. The above observation suggests the stability of both emulsions.
Figure 1.
Photomicrographs of (A) freshly prepared simple o/w emulsion; (B) 4 weeks old simple o/w emulsion; (C) freshly prepared simple w/o emulsion; (D) 4 weeks old simple w/o emulsion; (E) freshly prepared multiple w/o/w emulsion and (F) 4 weeks old multiple w/o/w emulsion (100 X magnification).
The photomicrographs of fresh (day of 1) and 4 weeks old samples of multiple w/o/w emulsions are presented in figure 1E and 1F, respectively. The internal dispersed droplets were highly colored due to the presence of amaranth whereas color in the external continuous phase was negligible. The micrographs indicated the presence of many internal aqueous droplets within the oily droplets. The typical light scattering birefringence characters of liquid crystal layers were clearly visible at the periphery of the internal aqueous and intermediate oily dispersed droplets. All the above photomicrographs of simple and multiple emulsions clearly indicated that the emulsions were stable even upon storage for 4 weeks after preparation.
Entrapment efficiency
The entrapment efficiency of different formulations (Table 2) varied from 84.37%–98.11% depending upon surfactant concentration and the ratio of oil and aqueous phases. Ratio 40:60 (C1–C3) of tween 80 and span 80 yielded higher entrapment efficiency in comparison to 60:40 ratios (C4–C6). In addition higher water/oil ratio was found to enhance the entrapment keeping the surfactant concentration constant i.e. C3 with water/oil ratio of 5.3 showed higher entrapment of the drug than C1 and C2 with water/oil ratio of 1.7 and 2.8, respectively. The high entrapment efficiency of these batches maybe attributed to the lipophilic nature of the drug which promotes the entrapment of drug in the oil phase.
Table 2.
Physical characteristics of w/o/w multiple emulsion formulation (Mean±S.D, n=3).
| Batch code | Entrapment efficiency (%) | Zeta potential | Poly dispersity index | Viscosity(cps) | EHLB value | Macroscopic properties | Microscopic observation | w/o ratio | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 20 rpm | 50 rpm | 100 rpm | ||||||||
| C1 | 87.44±4.87 | 28.07±1.59 | 0.327±0.03 | 7.9 | 5.9 | 4.8 | 8.49 | White, highly viscous | Multiple droplets | 1.7 |
| C2 | 90.89±3.87 | 27.87±1.09 | 0.256±0.03 | 7.1 | 5.1 | 3.9 | 8.49 | White, less viscous than C1 | Multiple droplets | 2.8 |
| C3 | 98.11±3.55 | 25.34±1.21 | 0.287±0.02 | 6.8 | 4.8 | 3.0 | 8.49 | White, less viscous than C2 | Multiple droplets | 5.3 |
| C4 | 84.37±3.18 | 24.89±1.24 | 0.365±0.02 | 7.3 | 5.2 | 4.5 | 10.53 | White, more viscous than C3 | Multiple droplets | 1.7 |
| C5 | 88.56±4.04 | 23.91±1.34 | 0.339±0.03 | 6.7 | 5.0 | 3.4 | 10.53 | White, less viscous than C4 | Multiple droplets | 2.8 |
| C6 | 90.65±3.78 | 23.46±1.17 | 0.310±0.02 | 6.2 | 4.9 | 2.5 | 10.53 | White, less viscous than C5 | Multiple droplets | 5.3 |
Zeta potential and polydispersity index
All formulations showed positive zeta potential values as can be seen in table 2. Zeta potential values of the emulsions were ranging from +23.46 to +28.07 which indicate a stable formulation. The polydispersity indices were found to be in the range of 0.256 and 0.365 which indicates low droplet size distribution.
Rheological analysis
The viscosity of the w/o/w multiple emulsions was measured at three different rpm (20, 50 and 100 rpm) and was found to decrease with increasing rate of shear stress (Table 2), which indicates non-Newtonian flow and shear thinning behavior. Increase in external phase volume (aqueous phase) also decreased viscosity (12). Increase in hydrophilic surfactant concentration decreased the viscosity whereas no significant difference was found in viscosity as a function of lipophilic surfactant percentage (13).
Some reports have described the bursting of multiple globules or phase inversion under shear stress (14, 15). However, such a phenomenon after viscosity measurement did not occur in this study, as it was determined by optical microscopy. It is concluded that shear stress of this study did not induce irreversible structural changes in multiple emulsions, such as coalescence or phase inversion.
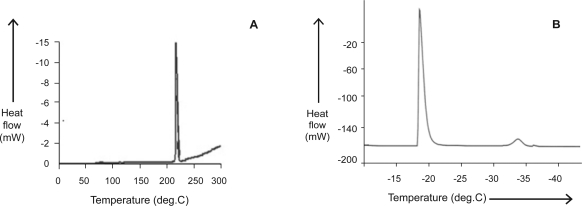
DSC studies
The DSC thermogram (Fig. 2A) of the pure drug shows a sharp endothermic peak at 217.83°C with a melting enthalpy of 116.58 J/g. The thermogram of w/o/w emulsion (Fig. 2B) with steady cooling shows two exothermic peaks typical to w/o/w multiple emulsions. The peaks demonstrate the presence of two aqueous phases, corresponding to the solidification of the external should be deleted and the inner aqueous phases which took place at different temperatures due to the barrier effect of the intermediate oil phase. The presence of the inner aqueous phase was indicated by the appearance of a second peak in the DSC curve. Any further decrease in area under the second peak or its disappearance would indicate the inner water loss during storage, probably due to multiple droplets breakdown (16). During the thawing of the frozen sample, recorded between −60 and +25°C, only one endothermic signal was observed which means entire quantity of the water melts at 0 °C (data not shown).
Figure 2.
DSC thermogram of (A) pure drug and (B) w/o/w multiple emulsion (C11).
Stability studies on different batches of multiple emulsions
Macro and microscopical characteristics of the multiple emulsions are shown in table 2. The stability and morphology of multiple globules were found to be affected not only by the type and concentration of surfactant, but also by the water to oil ratios which were used. The stability of the emulsions increased with the increase in water to oil ratio. Samples were considered macroscopically stable for the period of study, since no signs of instability such as creaming and phase separation were observed. The sedimentation ratio of the prepared multiple emulsions were in the acceptable range of 0.88 to 1.0. They were also considered microscopically stable, as the multiple droplet morphology showed no significant change during the pperiod of the study. It was observed that morphology of the multiple droplets was more influenced by the water/oil ratio than by the HLB values. Results of this study on stability are in agreement with earlier reports (8) where it was reported that most of the stable multiple droplets with Span and Tween series were obtained in samples with water/oil ratios varying from 2.8 to 18.0% (w/w).
In vitro drug release from different types of formulations
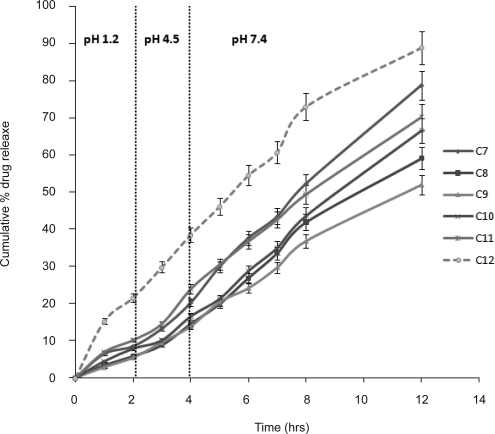
Standard curve of the drug was found to be linear in the range of 5–30 µg/ml for both acidic buffer pH 1.2 (r2 =0.9997) and phosphate buffer pH 7.4 (r2 =0.9989) having 2% (v/v) Tween 80 to maintain the sink condition. The in vitro release profiles from different formulations are shown in figure. The initial t10% (time for 10% cumulative drug release) values were found to be 2.05 hrs for aqueous drug suspension (C7), 3.24 hrs for oily drug suspension (C8), 3 hrs for o/w emulsion (C9), 3.17 hrs for w/o emulsion (C10) and 2.3 hrs for multiple w/o/w emulsion (C11) (Fig. 3). Burst release was not found in any of the formulation indicating proper loading of drug and absence of leakage from the formulations. Less than 20% of the drug was released in 4 hrs from formulations, and marketed product released drug to the extent of 40% in 4 hrs (C12). The t50% values of multiple w/o/w emulsions (8 hrs) were found to be less than that observed in the case of o/w emulsion (11.2 hrs) and w/o emulsion (12 hrs).
Figure 3.
In vitro release profiles of lamotrigine from aq. drug suspension (C7), oily suspension (C8), w/o emulsion (C9), simple o/w emulsion (C10), w/o/w multiple emulsion (C11), marketed formulation (C12) (Vertical bar represents S.D.).
The cumulative percent drug release was in the following order; marketed formulation >aqueous drug suspension >multiple emulsion >simple o/w emulsion >oily drug suspension >simple w/o emulsion. The difference in drug release from the above formulations may be attributed to the dissolved or undissolved state of drug, hydrophobic nature of the oil phase, affinity or partitioning into aqueous or non aqueous phases. In the case of aqueous drug suspension, the drug release was found to be 1.3 times higher than oily drug suspension which is due to the resistance offered by the oil phase to diffusion of drug into the release medium. In o/w emulsion, the drug is present in a suspended form in oil phase due to which negligible quantity of drug is available for partitioning between the oil and aqueous phases. Unlike w/o/w emulsion, the absence of pulling force from external surrounding resulted in slower rate of the drug release from o/w emulsion. However, drug release from o/w emulsion was higher than w/o emulsion which may be attributed to lower viscosity of o/w than w/o emulsion.
In the case of multiple emulsion, the external aqueous phase provides a gradient for the solubilized drug molecules present in the internal aqueous phase which is responsible for higher rate of drug release. Its lower viscosity is also an additional factor responsible for higher rate of drug release as compared to other formulations.
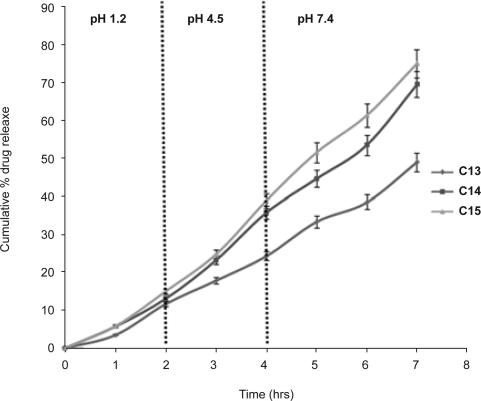
Effect of additives on drug release from multiple emulsions
The in vitro drug release data of different multiple emulsions containing 1% (C13), 3% (C14) and 5% (C15) (w/v) PVA in internal aqueous phase of w/o/w emulsion are shown in figure 4. The initial drug release from the formulations containing 3% and 5% PVA were found to be similar as is evident from the t10% values (1.6 hrs for C14 and 1.5 hrs for C15). Burst release was not found in any formulation. More than 20% of drug was released in 3 hrs from all formulations except Batch C13. Presence of PVA in internal aqueous phase might have weakened the interfacial films resulting in higher drug release i.e. 75% from the multiple emulsion (C15) as compared to 45% in multiple emulsion without additive (C11) in 7 hrs.
Figure 4.
In vitro release profiles of lamotrigine from different multiple emulsions containing PVA 1% (C13), 3% (C14) and 5% (C15) w/v (Vertical bar represents S.D.)
Influence of oil phases on the release of lamotrigine from multiple emulsions
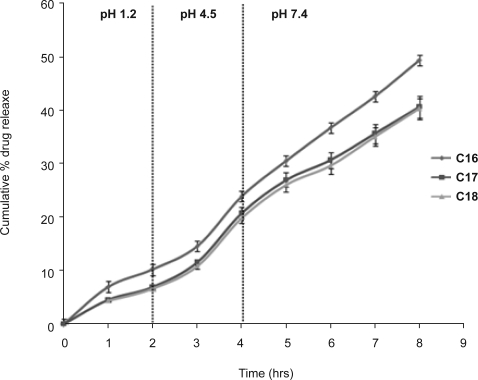
The in vitro release profiles from multiple w/o/w emulsions containing different oils, liquid paraffin (C16), sunflower oil (C17) and soya bean oil (C18), are shown in figure 5. The initial drug release from the formulations with sunflower oil (C17) and soya bean oil (C18) were found to be similar, probably due to the lack of appreciable difference in their viscosities (48.59 and 50.14 m Pa for soybean oil and sunflower oil, respectively) (17). This is evident from the t10% value which was found to be 2.85 hrs and 2.97 hrs for C17 and C18, respectively. With exception of batch C18, more than 20% of drug was released in 4 hrs from the other two formulations. The difference in drug release from formulation C16, C17 and C18 may again be attributed to viscosity of oil phase. Thus, it was inferred that the types of oil, its density and viscosity may influence the drug release characteristics from the multiple emulsions. A similar pattern has been reported for nitrofurantoin release from w/o/w emulsions formulated with soya and arachis oil (18).
Figure 5.
In vitro release profiles of lamotrigine from different multiple emulsions containing liquid paraffin (C16), sunflower oil (C17) and soya bean oil (C18). (Vertical bar represents S.D.)
Effect of drug location on release of lamotrigine from multiple emulsions
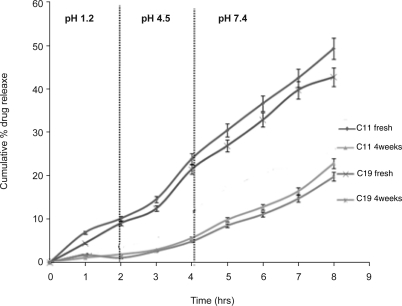
The in vitro release profiles of w/o/w emulsions (fresh, two and four weeks old) containing entire drug in the internal aqueous phase (Batch C11) and equally distributed drug in internal and external aqueous phase in the ratio of 1:1, (Batch C19) are shown in figure 6.
Figure 6.
In vitro release profiles of lamotrigine from freshly prepared wd/o/w emulsions (C11fresh), 4 weeks old wd/o/w emulsions (C114 weeks), freshly prepared wd/o/wd emulsions (C18 fresh) and 4 weeks old wd/o/wd emulsions (C184 weeks). d- Indicates presence of drug in that phase. (Vertical bar represents S.D.)
The t10% values were found to be 2, 3.87, 5.17, 2.46 and 5.34 hrs for C11fresh, C112 weeks, C114 weeks, C19fresh and C194 weeks, respectively. In comparison to the batch C11fresh, batch C19fresh gave slower drug release probably for two reasons. First, since Batch C19fresh contained half quantity of drug in the external aqueous phase which was in direct contact with the diffusion membrane where release of this drug might have clogged the surface of the membrane by precipitating in acidic pH of the release medium which resulted in initial lower drug release. However, release was rapid later because of higher pH of the diffusion medium, which caused more diffusion of drug. In the case of the emulsion C11fresh, the possibility of precipitation appears to be negligible as it contained the total drug in internal aqueous phase and only little amount of the drug was available in external aqueous phase. In addition, as compared to C11fresh, the concentration gradient between the inner and external phases in emulsion C19fresh was lower due to distribution of the drug between the aqueous phases which resulted in lower drug release.
Drug release profile modeling
In order to understand the mechanism of drug release, data were fitted to different models (Table 3). The best-fit model was selected on the basis of correlation coefficient (r) and the values of “K” and “n” which was shown by zero-order and Korsmeyer-Peppas model. This clearly indicates that drug release from the formulation followed zero-order mechanism.
Table 3.
Kinetics of drug release from different batches of multiple emulsions
| Model | BATCH CODE | |||||||
|---|---|---|---|---|---|---|---|---|
| C11(C3) | C13 | C14 | C15 | C16 | C17 | C18 | ||
| Zero order | r2 | 0.990 | 0.991 | 0.987 | 0.990 | 0.988 | 0.993 | 0.989 |
| KZ | 6.151 | 7.029 | 9.925 | 11.03 | 5.04 | 3.23 | 5.749 | |
| First order | r2 | 0.866 | 0.874 | 0.822 | 0.813 | 0.735 | 0.881 | 0.846 |
| kF | 0.135 | 0.170 | 0.170 | 0.174 | 0.181 | 0.204 | 0.153 | |
| Higuchi model | r2 | 0.751 | 0.871 | 0.910 | 0.908 | 0.743 | 0.882 | 0.856 |
| kH | 22.76 | 26.87 | 38.17 | 42.58 | 13.51 | 9.499 | 21.78 | |
| Weibull model | r2 | 0.861 | 0.792 | 0.892 | 0.895 | 0.880 | 0.751 | 0.686 |
| β | 1.103 | 1.457 | 1.518 | 1.60 | 1.415 | 1.502 | 1.251 | |
| Td | 1.73 | 2.25 | 1.98 | 6.79 | 3.09 | 1.95 | 2.42 | |
| Peppas model | r2 | 0.927 | 0.984 | 0.892 | 0.896 | 0.942 | 0.954 | 0.990 |
| kP | 0.062 | 0.073 | 0.105 | 0.059 | 0.016 | 0.08 | 0.041 | |
| n | 0.876 | 0.914 | 0.885 | 0.923 | 0.953 | 0.945 | 0.881 | |
| Hixon-Crowel model | r2 | 0.924 | 0.779 | 0.855 | 0.860 | 0.764 | 0.927 | 0.881 |
| kHC | 0.024 | 0.028 | 0.045 | 0.052 | 0.012 | 0.008 | 0.022 | |
| f1 | 26.73 | 12.13 | 22.06 | 26.29 | 31.87 | 25.81 | 11.56 | |
| f2 | 39.56 | 72.63 | 50.21 | 66.34 | 49.08 | 65.73 | 72.18 | |
C11 and C3 have similar formula but were prepared by two and one step emulsification techniques, respectively
Study of in vivo anticonvulsant activity in mice
Batch C11 at doses of 18 (T1), 36 (T2) and 54 (T3) µmol/kg were tested for their anticonvulsant activity in mice (19). The results obtained from this formulation at different doses are shown in terms of seizure latency duration, percent protection against seizure and lethality in both MES (Table 4) and strychnine induced convulsion (Table 5) whereas figure 7 shows seizure latency calculated with respect to control (vehicle).
Table 4.
Effect of lamotrigine formulations on maximal electroshock induced seizure (MES) test in mice.
| Treatment (No of animals) | Dose (µmol/kg) | Seizure latency (seconds) | Duration (seconds) | Animal HLTE (x/y) | % Protection against seizure |
|---|---|---|---|---|---|
| Vehicle (6) | – | 10.2±0.39 | 21.8±2.36 | 6/6 | 0 |
| T1 (6) | 18 | 8.65±1.26 | 25.9±3.26 | 5/6 | 83.34* |
| T2 (6) | 36 | – | – | 0/6 | 100* |
| T3 (6) | 54 | – | – | 0/6 | 100* |
| Standard(6) | 36 | – | – | 0/6 | 100* |
HLTE: Hind Limb Tonic Extensor. Vehicle was used as control. Where, x/y indicates ratio between number of non protected animals and number of animals which were used. (–) indicates as there was 100% protection, so no seizure latency. * Significantly different from control (vehicle) (p<0.01, n=6).
Table 5.
Effect of lamotrigine formulations on strychnine induced lethal seizure in mice.
| Treatment (No of animals) | Dose (µmol/kg) | Seizure latency (secs) | Clonic/Tonic Seizures | Lethality | % Protection against seizure |
|---|---|---|---|---|---|
| Vehicle (6) | – | 67.54±4.8 | 6/6 | 6/6 | 0 |
| T1 (6) | 18 | 272.50±18.21 | 6/6 | 6/6 | 0 |
| T2 (6) | 36 | 314.83±22.98 | 4/6 | 4/6 | 66.67* |
| T3 (6) | 54 | 425.33±23.73 | 5/6 | 5/6 | 83.34* |
| Standard (6) | 36 | 600 ±0 | 0/6 | 0/6 | 100* |
Seizure latency is the time required to start seizure. Lethality was estimated as the ratio between number of dead animals and number of animals showing convulsions. * Significantly different from control (vehicle) (p<0.01, n=6).
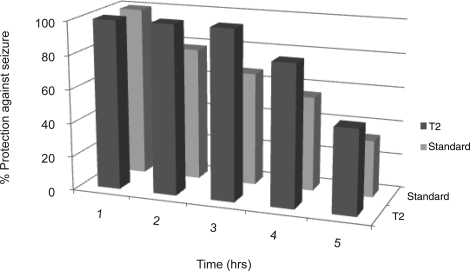
Figure 7.
w/o/w emulsion (T2) showing prolonged anticonvulsant effect.
MES induced seizures in mice
The results of this study are summarized in Table 4. Batch T1, T2 and T3 exhibited 83.34%, 100% and 100%, respectively, protection against seizure from hind limb tonic extensor induced by MES. The standard group (group administered with marketed formulation at the dose level of 36 µmol/kg) also exhibited 100% protection against MES induced HLTE phase. Mortality and lethality of T2 was also recorded at 1, 2, 4, 6 and 8 hrs. Percent protection against seizure by T2 and standard in 1, 2, 4, 6 and 8 hrs exhibited (100%, 100%), (100%, 78.64%), (100%, 66.67%), (83.34%, 55.67%) and (50%, 33.34%), respectively. From the percent protection against seizure vs. time data, it was concluded that T2 batch exhibited more prolonged anticonvulsant activity than marketed formulation used as standard and was significantly different from control (vehicle) (p<0.01, n=6) as shown in figure 7.
Strychnine induced convulsions in mice
Strychnine induced seizures were characterized by tremors and straub tail followed by myoclonic jerks of the limbs, leading to full generalized clonic/tonic seizures. Generally, death occurred within few minutes of the onset of clnic/tonic seizures or, in any case, within one hour. Vehicle and T1 failed to protect the mice from generalized clonic-tonic convulsions induced by strychnine. However, batch T2, T3 and standard formulation exhibited 66.67%, 83.34% and 100% protection against seizure, respectively, against generalized clonic-tonic seizures induced by strychnine in mice and the effect was significantly different from control (vehicle) (p<0.01, n=6) as shown in table 5. From the percent protection against seizure data, it was concluded that T3 batch exhibited comparable anticonvulsant activity against strychnine induced seizures as that of marketed formulation.
CONCLUSION
Multiple emulsions (w/o/w) were found to be suitable for the development of controlled release delivery systems for poorly water soluble drug like lamotrigine. Two promising w/o/w multiple emulsions prepared using optimized EHLB at 8.49 and 10.53 by one step emulsification technique exhibited anticonvulsant effect for sufficiently prolonged duration of time as compared to marketed formulation. The w/o/w multiple emulsions, by virtue of their sustained release characteristics, may avoid plasma drug level fluctuation, maintain plasma drug concentrations within therapeutic range for longer time and thereby reduce the onset of adverse events. Due to its liquid state, it may be used as a convenient and suitable dosage form for pediatric and geriatric patients and as a result may increase patient compliance.
ACKNOWLEDGMENT
The second author gratefully acknowledges the financial assistance provided by University Grants Commission (New Delhi, India) for this research work.
REFERENCES
- 1.Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16:165–170. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 2.Seizures and Epilepsy: Hope Through Research. National Institute of Neurological disorders and stroke. 2010. July 26. Available at: http://www.ninds.nih.gov/disorders/epilepsy/detail_epilepsy.htm (Assessed on 30th July 2010)
- 3.Rxlist. Avilable at: www.Rxlist.com. (Assessed on 30th July 2010)
- 4.Mishra B, Arya N, Tiwari S. Investigation of formulation variables affecting the properties of lamotrigine nanosuspension using fractional factorial design. DARU. 2010;18:1–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra B, Panyam J, Sharma AV. In vitro release of diclofenac sodium from multiple emulsions-effects of location of drug and pH of the aqueous phase. Acta Pharm Turc. 1999;41:42–45. [Google Scholar]
- 6.Mishra B, Panyam J, Sharma AV. Mechanism of drug release control by osmotic additives from multiple W/O/W emulsions containing diclodenac sodium. Acta Pharm Turc. 1999;41:58–61. [Google Scholar]
- 7.Garti N. Double emulsions scope, limitations and new achievements. Colloid Surface A. 1997;123:233–246. [Google Scholar]
- 8.Morais JM, Santos DH, Nunes JRL, Zanatta CF, Rocha-Filho PA. W/O/W multiple emulsions obtained by one step emulsification method and evaluation of the involved variables. J Disp Sci Tech. 2008;29:63–69. [Google Scholar]
- 9.Mishra B, Pandit JK. Multiple W/O/W emulsions as prolonged release formulations of pentazocine. J Controlled Rel. 1990;14:53–60. [Google Scholar]
- 10.Magdassi S, Garti N. A kinetic model for release of electrolytes from w/o/w multiple emulsions. J Controlled Rel. 1986;3:273–277. [Google Scholar]
- 11.Vogel GH. Drug discovery and evaluation: Pharmacological assays. 2nd Ed. Berlin Heidelberg: Spinger Verlag; 2002. pp. 388–575. [Google Scholar]
- 12.Khopade AJ, Jain NK. A stable multiple emulsion system bearing isoniazid: preparation and characterization. Drug Dev Ind Pharm. 1998;24:289–93. doi: 10.3109/03639049809085622. [DOI] [PubMed] [Google Scholar]
- 13.Tirnaksiz F, Kalsin O. A topical w/o/w multiple emulsions prepared with Tetronic 908 as a hydrophilic surfactant: Formulation, characterization and release study. J Pharm Pharm Sci. 2005;8:299–315. [PubMed] [Google Scholar]
- 14.Csoka I, Eros I. Stability of multiple emulsion. Int J Pharm. 1997;156:119–123. [Google Scholar]
- 15.Kawashima Y, Hino T, Takeuchi H, Niwa T, Horibe K. Rheological study of w/o/w emulsion by a cone-and-plate viscometer: Negative thixotropy and shear-induced phase inversion. Int J Pharm. 1991;72:65–77. [Google Scholar]
- 16.Kovács A, Csóka I, Kónya M, Csányi E, Fehér A, Eros I. Structural analysis of w/o/w multiple emulsions by means of DSC. J Ther Anal Calori. 2005;82:491–497. [Google Scholar]
- 17.Resa JM, González C, Fanega MA, Ortiz de Landaluce S, Lanz J. Enthalpies of mixing, heat capacities, and viscosities of alcohol (C1–C4)+olive oil mixtures at 298.15 K. J Food Engg. 2002;51:113–118. [Google Scholar]
- 18.Onyeji CO, Omotosho JA. Bioavailability of nitrofurantoin from multiple W/O/W emulsions in man and the influence of the oil phase of the emulsion. Indian J Pharm Sci. 1993;55:14–18. [Google Scholar]
- 19.Dalby NO, Nielsen EB. Comparison of the preclinical anticonvulsant profiles of tiagabine, lamotrigine, gabapentin and vigabatrin. Epi Research. 1997;28:63–72. doi: 10.1016/s0920-1211(97)00031-4. [DOI] [PubMed] [Google Scholar]