Abstract
Background and the purpose of the study
Gnidilatimonoein (Gn), a new diterpene ester from Daphne mucronata, possesses strong anti-metastasis and anti-tumor activities. In this study, its apoptosis and differentiation capabilities were evaluated by using the leukemia HL-60 cell line.
Material and methods
Cell prolifaration inhibition was estimated by MTT assay. The occurrence of apoptosis was evaluated by EtBr/AO double staining technique, cell cycle analyses and detection of apoptotic cells by Annexin V-FITC and propodium iodide (PI). Differentiation of the cells was determined by NBT reduction assay and the expression of specific cell surface markers such as CD14 and CD11b, were analyzed by flow cytometry.
Results
The drug decreased the growth of the cells dose- and time-dependently and the IC50 was found to be 1.3 µM. Our data suggested that Gn induced both monocytic differentiation and apoptosis among HL-60 cells. In addition, cell cycle analyses showed an increase in G1 phase population by 24 hrs, which was gradually replaced by Sub-G1 cell population (apoptotic cells) by 72 hrs.
Conclusion
Based on these data, the Gn-treated HL-60 cells displayed differentiation-dependent apoptosis. Thus, Gn might be a good candidate for differentiation therapy of leukemia, pending full biological evaluation of the compound among the wide array of leukemia cells.
Keywords: Apoptosis, Differentiation, Gnidilatimonoein, HL-60, Leukemia.
INTRODUCTION
A characteristic abnormality of some types of leukemia such as ALL, AML and APL is that they are blocked at an early stage of their developments and fail to differentiate into functional mature cells (1). Recently, several scientific achievements have emphasized on using differentiation therapy as an elegant alternative to chemotherapy with potent cytotoxic agents. This approach could theoretically limit the patient exposure to unwanted side effects of cytotoxic drugs and, more importantly, it would modulate the remission and the cure rates (2).
Human promyelocytic leukemia cells, derived from a patient with acute promyelocytic leukemia (APL), following treatment with respective inducers could be differentiated either along granulocytic or along monocytic lineage, and thus this cell line provide a good in vitro model for research and differentiation studies (3). HL-60 cells are induced to differentiate into mature functional neutrophils by dimethylsulfoxide (DMSO), retinoic acid (RA), anthracyclines, 6-thioguanine (6-TG), 5-azacytidine and tunicamycin (4–6) or into macrophage/monocyte-like cells by phorbol ester (12-O-tetradecanoylphorbol 13-acetate [TPA]), 1,25-dihydroxyvitamin D3 and cytosine arabinoside (Ara-C) (7). However, their clinical applications have always been associated with significant side effects (8). Therefore, in recent years significant efforts have been devoted to identify natural agents having differentiation capability and being devoid of general toxicities. In that respect, plants have been the center of attention for obtaining novel anti-leukemia agents (9). Many flavonoids, phenolic compounds and daphnane-type diterpene esters such as genkwadaphnin, yuanhuacine, genididin, catechin, epicatechin, taxifolin and pycnogenol (10, 11) have so far been evaluated and shown to induce differentiation and/or apoptosis among different leukemia cell lines. It has been also reported that the anti-leukemia property of these compounds is mainly due to their effects on different enzyme activities such as Inosin 5′-Monophosphate Dehydrogenase (IMPDH), thymidine kinase and DNA polymerase. IMPDH catalyses the rate limiting reaction of de novo guanine nucleotide biosynthesis and its activity has been associated with the regulation of cellular growth, differentiation and apoptosis (12). Results of previous studies have classified Gn as a strong anti-proliferative agent among several human cancer cell lines (13). It has been also found that Gn is capable of inhibiting IMPDH activity, which is usually up regulated in human leukemia cell lines (14). Studies of different independent groups have indicated that several inducers promote programmed cell death (apoptosis) in addition of causing terminal differentiation. In that line, it has been shown that the proportion of terminally differentiated cells was much higher than that of apoptotic cells (recorded in the same culture plate). This might suggest that a fraction of differentiated cells undergo apoptosis (15).
To further confirm the differentiation and apoptosis induction activities of Gn in leukemia cell lines, it was decided to do complementary studies in human promyeloid leukemia HL-60 cells having the following unique criteria: susceptibility to a variety of agents to induce differentiation by as much as 90%, the capacity to differentiate either to granulocyte-like or to monocyte/macrophage-like cells and finally bring a model of AML type 2 representing both differentiation and apoptosis activities (16). It was also aimed to determine whether anti-proliferative and cytotoxic effects of Gn in HL-60 cells can affect the apoptosis phenomenon and to evaluate possible differentiation effect(s) of this compound in human promyeloid leukemia HL-60 cells.
MATERIAL AND METHODS
Materials
The cell culture medium (RPMI-1640), fetal bovine serum (FBS) and penicillin–streptomycin were purchased from Gibco BRL (Life technology, Paisley, Scotland). Cell line was obtained from Pasteur Institute of Iran (Tehran, Iran). 12-O-tetradecanoyl porbol-13-acetate (TPA), propidium iodide (PI), RNase (DNase free), nitro blue tetrazolium (NBT) and sodium azide were purchased from Sigma Chem. Co. (Germany). Ethidium bromide (EtBr) and acridine orange (AO) were obtained from Pharmacia LKB Biotechnology AB Uppsala (Sweden). Annexin-V and anti CD14 and CD11b antibodies were purchased from IQ Products (Groningen, Netherlands).
Plant extraction and purification of Gn
Extraction and purification of Gn has been described in detail previously (17). The purity of the compound by 99% was confirmed by HPLC technique.
Cell culture
HL-60 cells (4×104 cells/ml) were grown in RPMI 1640 medium supplemented with 20% (V/V) FBS, 100 µg/ml streptomycin and penicillin (100 U/ml). The cultured cells were incubated under 5% CO2 humidified atmosphere at 37°C. For the various experiments, the cells were seeded at a density of 4×104 cells/ml in the presence or absence of Gn.
Cell proliferation assay
Cell proliferation was estimated by MTT assay (18). Briefly, HL-60 cells were seeded at a density of 4×104 cells/ml in 96 well plates. After being treated with various concentrations of Gn for different time intervals, 10 µl of MTT (5 mg/ml) was added to each well. The plates were incubated at 37°C for 4 hrs. Then the solution was removed and 100 µl of DMSO was added to each well. After rocking for 30 min, absorbance values were determined at 570 nm using a microplate reader (ELx 800 Microplate Reader, Bio-TEK).
Nitroblue tetrazolium (NBT) reduction assay
In order to evaluate the differentiation of HL-60 cells toward macrophage/monocyte lineage, NBT assay was performed as described by Sakashita et al. (19) with a slight modification. In brief, cells were harvested by centrifugation and re suspended in 200 µl RPMI 1640 containing NBT solution (4 mg/ml) and 2 µg/ml TPA, and the cell suspensions were then incubated at 37°C for 30 min. The differentiated cells were identified by their intracellular reduced dark blue formazan deposits.
Analyses of apoptotic cells by Annexin V-FITC and propodium iodide (PI)
The cells were treated with the drug for 72 and 96 hrs. After harvesting, the cells were washed twice with PBS and re-suspended in 100 µl binding buffer (10 mM HEPES/NaOH, of pH 7.4, 140 mM NaCl and 2.5 mM CaCl2). FITC-Annexin V (10 µl) was added to the cells followed by the addition of 10 µl of PI(50 µg/ml of PBS). The samples were incubated for 10 min in the dark at 4°C and then analyzed by flow cytometry.
Flow cytometric analyses of the cell surface expression antigens
Control and Gn-treated cells were collected and washed twice with ice-cold PBS. The cells were re-suspended in PBS containing 1% FBS and 0.1% sodium azide. Then 10 µl of PE-conjugated monoclonal antibody against CD14 and FITC-conjugated monoclonal antibody against CD11b were added to 100 µl of the cell suspension and incubated at 4°C for 30 min. For each population, a matched isotype control monoclonal antibody (mAb) was used to define the fluorescence background. Finally, after washing, at least 104 cells were analyzed by flow cytometry (Partec PAS) for the intended antigens.
Cell cycle analyses by flow cytometry
The distribution of cells among different phases of their growth cycles was achieved by using a published method with slight modification (20). Briefly, after washing the cells for twice with PBS and re-suspending in the hypotonic solution containing 10 mM Tris/HCl, 0.1% Triton X-100, 200 µg/ml RNase and 50 µg/ml propidium iodide, they were incubated at room temperature for 30 min. The stained cells were analyzed by flow cytometry.
Analyses of apoptotic cells by EtBr/AO double staining
Nuclear morphology of the cells was studied by fluorescence microscopy after staining with ethidium bromide/acridine orange (EtBr/AO). The cells (4×104 cells/ml) cultured in the presence or absence of the drug were collected and washed in cold PBS. Then the EtBr/AO solution (1:1 v/v) was added to the cell suspension at a final concentration of 100 µg/ml and then they were incubated at room temperature for 5 min. The stained cells were evaluated under a fluorescent microscope (Zeiss, Axioskope 2 plus, Germany).
Caspase-3 activity assay
Caspase-3 activity was evaluated according to the manufacturer's instruction of the relevant kit (Biosourse, Nirelles, Belgium). Control and the Gn-treated cells were harvested and lysed in 50 µl of the cell lysis buffer. Fifty microliters of the reaction buffer (containing 10 mM DTT) and 5 µl of the relevant conjugated substrate (DEVD-pNA) at a concentration of 4 mM were added to each lysate. The mixture was incubated at 37°C for 2 hrs and the absorbance was then measured at 405 nm.
Statistical analyses
Data are expressed as mean±SD. All experiments were performed in triplicates except for Caspase-3 activity assay which was performed in duplicates. For statistical analyses, the student's t-test was applied. P values of less than 0.05 were considered significant.
RESULTS
Gn inhibits proliferation of HL-60 cells
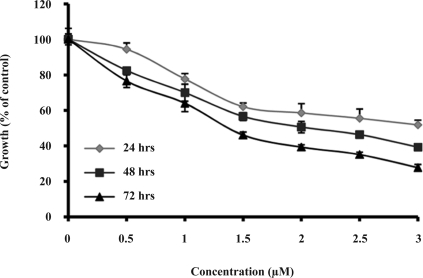
As it is shown in figure 1, the drug inhibited the proliferation of HL-60 cells in a time- and dose-dependent manner. After 72 hrs of treatment, the cell proliferation was reduced by 50% at 1.3 µM of the drug. Treatment of HL-60 cells with 0.5-3 µM of Gn for 3 days resulted in cell death by 24–72%.
Figure 1.
Effect of Gn on HL-60 cell proliferation. Cells were exposed to the indicated amounts of Gn (0.5–3 µM) for 24, 48 and 72 hrs. The cell growth inhibition was measured by MTT assay. Cell proliferation inhibition in each treatment is expressed as percentage of the treated cell number relative to the untreated control cells. Results are means±SD of three measurements (P< 0.05).
NBT assay
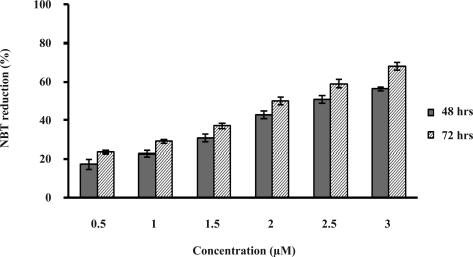
As shown in figure 2, HL-60 cells were differentiated by the drug in a time- and dose-dependent manner. Incubation of the cells with 0.5–3 µM of Gn for 48 to 72 hrs increased the cell differentiation by 17–56% and 24–66%, respectively.
Figure 2.
Changes in the extent of HL-60 cell differentiation induced by Gn at various concentrations. Differentiation was assayed by the NBT test. HL-60 cells were incubated with Gn (0.5–3 µM) for 48 and 72 hrs and NBT-positive cells were counted. Values are means±SD of triplicate measurements (P<0.05).
Effect of Gn on the expression of cell surface antigens
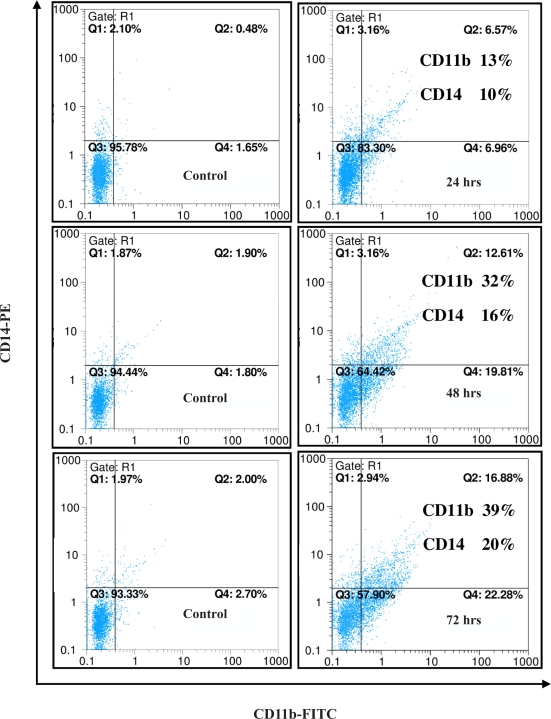
As it is evident in figure 3, treatment of the cells with a single dose (1.3 µM) of Gn resulted in gradual increase in the cell surface expressions of both antigens, CD14 and CD11b. Flow cytometric diagrams clearly indicated the enhanced expression of CD11b (by 13, 32 and 39%) and CD14 (by 14, 16 and 20%) after 24, 48 and 72 hrs, respectively.
Figure 3.
Effects of Gn on the expression of differentiation markers in HL-60 cells. HL-60 cells were treated with 1.3 µM of Gn for different time intervals. Differentiation was assessed by evaluating the extent of CD11b and CD14 expression using flow cytometry technique.
Gn induced G1 cell cycle arrest
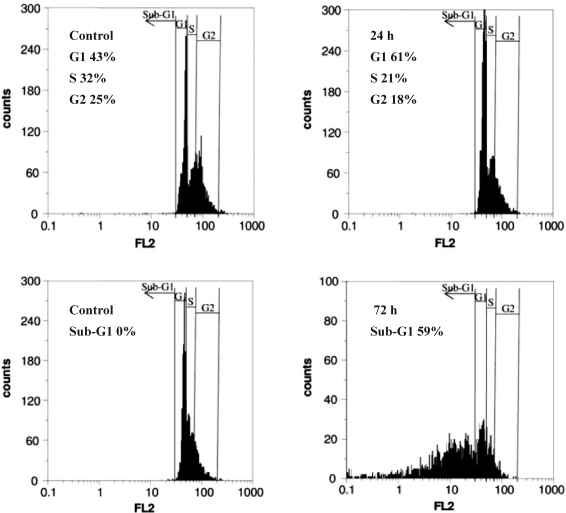
After treatment of HL-60 cells with 1.3 µM of the drug for 24 hrs, the cell population in G1 phase increased steadily from 43 to 61% which was accompanied with a slight decrease in S (11%) and G2 (7%) phases. However, longer exposures to the drug led to the appearance of Sub-G1 cell population which increased to 59% after 72 hrs (Fig 4). These results clearly indicated that Gn induced post G1-arrest apoptosis among the drug-treated cells.
Figure 4.
Cell cycle distribution of the cells upon Gn treatment. The cells were treated with 1.3 µM of the drug for 24 hrs and 72 hrs and after staining with propidium iodide (PI), analyzed by flow cytometry.
Induction of apoptosis by Gn in HL-60 cells
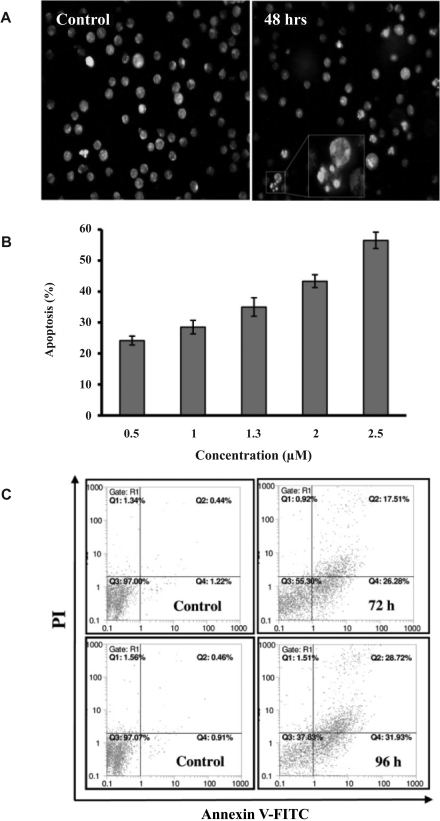
To verify the apoptotic nature of the cell death caused by Gn, we evaluated the nuclear changes by EtBr/AO double staining technique. Cultures exposed to the drug (1.3 µM) contained apoptotic cells with condensed and fragmented nuclei after 48 hrs (Fig 5A). In this figure, the nuclear region of viable cells is uniformly green and that of the apoptotic cells are orange with bright dots corresponding to nuclear chromatin fragmentation. Exposure of HL-60 cells to different concentrations of the drug induced apoptosis in a dose-dependent manner (Fig 5B). To further confirm the occurrence of apoptosis, the drug-treated cells were subjected to Annexin V/PI double staining followed by flow cytometry analyses. Exposure of the cells to 1.3 µM concentration of Gn induced time-dependent apoptosis among the cells (Fig 5C).
Figure 5.
Effects of Gn on apoptosis induction among HL-60 cells. (A) Gn-treated HL-60 cells (1.3 µM, 48 hrs) were stained with EtBr/ AO and then observed by a fluorescent microscope. Gn significantly induced chromatin condensation and nuclei fragmentation. Magnification 40x. (B) Cells were treated with different concentrations of Gn for 72 hrs. The percentage of apoptotic cells increased dose dependently. (C) HL-60 cells were treated with 1.3 µM of Gn for 72 and 96 hrs followed by FITC–Annexin V/PI flow cytometry analyses.
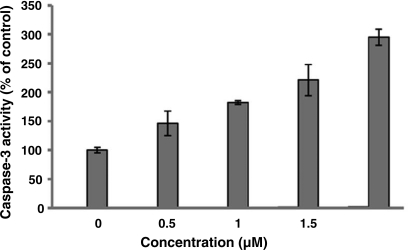
Activation of pro-caspase-3 by Gn
Apoptosis is often associated with caspase(s) activation. Among the caspase family, caspase-3 is responsible for proteolytic cleavage of a number of substrates and is the main key executioner of apoptosis. In this study, the drug-treated apoptotic cells showed, as indicated in figure 6, a significant increase in the activity of caspase-3 (1.5–3 folds) after 72 hrs of exposure to different concentrations (0.5–2 µM) of Gn, indicating that Gn-induced apoptosis among the treated cells is caspase-3 dependent.
Figure 6.
Effect of Gn on caspase-3 activation in HL-60 cells. HL-60 cells were treated with various concentrations of Gn (0.5-2 µM) for 72 hrs. After incubation time, the activity of caspase-3 enzyme was determined by DEVD-pNA. Results are means±SD of two independent measurements (P< 0.05).
DISCUSSION
The paradigm that some types of leukemias are characterized by the modification of 2 sets of genes, those that give the proliferative criterion and the second one that is associated with a block of differentiation, is still as valid today as it was 3 decades ago. To date, the hope that a variety of common chemicals would be identified for leukemia cells differentiation, similar to the successes of ATRA (All Trans Retinoic Acid) in APL, has not come to reality on a broad scale (21). This might be attributed to the fact that the candidate substances have been unable to target a disease-specific lesion, have short lives and have been accompanied by a variety of side effects such as drug-resistance and cytotoxicity (21). Based on these facts, a larg number of investigations are going on to find novel anti-leukemia agents from natural sources mainly from plant kingdom. Regarding the established mode of action of 3-HK and structural similarities between 3-HK and Gn, it was of interest to evaluate the differentiation and apoptosis activities of Gn relative to 3-HK. In the present investigation, it became evident that Gn induced G0/G1 cell cycle arrest, differentiation and apoptosis in HL-60 cells. To determine the anti-leukemia effect of Gn, the MTT assay was used. After treatment with Gn, the proliferation rate of HL-60 cells declined (Fig 1). Regarding differentiation, HL-60 cells have a dual potential to differentiate to monocytes or to granulocytes (19). Results of this study indicated that Gn significantly induced HL-60 cells to differentiate along the monocytic lineage, as indicated by morphological examination (data not shown), NBT reduction assay (Fig 2) and most importantly, the expression of both cell surface markers CD11b and CD14 (Fig 3). These observations were almost similar to our previous data on the effect of 3-HK on HL-60 cells (22), through the cytotoxic potency of Gn was found to be weaker (IC50=1.3 µM) compared to the IC50 of 3-HK which is around 10 nM.
The differentiation of the Gn-treated cells was associated with significant arrest of the cells in G1 phase of their growth progression cycle, which is in accordance to the present literatures data (23). However, after 24 hrs, the cell population at G1 phase decreased and the sub-G1 peak (representing apoptosis) appeared after 72 hrs (Fig 4) confirming the death of the Gn-treated cells by apoptosis. Morphological changes and also the appearance of Sub-G1 peak in Gn-treated cells clearly confirmed the occurrence of apoptosis among Gn- treated cells. To further verify the occurrence of apoptosis, the nuclear morphological changes following AO/EtBr staining and FITC-annexin V/PI treatment assay were investigated. Results confirmed apoptotic cell death among Gn-treated cells (Fig 5). In addition, results supported that apoptosis was caspase-3 dependent (Fig 6). This is in contrast to some present data in literature concerning the caspase-3-independent apoptotic effects of some anticancer drugs (24).
In conclusion, results of this investigation clearly showed that Gn is a natural anti-proliferative and differentiation-inducing agent which induced macrophage/monocyte differentiation-dependent apoptosis in human promyeloid leukemia HL-60 cells. Therefore, this new agent might be valubale candidate for pharmaceutical purposes.
ACKNOWLEDGMENT
The authors appreciate the financial support of this investigation by the Research Council of University of Tehran and also the authors thank Tahereh Shahrestani and Omran Asgarian for their assistance in flow cytometry analyses.
REFERENCES
- 1.Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukemia. Nature. 1978;274:535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- 2.Sell S. Leukemia: stem cells, maturation arrest, and differentiation therapy. Stem Cell Rev. 2005;1:197–205. doi: 10.1385/SCR:1:3:197. [DOI] [PubMed] [Google Scholar]
- 3.Zhong H, Wang HR, Yang S, Zhong JH, Wang T, Wang C, Chen FY. Targeting Smad4 links microRNA-146a to the TGF-beta pathway during retinoid acid induction in acute promyelocytic leukemia cell line. Int J Hematol. 2010;92:129–135. doi: 10.1007/s12185-010-0626-5. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz EL, Sartorelli AC. Structure-activity relationships for the induction of differentiation of human HL-60 acute promyelocyticleukemia cells by anthracyclines. Cancer Res. 1981;42:2651–2655. [PubMed] [Google Scholar]
- 5.Tarella C, Ferrero D, Gallo E, Pagliardi LG, Ruscetti FW. Induction of differentiation of HL-60 cells by dimethylsulfoxide: evidence for a stochastic model not linked to cell division. Cancer Res. 1982;42:445–449. [PubMed] [Google Scholar]
- 6.Christman JK, Mendelsohn N, Herzog D, Schneiderman N. Effect of 5-azacytidine on differentiation and DNA methylation in human promyelocytic leukemia cells (HL-60) Cancer Res. 1983;43:763–769. [PubMed] [Google Scholar]
- 7.Griffin J, Munroe D, Major P, Kufe D. Induction of differentiation of human myeloid leukemia cells by inhibitors of DNA synthesis. Exp Hematol. 1982;10:774–781. [PubMed] [Google Scholar]
- 8.Barber N, Belov L, Christopherson RI. All-trans retinoic acid induces different immunophenotypic changes on human HL60 and NB4 myeloid leukaemias. Leuk Res. 2008;32:315–322. doi: 10.1016/j.leukres.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Park BY, Min BS, Ahn KS, Kwon OK, Joung H, Bae KH, Lee HK, Oh SR. Daphnane diterpene esters isolated from flower buds of Daphne genkwa induce apoptosis in human myelocytic HL-60 cells and suppress tumor growth in Lewis lung carcinoma (LLC)-inoculated mouse model. J Ethnopharmacol. 2007;111:496–503. doi: 10.1016/j.jep.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Stanoeva E, He W, Kimpe ND. Natural and synthetic cage compounds incorporating the 2, 9, 10-trioxatricyclo [4.3.1.03,8] decane type moiety. Bioorg. Med. Chem. 2005;13:17–28. doi: 10.1016/j.bmc.2004.07.070. [DOI] [PubMed] [Google Scholar]
- 11.Huanga WW, Yangb JS, Linc CF, Hod WJ, Leec MR. Pycnogenol induces differentiation and apoptosis in human promyeloid leukemia HL-60 cells. Leuk Res. 2005;29:685–692. doi: 10.1016/j.leukres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Dairaku I, Han Y, Yanaka N, Kato N. Inhibitory effect of curcumin on IMP dehydrogenase, the target for anticancer and antiviral chemotherapy agents. Biosci Biotechnol Biochem. 2010;74:185–187. doi: 10.1271/bbb.90568. [DOI] [PubMed] [Google Scholar]
- 13.Mahdavi M, Yazdanparast R. Gnidilatimonoein from Daphne mucronata induces differentiation and apoptosis in leukemia cell lines. Arch Pharm Res. 2007;30:177–181. doi: 10.1007/BF02977692. [DOI] [PubMed] [Google Scholar]
- 14.Yazdanparast R, Sadeghi H. Nucleic acid synthesis in cancerous cells under the effect of gnidilatimonoein from Daphne mucronata . Life Sci. 2004;74:1869–1876. doi: 10.1016/j.lfs.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 15.Tsiftsoglou AS, Pappas IS, Vizirianakis IS. The developmental program of murine erythroleukemia cells. Oncol Res. 2003;13:339–346. doi: 10.3727/096504003108748546. [DOI] [PubMed] [Google Scholar]
- 16.Birnie G.D. The HL-60 cells line: a model system for studying human myeloid cell differentiation. Br J Cancer. 1988;58:41–45. [PMC free article] [PubMed] [Google Scholar]
- 17.Mianabadi M, Rivera E, Yazdanparast R. A new diterpene ester from the leaves of Daphne mucronata . J Trop Medl Plan. 2003;4:1–4. [Google Scholar]
- 18.Vistica DT, Skehan P, Scudiero D, Monks A, Pittman A, Boyd M R. Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res. 1991;51:2515–2520. [PubMed] [Google Scholar]
- 19.Sakashita A, Nakamaki T, Tsuruoka N, Honma Y, Hozumi M. Granulocyte colony-stimulating factor, not granulocyte-macrophage colony-stimulating factor, co-operates with retinoic acid on the induction of functional N-formyl-methionyl-phenylalanine receptors in HL-60 cells. Leukemia. 1991;5:26–31. [PubMed] [Google Scholar]
- 20.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Method. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 21.Daniel N, Daphne S, Phillip K. Differentiation therapy of leukemia: 3 decades of development. Blood. 2009;113:3655–3665. doi: 10.1182/blood-2009-01-198911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yazdanparast R, Moosavi MA, Mahdavi M, Sanati MH. 3-Hydrogenkwadaphnin from Dendrostellera lessertii induces differentiation and apoptosis in HL-60 cells. Planta Med. 2005;71:1112–1117. doi: 10.1055/s-2005-873165. [DOI] [PubMed] [Google Scholar]
- 23.Ding L, Liu B, Qi LL, Zhou QY, Hou Q, Li J, Zhang Q. Anti-proliferation, cell cycle arrest and apoptosis induced by a natural xanthone from Gentianopsis paludosa Ma, in human promyelocytic leukemia cell line HL-60 cells. Toxicol In Vitro. 2009;23:408–417. doi: 10.1016/j.tiv.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Hilchie AL, Furlong SJ, Sutton K, Richardson A, Robichaud MR, Giacomantonio CA, Ridgway ND, Hoskin DW. Curcumin-induced apoptosis in PC3 prostate carcinoma cells is caspase-independent and involves cellular ceramide accumulation and damage to mitochondria. Nutr Cancer. 2010;62:379–389. doi: 10.1080/01635580903441238. [DOI] [PubMed] [Google Scholar]