Abstract
Background and the purpose of the study
Functional constipation is prevalent in children. Recently polyethylene glycol has been introduced as an effective and safe drug to treat chronic constipation. There are only a few clinical trials on comparison of PEG and liquid paraffin in childhood constipation. The purpose of this study was to evaluate clinical efficacy and safety of PEG 3350 solution and liquid paraffin in the treatment of children with functional constipation in Sari Toba clinic during the period of 2008–2009.
Methods
Children with a history of functional constipation were subjects of this study. One hundred and sixty children of 2–12 years old with functional constipation were randomized in two PEG and paraffin treatment groups. Patients received either 1.0–1.5 g/kg/day PEG 3350 or 1.0–1.5 ml/kg/day liquid paraffin for 4 months. Clinical efficacy was evaluated by stool and encopresis frequency/week and overall treatment success rate was compared in two groups.
Results and major conclusion
Compared with the baseline, defecation frequency/ week increased significantly and encopresis frequency meaningfully decreased in two groups during the period of the study. Patients using PEG 3350 had more success rate (mean: 95.3%±3.7) compared with the patients in paraffin group (mean: 87.2%±7.1) (p=0.087). Administration of PEG 3350 were associated with less adverse events than liquid paraffin. In conclusion in treatment of pediatric functional constipation, regarding clinical efficacy and safety, PEG 3350 were at least as effective as liquid paraffin and but less adverse drug events.
Keywords: Defecation, Encopresis, Lactoluse, Mineral oil.
INTRODUCTION
Approximately 3% of general pediatric outpatient visits and 25% of pediatric gastroenterology consultations are related to perceived defecation disorders (1). More than 95% of children older than 1 year with constipation suffer from functional constipation (2). Liquid paraffin (mineral oil) is widely accepted and recommended as a fundamental component of regimens for the management of constipation in North America and Australia (3). Extensive experiences with long-term uses of mineral oil have shown its efficacy and safety (4) and North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGN) clearly identify liquid paraffin as a medication of first choice in the management of pediatric constipation (3). This drug combines ease of titration with tolerability and sustained effect despite prolonged uses and make it attractive for use in childhood constipation encopresis (5). Mineral oil is not recommended for infants less than 1 year old due to the risk of aspiration and development of lipoid pneumonia.
Some studies have shown PEG has at least equal efficacy and fewer adverse effects in comparison with lactulose (6, 7). A recent systematic review has demonstrated the efficacy of PEG as good as or even better than lactulose or milk of magnesia over a wide range of ages and treatment durations (8). There are only a few clinical trials on the comparison of PEG and liquid paraffin in childhood constipation. The purpose of this study was to evaluate clinical efficacy and safety of PEG 3350 solution and liquid paraffin in the treatment of children with functional constipation.
METHODS
Subjects
Patients eligible for enrolment were ambulatory 2 to 12 years old children with a history of functional constipation (at least 3 months) defined as less than 3 stools/week, more than 1 encopresis/week or palpable abdominal or rectal fecal mass on physical examination. The patients referred to Toba clinic initially were examined and followed during the study by a pediatric gastroenterologist.
Exclusion criteria were: Withdrawn the treatment during the first month, hypothyroidism, Hirschsprung disease; known cardiac, renal and neurologic disorders, any sign of organic causes, and history of colon surgery. At the time of enrolment, a full history details was obtained including pain or bleeding during defection, frequency of defecation, fecal impaction, and encopresis. Written informed consent was obtained from parents after full description of the study details. The ethics committee of Mazandaran University of Medical Sciences (MAZUMS) approved this open-labeled randomized study that accomplished in Sari Toba clinic affiliated to MAZUMS during the period of 2008–2009. At the beginning of the therapy, all patients and their parents were educated for appropriate diet, dairy consumption, toilet training and adverse drug reactions. Random table was used to randomize the patients in two groups.
Study Protocol
Patients were randomly assigned to receive either 1.0–1.5 g/kg/day PEG 3350 or 1.0–1.5 ml/kg/day liquid paraffin orally for 4 months. PEG 3350 powder was prepared as a 40% solution to trust reliable to apply the pediatric dosing and to increase compliance and liquid paraffin was provided from a pharmaceutical factory. For rectal disimpaction, bisacodyl suppositories were applied at the beginning of the study. After the first physical exam, the patients were visited at least on the days of 7, 14, 30, 60, 90, and 120 during the treatment period, repeatedly.
Assessment
Clinical efficacy was evaluated by stool and encopresis frequency/week and overall treatment success rate was compared in two groups. Patients that were withdrawn from the study due to lack of efficacy or adverse reaction of the drug placed in unsuccessful cases and remaining were considered as successful group. Adverse drug events were determined during examination or at the time of complain of patients and their parents.
Statistical Analyses
The statistical analysis was performed using SPSS-16 software and results were expressed as means±SD. Means differences between two groups were compared by independent samples t-test. P-values less than 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
One hundred and sixty children 2–12 years old with functional constipation were included in this study. Since 2 patients dropped out in paraffin group, the final data were reported of a total 158 patients (80 in the PEG and 78 in the paraffin groups). There was no significant difference with regard to age, and baseline frequencies of stool, encopresis, fecal impaction, rectal pain or bleeding during defecation between two treatment groups table 1.
Table 1.
Baseline characteristics of the patients
| Variable | PEG 3350 | Paraffin | P value |
|---|---|---|---|
| Gender (male/female) | 41/39 | 43/35 | 0.625 |
| Age (month) | 52.8±23.4 | 50.6±23.6 | 0.56 |
| Fecal impaction | 42 | 40 | 0.94 |
| Rectal bleeding during defecation | 11 | 13 | 0.61 |
| Pain during defecation | 71 | 70 | 0.84 |
Although all patients and their parents were educated for appropriate diet, dairy consumption and toilet training initially and during the study, the amount of patients’ compliance were not recorded and compared.
This randomized clinical trial demonstrated that both PEG 3350 and liquid paraffin were effective in increasing defecation frequency and decreasing encopresis significantly during the 4 month treatment period. The mean defecation rate significantly increased in PEG group from 1.6±0.8/week to 7.0±3.8/week and 1.4±.05/week to 6.3±3.1/week in paraffin group (Table 2). Patients using PEG and liquid paraffin had less encopresis in the 30th day of the treatment (from 27 cases to 12 and 28 cases to 10 in two groups respectively, p>0.05). As a result, with regard to defecation and encopresis frequency, PEG 3350 and liquid paraffin were equally effective.
Table 2.
Number of defecation/week at the beginning and during the treatment in two groups.
| Time | PEG 3350 (Mean±SD) | Paraffin (Mean±SD) | P value |
|---|---|---|---|
| At the beginning | 1.6±0.8 | 1.4±0.5 | 0.69 |
| 7th day | 4.1±1.7 | 3.3±0.8 | 0.19 |
| 14th day | 6.1±3.7 | 5.1±1.9 | 0.54 |
| 30th day | 7.0±3.8 | 6.3±3.1 | 0.70 |
| 60th day | 9.1±3.7 | 7.6±3.8 | 0.49 |
| 90th day | 8.9±3.8 | 7.6±3.4 | 0.51 |
| 120th day | 8.7±2.9 | 7.5±3.2 | 0.58 |
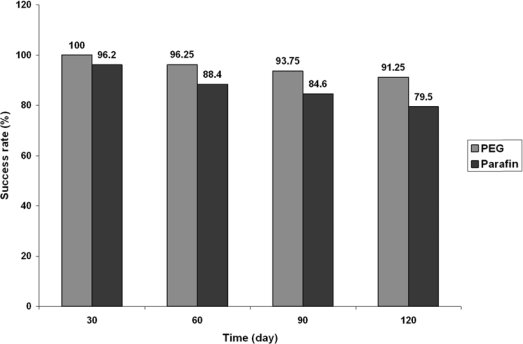
Success rates were compared at 30, 60, 90 and 120 days after the beginning of the therapy and are shown in figure 1. A higher number of patients in the PEG group (mean: 95.3%±3.7) were treated compared with the paraffin group (mean: 87.2%±7.1) (p=0.087). Although the mean differences were not significant, the trend of success rate showed that larger sample size could demonstrate better efficacy.
Figure 1.
Rate of the success (%) in the course of the treatment for two groups.
For the significant number of children with chronic constipation, maintenance therapy may be essential for longer period to regulate bowel movements without difficulty. A few studies have been performed to compare PEG vs. paraffin with regards to clinical efficacy and safety.
A crossover randomized clinical trial in 37 patients aged 2 to 16 years, PEG 3350 and lactulose increased stool frequency from 1.7±0.8/week to 14.8±1.4/week and 13.5±1.5/week respectively (6). Administration of PEG at the average dose of 1g/kg/day to 75 children 1–24 months with constipation increased mean defecation frequency from 3.7±3.2/week to 12.4±7.0/week in the initial 4 month of therapy (10). In a similar study, 74 children with chronic constipation, mentioned stool frequency increased significantly with PEG (from 2.8±0.3/week to 9.9±0.7/week) (p<0.001) (11). Significant decrease in the mean encopresis frequency/week was found in a study in Netherland (7). Similarly Karami et al reported that PEG 4000 was more effective than oral paraffin to increase stool frequency (from 2.6±1.4/week to 4.7±1.8/week and 3.0±1.5/week to 4.5±1.9/week respectively) (12).
In a case control study (13), children with constipation were treated with PEG or milk of magnesia. During one year follow up, bowel movement frequency increased and soiling events decreased significantly in both groups. In another report PEG 3350 and lactulose were compared in 100 children 6 months to 15 years with functional constipation for 8 weeks (7). It was found that 56% in PEG group were successfully treated compared to 29% in lactulose group and there were significant increase in the mean stool frequency/week and decrease encopresis frequency/week in both groups. A 3-month study with 96 patients aged 6–36 months showed the efficacy of PEG 4000 equal to or greater than lactulose (14).
A 2-week crossover study using either PEG 3350 or lactulose, reported that the success rate was significantly higher in PEG group (84%) than lactulose group (46%) (6). Also the treatment with PEG was more effective in terms of tolerability. Wang et al in their 2 weeks study have shown PEG 4000 is meaningfully more effective than lactulose (70% vs. 40%) (15). A randomized study that compared PEG 3350 without electrolytes with milk of magnesia for constipation and fecal incontinence concluded that PEG and milk of magnesia were equally effective in long-term treatment. (16)
All of adverse reactions (nausea, vomiting, flatulence, abdominal pain and dehydration), other than diarrhea, occurred more frequently in patients using liquid paraffin compared with PEG 3350 (p<0.05) (Table 3).. Rate of withdrawn for ADRs in those using paraffin were significantly higher than PEG 3350 (p<0.05).
Table 3.
Incidences of adverse drug reactions using PEG 3350 and paraffin.
| Adverse reaction | PEG 3350 (Mean±SD) | Paraffin (Mean±SD) | P value |
|---|---|---|---|
| Nausea | 4.5±2.4 | 12.3±2.6 | <0.01 |
| Vomiting | 0.2±0.4 | 2.5±1.9 | 0.014 |
| Diarrhea | 2±1.7 | 3.5±2.2 | 0.209 |
| Flatulence | 3.8±1.5 | 10.8±1.6 | <0.01 |
| Abdominal pain | 1.7±0.8 | 6.0±1.9 | <0.01 |
| Dehydration | 0.0±0.0 | 0.5±0.5 | 0.049 |
| Withdraw for ADR | 0.3±0.5 | 1.5±1.1 | 0.035 |
| Withdraw for no efficacy | 0.8±0.9 | 1.2±1.1 | 0.61 |
| Need to additive drugs | 1.2±1.1 | 2.2±1.5 | 0.22 |
In agreement with results of other reports (10, 13, 17, 18), in the present study, patients or their parents addressed mild adverse events and did not report any serious or new onset adverse reactions. In an eight weeks study period (7) PEG 3350 had no serious or significant adverse events and less adverse reactions occurred compared with patients using lactulose. Although it has been reported that liquid paraffin is more effective with fewer side effects, (17, 19), the present study showed that treatment of chronic constipation with PEG 3350 had less adverse events compared with liquid paraffin. These results were similar to others that reported PEG was safe for long term treatment of children with constipation. (16, 18).
CONCLUSION
The results of this study demonstrated that PEG 3350 was at least as effective as liquid paraffin to treat pediatric functional constipation and with less adverse drug events. Reviewing the results of the present investigations and other studies with regard to clinical efficacy and safety, revealed that PEG 3350 can be drug of choice for treatment of chronic constipation in majority of children.
ACKNOWLEDGEMENT
The authors would like to appreciate Sepidaj Pharmaceutical Co. to provide polyethylene glycol 3350 powder and Toba pharmacy members affiliated to Mazandaran University of Medical Sciences to supply PEG solution to patients.
REFERENCES
- 1.Clinical Practice Guideline. Evaluation and Treatment of Constipation in Infants and Children: Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Journal of Pediatric Gastroenterology and Nutrition. JPGN. 2006 Sep;43:e1Ye13. doi: 10.1097/01.mpg.0000233159.97667.c3. [DOI] [PubMed] [Google Scholar]
- 2.Felt B, Brown P, Coran A, Kochhar P, Opipari-Arrigan L. Functional constipation and soiling in children. University of Michigan Health System guidelines for clinical Care. 2003. Accessed online February 2, 2005, at: http://cme.med.umich.edu/pdf/guideline/peds03.pdf.
- 3.Baker SS, Liptak GS, Colletti RB, Croffie JM, Di Lorenzo C, Nurko S. Constipation in infants and children: evaluation and treatment. A medical position statement of the North American Society for Pediatric Gastroenterology and nutrition. J Pediatr Gastroenterol Nutr. 1999;29:612–626. doi: 10.1097/00005176-199911000-00029. [DOI] [PubMed] [Google Scholar]
- 4.McClung HJ, Boyne LJ, Linsheid T, Heitlinger LA, Murray RD, Fyda J, Li B U.K. Is combination therapy for encopresis nutritionally safe? Pediatr. 1993;91:591–594. [PubMed] [Google Scholar]
- 5.Sharif F, Crushell E, O'Driscoll K, Bourke B. liquid paraffin: a reappraisal of its role in the treatment of constipation. Arch Dis Child. 2001;85:121–124. doi: 10.1136/adc.85.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gremse DA, Hixon J, Crutchfield A. Comparison of polyethylene glycol 3350 and lactulose for treatment of chronic constipation in children. Clin Pediatr (Phila) 2002;41:225–229. doi: 10.1177/000992280204100405. [DOI] [PubMed] [Google Scholar]
- 7.Voskuijl W, de Lorijn F, Verwijs W, Hogeman P, Heijmans J, Makel W, Taminiau J, Beninga M. PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomized, controlled, multicentre trial. Gut. 2004;53:1590–1594. doi: 10.1136/gut.2004.043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Candy D, Belsey J. Macrogol (polyethylene glycol) laxatives in children with functional constipation and faecal impaction: a systematic review. Arch Dis Child. 2009;94:156–160. doi: 10.1136/adc.2007.128769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loening-Baucke VA. Sensitivity of the sigmoid colon and rectum in children treated for constipation. J Pediatr Gastroenterl Nutr. 1984;3:454–459. doi: 10.1097/00005176-198406000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Loening-Baucke V, Krishna R, Pashankar DS. Polyethylene glycol 3350 without electrolytes for the treatment of functional constipation in infants and toddlers. J Pediatr Gastroenterol Nutr. 2004;39:536–539. doi: 10.1097/00005176-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Pashankar DS, Bishop WP, Loening-Baucke V. Long-term efficacy of polyethylene glycol 3350 for the treatment of chronic constipation in children with and without encopresis. Clin Pediatr (Phila) 2003;42:815–819. doi: 10.1177/000992280304200907. [DOI] [PubMed] [Google Scholar]
- 12.Karami H, Khademlo M, Niari P. Polyethylene glycol versus paraffin for the treatment of children functional constipation. Iran J Pediatr. 2009;19:255–261. [Google Scholar]
- 13.Loening-Baucke V. Polyethylene glycol without electrolytes for children with constipation and encopresis. J Pediatr Gastroenterol Nutr. 2002;34:372–377. doi: 10.1097/00005176-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Dupont C, Leluyer B, Maamri N, Morali A, Joye JP, Fiorini JM, Abdelatif A, Baranes C, Benoît S, Benssoussan A, Boussioux L, J, Boyer P, Brunet E, Delorme J, FC S, Gottrand F, Grassart M, Hadji S, Kalidjian A, Languepin J, Leissler C, Lejay D, Livon D, Lopez P, J, Mougenot F, J, Risse C, J, Rizk C, Roumaneix D, Schirrer J, Thoron B, Kalach N. Double-blind randomized evaluation of clinical and biological tolerance of polyethylene glycol 4000 versus lactulose in constipated children. J Pediatr Gastroenterol Nutr. 2005;41:625–633. doi: 10.1097/01.mpg.0000181188.01887.78. [DOI] [PubMed] [Google Scholar]
- 15.Wang B-X, Wang M-G, Jiang M-Z, Xu CD, Shao CH, Jia LY, Huang ZH, Xu XH. Forlax in the treatment of childhood constipation: a randomized, controlled, multicentre clinical study. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:429–432. [PubMed] [Google Scholar]
- 16.Loening-Baucke V, Pashankar DS. A randomized, prospective, comparison study of polyethylene glycol 3350 without electrolytes and milk of magnesia for children with constipation and fecal incontinence. Pediatrics. 2006;118:528–535. doi: 10.1542/peds.2006-0220. [DOI] [PubMed] [Google Scholar]
- 17.Farahmand F. A randomized trial of liquid paraffin versus lactulose in the treatment of chronic functional constipation in children. Acta Medica Iranica. 2007;45:183–188. [Google Scholar]
- 18.Michail S, Gendy E, Preud'Homme D, Mezoff A. Polyethylene glycol for constipation in children younger than eighteen months old. J Pediatr Gastroenterol Nutr. 2004;39:197–199. doi: 10.1097/00005176-200408000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Urganci N, Akyildiz B, Polat TB. A comparative study: The efficacy of liquid paraffin and lactulose in management of chronic functional constipation. Pediatrics international. 2005;47:15–19. doi: 10.1111/j.1442-200x.2004.02001.x. [DOI] [PubMed] [Google Scholar]