Abstract
Background and the purpose of the study
Mechanical properties of films prepared from aqueous dispersion and organic solutions of Eudragit RL were assessed and the effects of plasticizer type, concentration and curing were examined.
Methods
Films were prepared from aqueous dispersion and solutions of Eudragit RL (isopropy alcohol-water 9:1) containing 0, 10 or 20% (based on polymer weight) of PEG 400 or Triethyl Citrate (TEC) as plasticizer using casting method. Samples of films were stored in oven at 60°C for 24 hrs (Cured). The stress-strain curve was obtained for each film using material testing machine and tensile strength, elastic modulus, %elongation and work of failure were calculated.
Results and major conclusion
The films with no plasticizer showed different mechanical properties depending on the vehicle used. Addition of 10% or 20% of plasticizer decreased the tensile strength and elastic modulus and increased %elongation and work of failure for all films. The effect of PEG 400 on mechanical properties of Eudragit RL films was more pronounced. The differences in mechanical properties of the films due to vehicle decreased with addition of plasticizer and increase in its concentration. Curing process weakened the mechanical properties of the films with no plasticizer and for films with 10% plasticizer no considerable difference in mechanical properties was observed before and after curing. For those with 20% plasticizer only films prepared from aqueous dispersion showed remarkable difference in mechanical properties before and after curing. Results of this study suggest that the mechanical properties of the Eudragit RL films were affected by the vehicle, type of plasticizer and its concentration in the coating liquid.
Keywords: Eudragit RL, Aqueous polymeric dispersion, Plasticizer, Polymeric film
INTRODUCTION
Polymeric coatings have been applied to solid dosage forms in order to mask the bitter taste of the drug, to protect the sensitive drug substances, to control drug release and/or to impart aesthetic nature to dosage form (1)
Acrylates are among the polymers which have been used widely in production of coated controlled release dosage forms (2). Eudragit RL and Eudragit RS are water insoluble derivatives of acrylate polymers with widespread use in preparation of coated sustained release dosage forms (3). Traditionally, water insoluble polymers had been applied from their solutions in organic solvents (4). However several problems associated with the use of organic solvents namely flammability, toxicity, environmental contamination, explosion hazards and expenses shifted the pharmaceutical industry to the use of water based coating formulations (1). The use of aqueous polymeric dispersions of water insoluble polymers has been preferred over organic solutions due to their advantages (5).
The mechanism of film formation from organic solutions of polymers differs from polymeric aqueous dispersions (6). In organic solutions, evaporation of the solvent leads to increase in polymer concentration, interdiffusion of polymeric chains and gel formation at high polymer concentration. Eventually further evaporation of solvent results in a solvent free film. However, film formation from aqueous polymeric dispersions is a complex phenomenon. During film formation the colloidal polymer particles are deposited on the surface of the substrate and upon evaporation of water, arrange themselves, in a closed-packed array due to interfacial tension between water and polymer. The coalescence of particles is then happened due to capillary forces following evaporation of tiny layer of water surrounding the particles. To achieve uniform and effective coating, the complete coalescence of the dispersed polymeric particles is necessary (7). This process is called further gradual coalescence or curing (8). The extent of coalescence of polymeric particles which starts during the coating process depends on the formulation of aqueous coating dispersion and the condition of coating procedure and may not be complete during coating process. Therefore, the products coated with aqueous polymeric dispersions are exposed to elevated temperatures after coating to ensure complete coalescence of polymer particles (7). The curing process is both time and temperature dependent. The nature and concentration of the plasticizer incorporated into the coating formulation also play an important role during this process. Overall, plasticizers play an important role in the mechanical, adhesive and dissolution properties of films and film coated products irrespective of the solvent or vehicle used.
Performance of organic and aqueous dispersion based coatings on coated products has been focus of several investigations. The aqueous and organic coating techniques for polymer blends have been compared to study the effect of type of coating on drug release (5). The performance of three ethyl cellulose based film coatings (one organic solution and two commercially available aqueous dispersions) on the release of propranolol HCl from coated pellets has been examined and it was shown that the drug release profile was dependent on the solvent or vehicle used for coating (9). In another study, it was shown that dissolution rate of 5-amino salicylic acid was slower from pellets coated with organic solution of Eudragit S100 compared to those coated with aqueous dispersion of Eudragit S100 (10).
While comparison of organic and aqueous polymeric dispersion on performance of coated dosage forms has been well documented, limited knowledge is available regarding the comparison of mechanical properties of free films prepared from organic solutions and aqueous dispersion of polymers. The physicochemical properties of plasticized films composed of Eudragit S100:L100 (1:1) and prepared from aqueous dispersions and organic solutions has been evaluated and it is shown that a change from organic solutions to aqueous dispersions would impact film properties (11).
In this study, the mechanical properties of the films from organic solutions of Eudragit RL were compared to those from commercially available aqueous dispersion of this polymer prepared under the same conditions, and the effect of type of plasticizer and its concentration on these properties were examined. The effect of curing on the changes in the mechanical properties of the films prepared from aqueous polymeric dispersions was also investigated.
MATERIAL AND METHOD
Materials
Eudragit RL 100 and Eudragit RL 30D supplied by Rohm Pharma (Germany) Isopropyl alcohol (IPA), polyethylene glycol 400 (PEG 400) and triethylcitrate (TEC) obtained from Merck (Germany) were used in this study.
Methods
Preparation of cast films from organic solution of Eudragit RL
To prepare films from organic solutions, 3.6 g of Eudragit RL 100 was dissolved in appropriate volume of isopropyl alcohol:water mixture (9:1) so that the final concentration of polymer in the solution was 12%. Free films were prepared by casting pre-determined amounts of polymeric solution on leveled 15 cm diameter polytetrafluoroethylene (PTFE) containers which were stored in oven at 40°C until constant weight was achieved. To prepare films containing 10 or 20% plasticizers based on polymer weight, appropriate amounts of each plasticizer (PEG 400 or TEC) was added to polymer solution and stirred for at least one hour before casting in pan. The above procedure was performed to prepare the films containing plasticizers. After evaporation of the solvent the casting container was placed in a desiccator containing water to make films more flexible and to facilitate separation of films from their casting container. Then the films were stored at ambient temperature for one week before mechanical testing.
Preparation of cast films from Eudragit RL aqueous dispersion
Eudragit RL 30D was commercially available as 30% aqueous dispersion. To prepare free films, 12 g of Eudragit RL 30D was weighed in a glass beaker (3.6 g polymer is present in each 12 g of Eudragit RL 30D) and diluted with distilled water till final concentration of polymer reached to 12%. The mixture was stirred with magnetic stirrers for 15 min and casted as above.
To prepare films containing 10 or 20% plasticizers based on polymer weight, appropriate amounts of each plasticizer (PEG 400 or TEC) was added to 12g of Eudragit RL 30D and mixed for at least 2 hrs with magnetic stirrer to ensure proper partitioning of the plasticizers into the polymer. The mixture was diluted with distilled water until the final concentration of polymer reached to 12% and stirred for another 15 min before casting in PTFE containers. The contents of the containers were then dried in an oven at 40°C until constant weight was achieved.
All films prepared in this study along with their identifying codes are listed in table 1.
Table 1.
Identifying codes of the prepared films.
| Film | Identifying code |
|---|---|
| Film prepared from IPA-water mixture in the absence of plasticizer | IPA |
| Film prepared from IPA-water mixture with 10% PEG 400 | IPA+10% PEG 400 |
| Film prepared from IPA-water mixture with 20% PEG 400 | IPA+20% PEG 400 |
| Film prepared from aqueous dispersion in the absence of plasticizer | Aq |
| Film prepared from aqueous dispersion with 10% PEG 400 | Aq+10% PEG 400 |
| Film prepared from aqueous dispersion with 20% PEG 400 | Aq+20% PEG 400 |
| Film prepared from IPA-water mixture with 10% TEC | IPA+10% TEC |
| Film prepared from aqueous dispersion with 20% TEC | IPA+20% TEC |
| Film prepared from aqueous dispersion with 10% TEC | Aq+10% TEC |
| Film prepared from aqueous dispersion with 20% TEC | Aq+20% TEC |
Film samples
The film samples were cut into strips of 7.5 cm length and 1.5 cm width using a sharp blade and examined visually and microscopically using stereomicroscope (Kyowa, Japan) for transparency and physical defects such as cracks or fissures and air bubbles. Film with defects on its surface was discarded and was not used in mechanical testing. The thickness of the samples was measured in 5 points using digital micrometer (Mitutoyo, Japan) and the mean was recorded. The samples with mean thickness of 250-300 µm were used for mechanical analysis.
Curing of films
Samples of the each film were stored in oven at 60°C for 24 hrs in order to study the effect of curing on mechanical properties of the free films.
Mechanical testing of the films
The mechanical properties of the films were evaluated using material testing apparatus (Hounsfiled, England) mounted with a 1KN capacity load cell. The film samples (7.5 cm×1.5 cm) were held on place using flat-faced metal grips. The initial length of the film between grips was 5 cm and the extension speed was adjusted on 1mm/min. All samples were subjected to mechanical testing following 1 week of storage at ambient temperature and tests were carried out at ambient conditions. The stress-strain curves were recorded for each sample, and tensile strength at break (the highest value for stress before fracture), % of elongation at the break (the fractional increase in film length at the point of fracture), elastic modulus (slope of the linear portion of the stress–strain profile) and the work of failure (the area under the stress–strain curve) were calculated. Tensile strength represents the film strength and higher values correspond to stronger films. Elastic modulus shows the elasticity of the film with lower values correspond to higher elasticity. Tensile strength/elastic modulus ratio which indicates a crack resistance was also calculated from the results. Ideally a film coating must be strong and elastic and therefore the higher tensile strength/elastic modulus ratio would be more desirable (1). The higher values for this ratio are indicative of a lower tendency of the films to crack. At least 5 samples were tested for each formulation and the average was calculated.
Statistical analyses
The data of mechanical testing were compared statistically using one way analysis of variance ANOVA and p value less than 0.05 was considered significant.
RESULT AND DISCUSSION
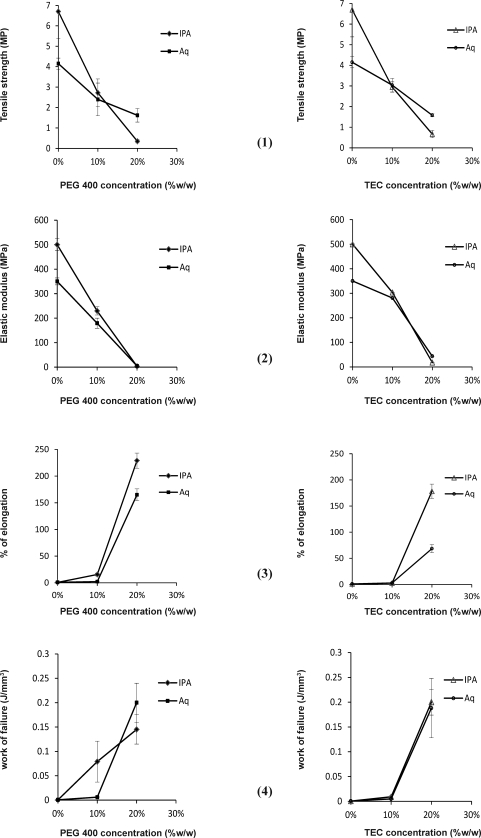
The results of tensile strength, elastic modulus, %elongation and work of failure of the films at different plasticizer concentrations are shown in figures 1-4.
Figure 1-4.
Effects of the type of plasticizer a€nd concentration on: tensile strength (1), elastic modulus (2), %elongation (3), the work of failure (4) of the Eudragit RL films prepared from organic solution (IPA) and aqueous dispersion (Aq).
The ratio of tensile strength to elastic modulus are shown in table 2 and results for tensile strength and elastic modulus of films after curing are shown in table 3.
Table 2.
The ratio of tensile strength to elastic modulus.
| Film type | Tensile strength /Elastic modulus |
|---|---|
| IPA | 0.013 |
| IPA+10%PEG 400 | 0.011 |
| IPA+20%PEG 400 | 0.106 |
| Aq | 0.011 |
| Aq+10%PEG 400 | 0.013 |
| Aq+20%PEG 400 | 0.362 |
| IPA+10%TEC | 0.009 |
| IPA+20% TEC | 0.038 |
| Aq+10%TEC | 0.010 |
| Aq+20%TEC | 0.036 |
Table 3.
Tensile strength and elastic modulus of the films after curing
| Film | Tensile strength (MP) | Elastic modulus (MPa) |
|---|---|---|
| IPA | 3.70±1.2 | 393.0±32.4 |
| IPA+10% PEG 400 | 3.40±1.6 | 204.6±19.9 |
| IPA+20% PEG 400 | 0.12±0.06 | 1.9±1.1 |
| Aq | 2.54±0.98 | 290.0±21.1 |
| Aq+10% PEG 400 | 1.87± 0.45 | 164.4±17.2 |
| Aq+20% PEG 400 | 2.30±1.09 | 8.8±1.4 |
| IPA+10% TEC | 2.30±0.87 | 305.5±25.1 |
| IPA+20% TEC | 0.53±0.16 | 15.1±3.7 |
| Aq+10% TEC | 1.83±0.26 | 255.3±15.1 |
| Aq+20% TEC | 2.50±1.21 | 117.0±25.0 |
An ideal film coating should be hard and tough without being brittle (12). A film with higher ratio of tensile strength to elastic modulus is more desirable due to lower incidence of coating defects (1). There are reports indicating that both plasticizer and casting solvent could influence mechanical properties of some polymeric films (11). The importance of casting solvent on mechanical properties of films could be partly due to its effect on development of internal stresses during drying steps. The higher differences between the volume fraction of solvent at the solidification point of the film and the volume fraction of solvent in an air dried film could lead to build up of more internal stresses in the film (1). This difference is higher for poor solvents.
The results of this study showed that in the absence of plasticizers, mechanical properties of the films prepared from aqueous dispersion are different from those prepared from organic solution of Eudragit RL. Films prepared from organic solution had higher tensile strength and elastic modulus (p<0.05) but lower percent of elongation (p<0.05). Similarly it has been reported that tensile strength of the films casting from organic solution of Eudragit S100 and L100 were significantly higher and its percent of elongation was lower than films casted from aqueous dispersions (11). These results may indicate the stiffer nature and higher resistance to deformation for films prepared from organic solutions. However the low energy required for breaking both types of films indicate the high brittleness and low toughness. The external forces and/or mechanical stresses could results in breaking of the films with high brittleness following small strains (13). In addition the ratio of tensile strength to elastic modulus is very low and nearly the same for both types of the films indicating that both films are unsuitable.
From the results of mechanical testing it appears that all films with no plasticizer were very brittle and therefore addition of plasticizer into their formulations is necessary. Among the additives used in formulation of coating liquids, plasticizers are of prime importance (14).
The results showed that following addition of, 10% PEG 400 into films prepared from organic solutions elastic modulus and tensile strength decreased while percent of elongation and work of failure increased significantly (p<0.05). Similar results were observed for films prepared from aqueous dispersion but the effect of plasticizers was more pronounced for films from organic solutions. It has been reported that inclusion of triacetin into the Eudragit E100 films also decreased the tensile strength and elastic modulus of the films (15).
A comparison between two types of films at 10% PEG 400 level showed that the differences between mechanical properties of the films prepared from organic solutions and aqueous dispersion where reduced.
Increase in concentration of PEG 400 to 20% decreased the tensile strength and elastic modulus and increased the percent of elongation and work of failure dramatically for both types of the films (p<0.05). The ratio of tensile strength to elastic modulus also increased significantly indicating an obvious improvement in mechanical properties of films. A comparison of two types of films revealed that at high PEG 400 concentration, films, prepared from aqueous dispersion of the polymer have similar mechanical properties from the point of view of tensile strength and elastic modulus and even better properties when work of failure and the ratio of tensile strength to elastic modulus are considered.
Addition of 10% TEC as a plasticizer into Eudragit RL films produced similar changes in mechanical properties of the films. However the changes in mechanical properties of the films were less than that observed following addition of 10% PEG 400. Therefore by comparing two plasticizers it may be concluded that for each film at 10% plasticizer level, PEG 400 has been more effective than TEC. The ratios of tensile strength to elastic modulus of the films were also higher for films prepared with PEG 400 as plasticizer. It has been reported that the plasticizing effect of PEG 400 on Eudragit RS films was due to the interaction between carbonyl groups in trimethylammonioethyl methacrylate chloride segment of Eudragit RS and hydroxyl groups of PEG 400 (16). The potential for interaction between Eudragit RL and PEG 400 would be even more due to higher percentage of trimethylammonioethyl methacrylate chloride segment in Eudragit RL.
An interesting finding of this study was that in films without plasticizer, differences between mechanical properties of the Eudragit RL films prepared from organic solutions and aqueous dispersion was noticeable. For example at 10% TEC, films prepared from organic solutions were stronger than those prepared from aqueous dispersions. The same results could be observed for films containing 10% PEG 400. However these differences were reduced by increase in plasticizer concentration in the way that at 20% plasticizer levels both types of films showed similar mechanical properties.
The effect of curing at 60°C for 24 hrs on tensile strength and elastic modulus of the films was also investigated. When polymeric films prepared from aqueous dispersions are stored at temperatures higher than their glass transition temperature (Tg) the polymeric particles would coalesces and therefore a uniform film could be obtained (17). It has been reported that tensile strength and elastic modulus of the films are affected more than those of percent of elongation and work of failure by curing process and in most cases the elastic modulus and tensile strength of the films increased following curing (12).
The time required for formation of physically stable films depends on several factors such as type and concentration of plasticizer and also the temperature at which the films are stored (18).
The tensile strength and elastic modulus of the films after curing (Table 3) and its comparison with their corresponding values before curing showed that curing resulted in decrease in tensile strength and elastic modulus of films prepared from either organic solutions or aqueous dispersion with no plasticizer. The more brittle nature of the films with no plasticizer after curing could be attributed to evaporation of residual solvent or vehicle in the films at 60°C and development of internal stresses (19) which could not be relieved due to absence of plasticizer and therefore result in weaker films.
For films with 10% PEG 400 or TEC and films with 20% plasticizer prepared from organic solution curing did not have any remarkable effect on tensile strength and elastic modulus of the films. However for films prepared from aqueous dispersion and containing 20% plasticizer tensile strength and elastic modulus increased significantly (p<0.05) after curing. The results are in agreement with those reported for ethylcellulose aqueous dispersion (12) and Eudragit RS films (7). These results could be explained by interdiffusion of polymeric chains at 60°C for films prepared from aqueous dispersion of Eudragit RL and containing 20% plasticizer. However films prepared from Eudragit RL 30D with 10% plasticizer concentration did not show any significant difference in tensile strength and elastic modulus after curing. The higher concentration of plasticizer in films with 20% plasticizer level could provide the possibility of further gradual coalescence of the polymeric film at curing temperature for the time period used. Study on the effect of time and temperature on the elongation of films consisting of a 1:1 blend of Eudragit NE 30D/RS 30D showed that for films stored at 25°C for a period of one month no significant difference could be found in percent of elongation. However, films stored at 40°C exhibited a significant decrease in percent of elongation over one month testing period (18).
CONCLUSION
Overall the mechanical properties of Eudragit RL films in absence of plasticizer was dependent on solvent or vehicle and films prepared from organic solution were stronger than those prepared from aqueous dispersion. However in films containing plasticizer differences were not remarkable. The PEG 400 was a better plasticizer for Eudragit RL films.
ACKNOWLEDGEMENT
Financial support of vice chancellor for research of Mashhad University of Medical Sciences is greatly appreciated. The results presented here was a part of a Pharm.D. student thesis proposal.
REFERENCES
- 1.Porter SC., Felton LA. Techniques to assess film coatings and evaluate film-coated products. Drug Dev Ind Pharm. 2010;36:128–142. doi: 10.3109/03639040903433757. [DOI] [PubMed] [Google Scholar]
- 2.Kucera H., Shah NH., Malick AW., Infeld MH., McGinity JW. Influence of an acrylic polymer blend on the physical stability of film-coated theophylline pellets. AAPS PharmSciTech. 2009;10:865–871. doi: 10.1208/s12249-009-9275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaessl B., Siepmann F., Tucker I., Siepmann J., Rades T. Characterisation of quaternary polymethacrylate films containing tartric acid, metoprolol free base or metoprolol tartrate. Euro J Pharm Biopharm. 2009;73:366–372. doi: 10.1016/j.ejpb.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Bodmeier R., Paeratakul O. Dry and wet strength of polymeric films prepared from an aqueous colloidal polymer dispersion, Eudragit RS30D. Int J Pharm. 1993;96:129–138. [Google Scholar]
- 5.Lecomte F., Siepmann J., Walther M., MacRae R.J., Bodmeier R. Polymer blends used for the coating of multiparticulates: comparison of Aqueous and organic coating technique. Pharm Res. 2004;21:882–890. doi: 10.1023/b:pham.0000026443.71935.cb. [DOI] [PubMed] [Google Scholar]
- 6.Nimkulrat S., Suchiva K., Phinyocheep P., Puttipipatkhachorn S. Influence of selected surfactants on the tackiness of acrylic polymer films. Int J Pharm. 2004;287:27–37. doi: 10.1016/j.ijpharm.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharjya S., Wurster DE. Investigation of the drug release and surface morphological properties of film-coated pellets, and physical, thermal and mechanical properties of free films as a function of various curing conditions. AAPS Pharm Sci Tech. 2008;9:449–457. doi: 10.1208/s12249-008-9058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris M.R., Ghebre-Sellassie I. A water based coating process for sustained release. Pharm. Technol. 1986;10:102–107. [Google Scholar]
- 9.-Iyer U., Hong WH., Das N., Ghebre-Sellassie I. Comparative evaluation of three organic solvent and dispersion-based ethylcellulose coating formulations. Pharm. Technol. 1990;14:68–86. [Google Scholar]
- 10.Rudolph MW., Klein S., Beckert TE., Petereit HU., Dressman JB. A new 5-aminosalicylic acid multi-unit dosage form for the therapy of ulcerative colitis. Eur J Pharm Biopharm. 2001;51:183–190. doi: 10.1016/s0939-6411(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 11.Bando H., McGinity W. Physicochemical properties of enteric films prepared from aqueous dispersions and organic solutions. Int J Pharm. 2006;313:43–48. doi: 10.1016/j.ijpharm.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Heng PWS., Chan LW., Ong CKT. Influence of storage conditions and type of plasticizers on ethylcellulose and acrylate films formed from aqueous dispersions. J Pharm Pharmaceut Sci. 2003;6:334–344. [PubMed] [Google Scholar]
- 13.Gal A., Nussinovitch A. Plasticizers in the manufacture of novel skin-bioadhesive patches. Int J Pharm. 2009;370:103–109. doi: 10.1016/j.ijpharm.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Zelko R., Orban A., Suvegh K., Riedl Z., Racz I. Effect of plasticizer on the dynamic surface tension and the free volume of Eudragit systems. Int J Pharm. 2002;244:81–86. doi: 10.1016/s0378-5173(02)00317-4. [DOI] [PubMed] [Google Scholar]
- 15.Elgindy N., Samy W. Evaluation of the mechanical properties and drug release of cross-linked Eudragit films containing metronidazole. Int J of Pharm. 2009;10:10–16. doi: 10.1016/j.ijpharm.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Fujimori J., Yoshihashi Y., Yonemochi E., Terada Application of Eudragit RS to thermo- sensitive drug delivery systems:II. Effect of Eudragit RS/PEG400 blend polymers. J Control Rel. 2005;102:49–57. doi: 10.1016/j.jconrel.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Wurster DE, Bhattacharjya S, Flanagan DR. Effect of curing on water diffusitives in acrylate free films as measured via a sorption technique. AAPS PharmSciTech. 2007:E1–E6. doi: 10.1208/pt0802036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucera S., Shah N.H., Malick A.W., Infeld M.H, McGinity J.w. Influence of an acrylic polymer blend on the physical stability of film-coated theophylline pellets. AAPS PharmSciTech. 2009;10:864–871. doi: 10.1208/s12249-009-9275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe RC. Correlations between the in-situ performance of tablet film coating formulations based on hydroxypropyl methylcellulose and data obtained from tensile testing of free films. Acta Pharm Technol. 1983;29:205–207. [Google Scholar]