Abstract
Achillea L. (Compositae or Asteraceae) is a widely distributed medicinal plant throughout the world and has been used since ancient time. Popular indications of the several species of this genus include treatment of wounds, bleedings, headache, inflammation, pains, spasmodic diseases, flatulence and dyspepsia. Phytochemical investigations of Achillea species have revealed that many components from this genus are highly bioactive. There are many reports on the mentioned folk and traditional effects. Although, the medicinal properties of Achillea plants are recognized worldwide, there are only one review article mainly about the structures of the phytochemical constituents of Achillea. The present paper reviews the medicinal properties of various species of Achillea, which have been examined on the basis of the scientific in vitro, in vivo or clinical evaluations. Various effects of these plants may be due to the presence of a broad range of secondary active metabolites such as flavonoids, phenolic acids, coumarins, terpenoids (monoterpenes, sesquiterpenes, diterpenes, triterpenes) and sterols which have been frequently reported from Achillea species.
Keywords: Achillea, Asteraceae, Bioactive compounds.
INTRODUCTION
The genus Achillea L. belongs to Asteraceae (Compositae), the largest family of vascular plants. Asteraceaeous plants are distributed throughout the world and most common in the arid and semi-arid regions of subtropical and lower temperate latitudes. Achillea contains around 130 flowering and perennial species and occurs in Europe and temperate areas of Asia and a few grow in North America. These plants typically have hairy and aromatic leaves and flat clusters of small flowers on the top of the stem. Since these flowers have various colors, a number of species are popular garden plants (1–4). The basic chromosome number of this genus is X=9 and most of the species are diploid with great ecological ranges from desert to water-logged habitats (5).
The name of Achillea is referred to the Achilles in the literary Trojan War of the Iliad who used yarrow to treat the soldiers’ wounds (6). The majority of the Achillea species are as the medicinal plants which have therapeutic applications (4). There are few review papers on the different aspects of Achillea as a noteworthy and medicinal genus. Recently, Si and co-authors (7) published a review article mainly about the structures of phytochemical constituents and a brief section of biological properties of Achillea (7). Literature reviews show that there are many reports on pharmacological, immunological, biological and other therapeutic activities of these valuable herbs which are reviewed in this article.
Traditional usages
Since Achillea genus is widespread all over the world, its species have been used by local people as folk or traditional herbal medicines. Bumadaran is a popular name for several species of Achillea in Persian language. They are reported as tonic, anti-inflammatory, anti-spasmodic, diaphoretic, diuretic and emmenagogic agents and have been used for treatment of hemorrhage, pneumonia, rheumatic pain and wounds healing in Persian traditional literature (8, 9).
In Spanish-speaking New Mexico and southern Colorado, A. millefolium L. is called plumajillo, or “little feather”, because of the shape of the leaves. Native Americans and early settlers used yarrow for its astringent qualities that made it effective in wound healing and anti-bleeding (10).
Achillea species are the most important indigenous economic plants of Anatolia. Herbal teas prepared from some Achillea species are traditionally used for abdominal pain and flatulence in Turkey (11). Dioscorides also used Achillea for dysentery, whether associated with cholera or other causes, which killed as many soldiers as did steel and lead. In terms of Chinese medicine, Achillea can be said to have three main actions: clear Exterior Wind (diaphoretic), Tonify Deficiency (tonic) and clear Heart Phlegm (anti-hypertention) (12).
Many of these therapeutic usages have been confirmed by new experimental and clinical studies. The consumption of herbal teas from different species of Achillea, especially for treatment of the gastrointestinal tract, is common in folk medicine (13). However, there are still several unknown aspects of Achillea plants that need more attention.
Phytochemical constituents
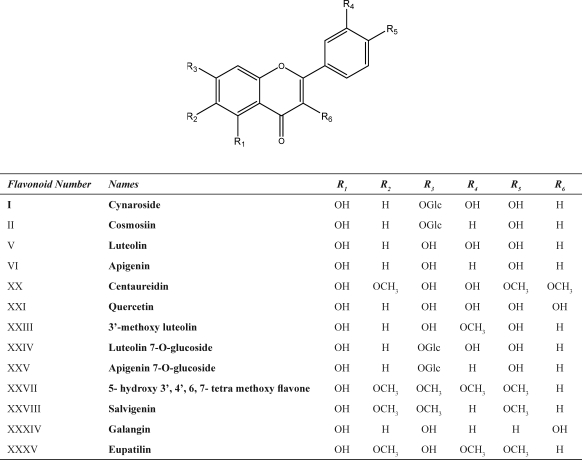
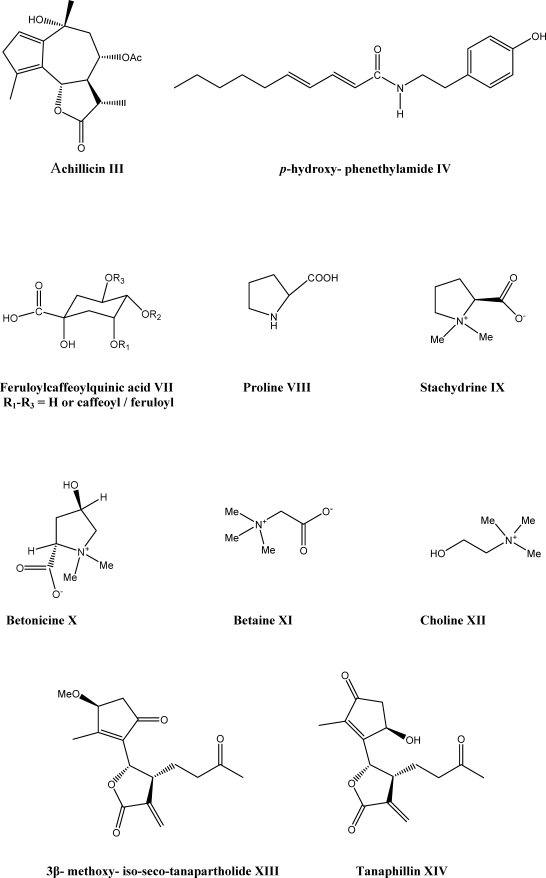
Phytochemical investigations of Achillea species have revealed that many components from this genus are highly bioactive. The first anti-spasmodic flavonoids, cynaroside I and cosmosiin II (Scheme 1) were isolated from A. millefolium L. (14), and the first natural proazulene, achillicin III (Scheme 2) was identified from the genus Achillea (15). Literature search shows that the, flavonoids, terpenoids, lignans, amino acid derivatives, fatty acids and alkamides such as p-hydroxyphenethylamide IV (Scheme 2) have been identified in Achillea species. The main constituents of the most species have been previously reviewed (7). Therefore, in this article some other minor or rare compounds and especially their medicinal or industrial usages which have been less described are reviewed. Among them,alkamides, the lipophilic and nitrogen containing compounds, are responsible for insecticide, anti-inflammation and some immunological activities of Achillea and Echinacea plants (16). The genus Achillea comprises flavored species which produce intense essential oils. The volatile oils of Achillea contain monoterpenes as the most representative metabolites. However, there are reports on high levels of sesquiterpenes compared with monoterpenes (17, 18). There are several pharmacological actions which have been mostly attributed to the presence of azulenogenous sesquiterpene lactones in the essential oil of Achillea. Results of studies have indicated that tetraploid species are accumulating proazulenes such as achillicin III (Scheme 2) (19).Except for the essential oil constituents, yarrow (A. tenuifolia Lam.) seeds consist of the high oil content which is rich in linoleic acid, an essential polyunsaturated fatty acid. This makes yarrow seed as a potential source of edible oil for human consumption (20). Recently, A. millefolium has been introduced as a new source of natural dye for wool dyeing due to the presence of the flavonoids, luteolin V and apigenin VI (Scheme 1). A. millefolium was found to have good agronomic potential as a natural dye in Iran (21). In the plant kingdom, hydroxycinnamoyl conjugates of quinic acid represent common end metabolites of the shikimate-phenylpropanoid pathway, and feruloylcaffeoylquinic acid derivates VII have been isolated only from two species of genus Achillea so far (22). From the aerial parts of Achillea species, proline VIII, stachydrine IX, betonicine X, betaine XI and choline XII have been isolated as the major nitrogen containing compounds (Scheme 2) (23, 24). Betaines, containing the permanent positive charge on the quaternary ammonium moiety, belong to an important class of naturally occurring compounds that function as compatible solutes or osmoprotectants (25). These compounds have shown immunosuppressive activity in the experimental animals (26, 27).
Scheme 1.
Structures of the isolated flavonoids from various species of Achillea.
Scheme 2.
Structures of the isolated terpenoids amins and phenolic compounds from the various species of Achillea.
Medicinal properties of Achillea species Wound healing activity
Nowadays, the traditional usage of medicinal plants for wound healing has received attention by the scientific community (28). Wound healing is a complex process characterized by homeostasis, re-epithelization, and granulation tissue formation and remodeling of the extracellular matrix. Medicinal plants may affect various phases of the wound healing process, coagulation, inflammation and fibroplasia (29). Aqueous extract of the flowers of A. kellalensis Boiss. & Hausskn., applied topically, has shown significant wound healing activity in rats. The wound sizes of the test compared to control groups were reduced faster (30).
Protective activity
The protective activity of natural antioxidants in biological systems has received attention. Some medicinal plants have proved free radical scavenging or antioxidant activities (31). The infusions of Achillea species were tested on antioxidant enzyme systems of erythrocytes and A. falcata L. was the most effective one against CAT (catalase), GPx (glutathione peroxidase) and SOD (superoxide dismutase) enzyme systems of erythrocytes. Among the plant infusions, highest activities on leucocyte enzymes were by A. crithmifolia Waldst. & Kit. and A. nobilis L. subsp. neilrechii on CAT, by A. millefolium subsp. pannonica on SOD, by A. teretifolia Willd. on GPx and by A. nobilis subsp. sipylea on LPO (lactoperoxidase). Therefore, Achillea species may be of potential sources of natural antioxidants for treatment or prevention of related diseases (32).
The influence of the extracts of A. alexandri-regis Bornm. & Rudsky on hydroxyl and superoxide radicals’ quantity in different in vitro systems have been determined. The ethyl acetate extract exhibited hydroxyl radical scavenging activity in all tested biological systems (liver homogenate, hemolyzed blood, serum and post mitochondrial liver fraction), whereas butanol extract reduced hydroxyl radicals significantly only in the post mitochondrial liver fraction (a homogenate of liver cells remaining after sedimentation of the mitochondrial fraction by centrifugation). Both extracts affected only hemolysed blood (33).
The hydroalcoholic extract of A. santolina L. was studied on various in vitro antioxidative systems and it has been reported that the extract prevented formation of thiobarbituric acid reactive substances in Fe2+ascorbate induced lipid peroxidation in rat liver tissue. Free radical induced protein oxidation has also been suppressed significantly by high concentration (1000 µg/ml) of the extract (34). Ethanol extracts of eight wild samples of A. ligustica All., and one sample of cultivated A. millefolium were evaluated for radical scavenging activites including DPPH test. The TEAC (the concentration of a Trolox solution having an antioxidant capacity equivalent to that of the diluted hydroalcoholic extract) were in the range of 4.18 and 12.3 mM. The ability of the extracts to inhibit non-enzymatic lipid peroxidation using an in vitro system of linoleic acid oxidation has been investigated. Five of the nine extracts had a protective effect at the lowest tested amount (5 µg). Protection on CaCo-2 intestinal cells against TBH-induced toxicity was also investigated and two of the tested ethanolic extracts of A. ligustica showed protection against the oxidative stress (35). The antioxidant capacity and cytoprotective activity of A. collina Becker ex Rchb. infusions against oxidative stress were investigated by chemical (DPPH and Folin Ciocalteu assay) and biological assays (in vitro model of cytotoxicity and lipid peroxidation in PC12 cells line) and it has been shown that the infusions of leaves had the highest antioxidant and cytoprotective activity, where antioxidant capacity was significantly correlated with the total phenolic content but not with the cytoprotective profile (36).
Esterogenic activity
A. millefolium is used in folk medicine as an emmenagogue (8). A crude extract of the aerial parts of A. millefolium has shown estrogenic activity based on recombinant MCF-7 cells (37, 38). Evaluation of the isolated and identified compounds from this plant indicated that luteolin V and apigenin VI (Scheme 1) were the most important estrogenic compounds among tested compounds. Apigenin can also stimulate ERs-dependent biological pathways, but less than the endogenous hormone. Both α and β receptors of estrogen could be activated by apigenin. Luteolin seems to have a very slight effect on β and does not seem to activate α receptor at all, while many phytoestrogens appear to have a stronger binding affinity with β estrogen receptors than estradiol (39).
Anti-diabetic activity
Oxidative stress is produced under diabetic condition and is likely involved in progression of pancreatic damage in diabetes. The effect of A. santolina (hydro alcoholic extract) on blood glucose level, serum NO (nitric oxide) concentration and the oxidative stress in rat pancreatic tissue have been evaluated. This herbal treatment could reduce blood glucose level, serum NO, pancreatic MDA (Malondialdehyde), PCO (Protein Carbonyls) and AOPP (Advanced Oxidation Protein Products) levels. In addition, the content of GSH (Reduced Glutathione) was restored to the normal level of the control group. Furthermore, CAT and SOD activities in the treated rats were increased significantly. In conclusion, A. santolina have a high hypoglycemic activity which may be due to its antioxidative potential (40).
Antispermatogenic effect
Ethanolic (intraperitoneally) and hydroalcoholic extracts (orally) of A. millefolium were administered to Swiss mice to evaluate the effect on spermatogenesis. Observation of morphological characteristics using light and electron microscopes revealed exfoliation of immature germ cells, germ cell necrosis, and seminiferous tubule vacuolization. The extract treated animals had an increased number of metaphases in the germ epithelium which should be due to substances stimulating cell proliferation (41) .
Antiulcer activity
A. millefolium is a widespread medicinal plant used in folk medicine to treat inflammation, pain and gastrointestinal disorders. Screening of gastroprotective potential against acute and chronic ulcers has shown positive correlation with its uses in folk medicinal. The aqueous extract of A. millefolium showed effectiveness in protecting the gastric mucosa against acute gastric lesions induced by ethanol and indomethacin and in healing chronic gastric lesions induced by acetic acid (ED50= 32 mg/kg, orally). Reviewing literature reveals that the antiulcer potential of A. millefolium is not accompanied by any sign of toxicity even by long chronic exposure. Oral administration (30, 100 and 300 mg/kg) of the hydroalcoholic extract inhibited ethanol-induced gastric lesions by 35, 56 and 81%, respectively. Oral treatment with this extract (1 and 10 mg/kg) reduced the chronic gastric ulcers induced by acetic acid by 43 and 65%, respectively, and promoted significant regeneration of the gastric mucosa after ulcer induction denoting increased cell proliferation (42, 43). It has been reported that A. millefolium protected rats against ulcers induced by ethanol and restraint-in-cold-stress, but not against indomethacin induced ulcers. When hot water extract was injected into duodenal lumen it could inhibit the basal acid secretion. It seems that the antiulcer activity of A. millefolium is related either to inhibition of gastric secretion or increase in protective factors (such as blood flow) in gastric mucosa. Anyhow, further study is required to clarify the mechanism of action (44). There are some reports on gastrointestinal effects of Achillea, such as antiulcer, antibacterial, hepatoprotective, choleretic, and antispasmodic. The effects of aqueous ethanol extract of A. wilhelmsii on rat's gastric acid output in basal, vagotomized (VX), and vagal-stimulated conditions have been investigated. Result of study showed that introduction of one milliliter of 3 doses (0.5, 1, and 2 mg/kg) A. wilhelmsii C. Koch into the stomach of each rat in the test group compared with introduction of the same volume of saline in the control group resulted in an inhibitory effect on acid output in basal condition. The inhibitory effect of the extract (at doses 1 and 2 mg/kg) was exerted via gastric vagal parasympathetic nerve. At VX condition, not only this inhibitory effect on acid output disappeared, but also the acid output significantly increased. The extract showed a reduction in the acid output in vagal-stimulated condition at doses of 1 and 2 mg/kg, which were not statistically significant (45).
Cytotoxicity effect
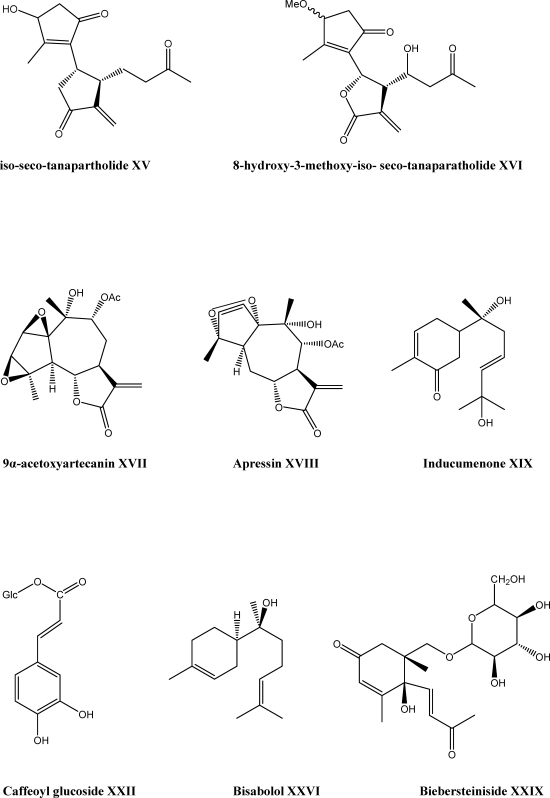
There are some reports about the anti-proliferative activity of the isolated constituents from A. falcata and A. clavennae. L.Four sesquiterpene lactones have been isolated from A. falcata, which had significant ability to inhibit HaCaT-cell growth and identified as 3β-methoxy-iso-seco-tanapartholide XIII, tanaphillin XIV, iso-seco-tanapartholide XV, and 8-hydroxy-3-methoxy-iso-seco-tanaparatholide XVI. These compounds have been found to decrease keratinocyte cell viability significantly (Scheme 2). Statistical analyses confirmed an enhanced potency of the β-OH iso-seco-tanapartholide over the α,β-OH diastereoisomeric mixture. The enhancement of the lipophilicity of the molecule resulted in the highest potency (46). The aerial part of A. clavennae was used for isolation of the phytoconstituents and the antiproliferative activity of the compounds was tested to HeLa, K562 and Fem-X human cancer cell lines. Guaianolides, 9α-acetoxyartecanin XVII and apressin XVIII showed significant cytotoxic effects in all tested cell lines. A bisabolene, inducumenone XIX exhibited a moderate activity (Scheme 2). The most active compound was a flavonol, centaureidin XX (Scheme 1), which was already known as cytotoxic agent (47).
Immunosuppressive activity
The aqueous extract of A. talagonica Bioss. was studied on humoral antibody responses in BALB/c mice and albino rabbits. Intraperitoneal administration of the extract to mice, prior to immunization with sheep red blood cells, resulted in a significant dose dependent decrease in haemagglutinating antibody (HA) titer. In rabbits after intrascapular injection of the extract, a significant decrease in typhoid-H antibody (anti-HD) titer was found, but no change was observed in secondary response (48).
Methanol and aqueous methanol (80% and 50% v: v) extracts of A. talagonica have been examined to find its immunosuppressive components. Guided by anti-SRBC (sheep red blood cells) assay, active principles were isolated by chromatographic methods and identified as choline XII (Scheme 2), quercetin XXI (Scheme 1) and caffeoyl glucoside XXII (Scheme 2). These compounds compared to the control groups decreased anti-SRBC titer significantly. Alongside these compounds, 3’-methoxy luteolin XXIII (Scheme 1) and proline VIII (Scheme 2) has been also reported from this plant (49).
Methanol extract and some other fractions of A. millefolium were studied on humoral immunity in BALB/c mice by microhaemagglutination test. Only two fractions showed a significant decrease in the anti- SRBC titer of mice. The immunological properties may be related to presence of glycosylated derivatives of caffeic acid, because caffeic acid glucoside XXII (Scheme 2) was isolated and identified from the active fractions. Some known compounds including, luteolin 7-O-glucoside XXIV and apigenin 7-O-glucoside XXV (Scheme 1) have also been reported from this species (50).
Effects of the essential oils of A. talagonica and A. millefolium have been studied on humoral immune responses in BALB/c mice. The oil isolated from A. millefolium ssp. millefolium possessed a high percentage of sesquiterpenes (55.4%) in which bisabolol XXVI (Scheme 2) was the main compound. The volatile oil of A. millefolium decreased the anti-SRBC antibody titer, but the oil of A. talagonica was not effective. High percentage of sesquiterpenes and presence of proazulene in A. millefolium together with the lack of these compounds in A. talagonica could account for the different immunological effects of these plants (51).
Biological effects
Ethyl acetate extract of A. talagonica showed toxicity in BST (brine shrimp lethality test) and on the basis of results only 5- hydroxy 3’, 4’, 6, 7- tetra methoxy flavone XXVII (Scheme 1) showed toxicity (LC50=15 µg/ml) against Artemia salina larvae. Another separated flavonoid named salvigenin XXVIII (Scheme 1) showed no activity (52).
It is reported that the essential oil of A. biebersteinii Afan. exhibited antimicrobial activity against 8 bacteria, 14 fungi and one yeast namely C. albicans, whereas methanolic extract was inactive (53). The antimicrobial activity of the essential oil of A. ligustica was evaluated by the broth micro-dilution method on 6 microbial strains and it showed to be effective against Streptococcus mutans (54). In another report, antibacterial activity of the extracts (hexane: ether: methanol=1:1:1) of the aerial parts of A. clavennae, A. holosericea Sm., A. lingulata and A. millefolium were evaluated against five bacteria (S. aureus, E. coli, K. pneumoniae, P. aeruginosa and Salmonella enteritidis) and two fungi (A. niger and C. albicans) and it was found that the extracts of all four species possessed a broad spectrum of antimicrobial activity against all tested strains (55). Recently, the oil of A. millefolium was evaluated on heterozygous diploid strain of Aspergillus nidulans, with green conidia and a significant increase in the number of yellow and white mitotic recombinants (per colony) of the diploid strain was observed when it was treated with 0.19 and 0.25 µl/ml of the oil. The induction of mitotic non-disjunction may cause the genotoxicity (56).
E. coli contains certain strains that can cause resistant infections to antibiotics. Multidrug-resistant E.coli produces extended-spectrum β lactamases (ESBLs) and is an important cause of urinary tract (UTIs) and bloodstream infections. Activity of nineteen Jordanian plants against multidrug-resistant E.coli has been reported. The methanolic extract of A. santolina (one of 19 species) was combined with antibiotics of different classes (chloramphenicol, neomycin, doxycycline, cephalexin and nalidixic acid) and tested against both the standard and resistant strain of E. coli. The results showed that the activity of all tested antibiotics especially doxycycline on the resistant strain was enhanced when it was used in combination with plant material. The enhanced activity of cephalexin against the standard strain has been reported to be higher than resistant strain (57) Also, the extracts of 13 Brazilian medicinal plants were screened for their antimicrobial activity against bacteria (E. coli, P. aeruginosa, B. subtilis and S. aureus) and yeasts (Candida albicans, C. krusei, C. parapsilosis, and C. tropicalis) and the ethanol-water extract (90% v/v) of A. millefolium was considered inactive (58). The in vitro antimicrobial efficacy of 39 water and 39 methanol extracts of 27 indigenous wild plant species that have been commonly used in Lebanese folk medicine has been reported on nine test microorganisms (E. coli, Proteus sp., P. aeruginosa, S. dysenteria, S. enteritidis, S. typhi, S. aureus, S. faecalis, and C. albicans) by the single disk diffusion method. The percentage of test organisms, which were susceptible (20 µl /disc) to methanol extract of A. damascena DC., was 88.8%. The methanol extract of A. damascena showed different efficacy against the tested microorganisms when harvested from two different locations. The MIC of A. damascena range for S. aureus, Proteus sp., and S. dysenteriae were 1-3.5 and for C. albicans, S. enteritidis, and S. faecalis were 1-2.5. These differences were explained by the nature and level of the antimicrobial agents present in the extracts and their modes of actions on the different test microorganisms (59).
In a recent investigation, the in vitro susceptibility of 15 H. pylori strains to botanical extracts was evaluated. The minimum inhibitory concentration (MIC) of the methanol extract of A. millefolium is reported as 50 µg/ml (60).
Besides the antimicrobial effects of Achillea plants, the in vitro anti-epimastigote activity of some extracts of A. biebersteinii and A. millefolium have been reported. Diethyl ether extracts of the above Achillea species showed activity (MLC=12.5 µg/ml) against the epimastigotes of Trypanosoma cruzi, the etiological agent of Chagas disease. Aqueous and methanol extracts were not so effective (61). In another study, the ethyl acetate extracts of A. talagonica and A. tenuifolia showed a moderate activity against the epimastigotes of T. cruzi (62).
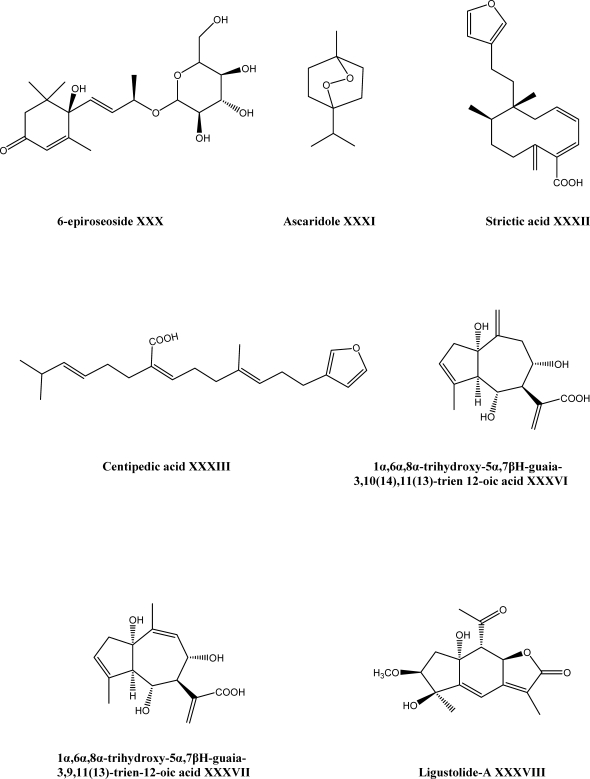
Forty-two Egyptian medicinal plant species were subjected to antiviral screening bioassay to evaluate their biological activities. Hydro-alcoholic extracts of each species were prepared and tested against three viruses, herpes simplex-1 virus (HSV), poliomyelitis-1 virus (POLIO) and vesicular stomatitis virus (VSV). The antiviral activity were determined by means of the end point titration technique (EPTT) that depends on the ability of diluted plant extract to inhibit the produced cytopathogenic effect (CPE) and was expressed as reduction factor (Rf) of the viral titer. A. fragrantissima (Forssk) Sch. Bip. showed the highest antiviral activity (among these species) against POLIO in a concentration dependent manner at complete non-toxic concentration range (10–100 µg/ml) and the highest detected antiviral activity was recorded at Rf of 106. It seems that the interesting antiviral activity of A. fragrantissima against POLIO may be attributed to of essential oil content which has been traditionally used as an antiseptic agent (63). Furthermore, a new ionone glucoside, biebersteiniside XXIX, together with four known compounds 6-epiroseoside XXX, ascaridole XXXI, strictic acid XXXII and centipedic acid XXXIII (Scheme 2) were reported from the aerial parts of A. biebersteinii. The compounds XXX-XXXIII were reported for the first time from A. biebersteinii. Also, antifungal activity was observed from the compounds XXIX and XXXI-XXXIII (64).
Antispasmodic activity
The use of herbal teas from different species of the A. millefolium group against the gastrointestinal disorders, especially as an antispasmodic and anti-inflammatory, is quite common in folk medicine. The antispasmodic effect of A. nobilis subsp. sipylea on rat duodenum has been reported recently. The total of papaverine, but not to that of atropine on the dose-response curves. The extract also reduced the maximal response in curves induced by CaCl2 (in a similar manner to verapamil) (65). The antispasmodic effects of Achillea species might be due to the flavonoid constituents of the plant. Galangin XXXIV, quercetin XXI and eupatilin XXXV (Scheme 1), which are found commonly in Achillea, are reported to cause a potent relaxation of the ileum (66, 67).
The effect of A. millefolium hydro-alcoholic extract on the contractile responses of the isolated guinea-pig ileum at five concentrations ranging from 0.05 to 5 mg/ml has been reported. Changes in contraction of tissues were monitored using force displacement transducer amplifier connected to physiograph. Each segment served as its own control. Results showed that the contractile response was inhibited by extract in a dose-dependent manner (EC50=1.5 mg/ml). Those results demonstrated that in vitro evaluation of A. millefolium extract resulted in inhibition of electrical induced contractions of the guinea-pig ileum (68).
Anti-inflammatory activity
As shown in traditional usage, Achillea species are well known as the anti-inflammatory plants. Besides the alkamides, as the noteworthy active anti-inflammatory compounds (16), sesquiterpenes are introduced as another effective group of the secondary metabolites. After the last review (7) on photochemistry of Achillea, isolation of some other sesquiterpenes have been reported as follows:
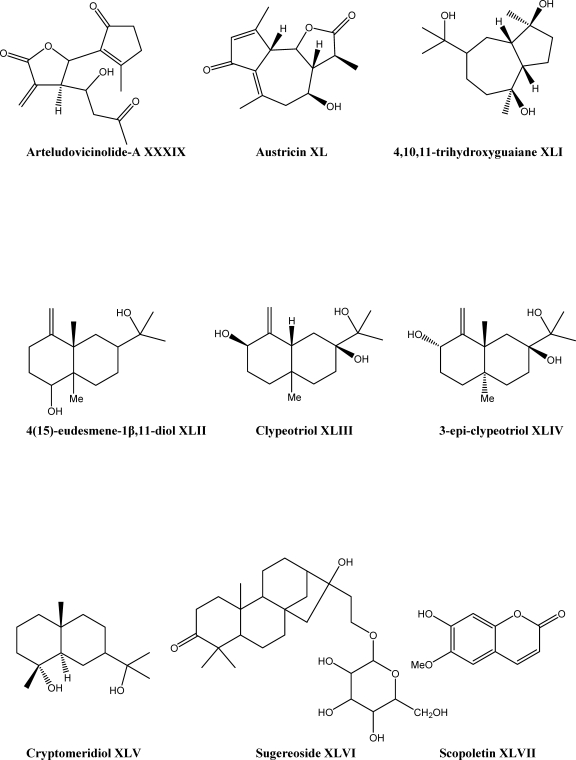
The methylene chloride-methanol extract of aerial parts of A. coarctata was investigated by chromatographic analysis and resulted in isolation of two new guaiane acid derivatives, 1α,6α,8α-trihydroxy-5α,7βH-guaia-3,1014,1113-trien-12-oic acid XXXVI and 1α,6α,8α-trihydroxy-5α,7βH-guaia-3,9,1113-trien-12-oic acid XXXVII, in addition to three known compounds, ligustolide-A XXXVIII, arteludovicinolide-A XXXIX and austricin XL (Scheme 2) (69). They also reported that the compounds XXXVI and XXXVII enhanced the proliferation of beneficial macrophages significantly and compounds XXXVII and XXXIX exhibited anti-inflammatory properties (69). Another article has reported that chromatographic separation on dichloromethane extract of A. clypeolata resulted in one guaiane 4,10,11-trihydroxyguaiane XLI, four eudesmanes 415-eudesmene-1β,11-diol XLII, clypeotriol XLIII, 3-epi-clypeotriol XLIV, cryptomeridiol XLV, one diterpene sugereoside XLVI (Scheme 2) and two phenolics centaureidin XX (Scheme 1) and scopoletin XLVII (Scheme 2). The compounds XLI and XLVI have been reported for the first time (70).
Adverse effects and safety
Adverse reaction of herbal medicines is an important point which needs further systematic investigation. Adverse drug reactions (in association with complementary and alternative medicine substances) have been spontaneously reported therefore, such a data could be used in monitoring the safety of these products. By analyzing such data (in Sweden), it has been found that A. millefolium (in combination with hawthorn, peppermint, and paprika, seed of pumpkin, rosemary and vitamins) showed urticarial and skin reactions which have been poorly documented (71).
Because A. millefolium is effective in protection of gastric mucosa against acute gastric lesions (ED50=32 mg/kg, p.o.), safety studies were performed in female and male Wistar rats by daily treatment with aqueous extract of A. millefolium (0.3-1.2 g/kg, p.o./day) or vehicle (water, 10 ml/kg/day) for 28 or 90 consecutive days. Slight changes in liver weight, cholesterol, HDL-cholesterol and glucose were observed in male and female animals which were not correlated with dose or time of exposure of the animals to the plant (72).
Ethnomedicinal and pharmaceutical usage
There are many botanical remedies, consisting powdered plant material or extracts of Achillea species, which are used for the treatment of skin and soft tissue infections, visceral pain, gastrointestinal disorders and inflammations. Literature review indicated that there is a patent for treatment of dermatose, by topical application of botanical medicinal compounds (from Achillea), eczema, atopic dermatitis, non-allergic dermatitis, psoriasis and rosacea, or any inflammation of the skin (73). A medicinal combination, named Sedospasmil®, for the treatment of chronic colitis was prepared from medicinal plants including A. millefolium, Matricaria chamomillae, Hypericum perforatum and Valeriana officinalis. Normalization of the intestinal functions, tranquilization, spasmolytic and analgesic activity of a combination made with A. millefolium and some other medicinal plants has been reported for this medicine (74). Also, a Chinese medicinal preparation for relieve of pain and inflammation of some medicinal plants including Achillea with gelatin, in the form of ointment, pellicle, or powder for external use is reported. The formulation is suggested to be used for treatment of soft tissue injury, fracture, dislocation, carbuncle furuncle, and gout (75). In addition, a medicine for treatment of hysteromyoma, prepared from A. millefolium together with Inula, Calami, Urtica, Arnica, Capsella and some other medicinal plants, has been reported. The medicine is suggested to be useful for treatment of hysteromyoma, particularly hormone-dependent tumor (76).
CONCLUSION
Achillea has been used in popular medicine for its anti-hemorrhagic, healing, and analgesic properties in the various regions throughout the world. It was used by northern European and North American native people as a contraceptive, abortifacient, and emmenagogue. Some of these traditional and folk usages have been evaluated showing the potential medicinal use of the plant. The medicinal properties of A. millefolium are worldwide recognized and the plant is included in the national Pharmacopoeias of countries such as Germany, Czech Republic, France and Switzerland. As it is reviewed in this paper, antioxidant and protective activity is of various species of Achillea is reported frequently. This might be due to high content of flavonoids and phenolics in these plants. It is noteworthy that oxidative stress is produced under diabetic condition and Achillea plants are considered for high hypoglycemic activity. Among the medicinal properties of Achillea, their cytotoxic and antiulcer effects are important especially when the species contain immunomodulatory constituents. The activity of these plants against different bacteria, fungi and parasites might be due to the presence of a broad range of secondary active metabolites such as flavonoids, phenolic acids, coumarins, terpenoids (monoterpenes, sesquiterpenes, diterpenes, triterpenes) and sterols which have been isolated. Finally, presence of anti-inflammatory compounds such as sesquiterpenes and alkamides is another reason for importance of these plants as the potential source of medicinal compounds and drugs in future.
REFERENCES
- 1.Bremer K. Asteraceae: Cladistics and Classification. Oregon: Timber Press; 1994. [Google Scholar]
- 2.Mozaffarian V. A. Dictionary of Iranian Plant Names. Tehran: Farhang Moaser publisher; 1996. pp. 11–12. [Google Scholar]
- 3.Huber-Morath A. Achillea. In: Rechinger KH, editor. Flora Iranica. No.158. Graz: Ackademiche Druck-U. Verlagsansfalt; 1989. pp. 57–58. [Google Scholar]
- 4.Sheidai M, Azanei N, Attar F. New chromosome number and unreduced pollen formation in Achillea species (Asteraceae) Acta Biol Szegediensis. 2009;53:39–43. [Google Scholar]
- 5.Dabrowska J. The chromosome numbers of several taxa of the genus Achillea L. in relation to the distribution of the genus. Prace Bot. 1992;49:1–83. [Google Scholar]
- 6.Achillea. Index Nominum Genericorum. Int Association for Plant Taxonomy 2006-02-20. http://botany. si.edu/ing/INGsearch.cfm?searchword=Achillea. Retrieved 2008-05-21, access date 2006-02 -20.
- 7.Si XT, Zhang ML, Shi QW, Kiyota H. Chemical Constituents of the Plants in the Genus Achillea . Chem Biodivers. 2006;3:1163–1180. doi: 10.1002/cbdv.200690119. [DOI] [PubMed] [Google Scholar]
- 8.Zargari A. Medicinal Plants. 4th ed. Tehran: Tehran University Publication; 1996. pp. 106–117. [Google Scholar]
- 9.Saeidnia S, Gohari AR, Yassa N, Shafiee A. Composition of the volatile oil of Achillea conferta DC, from Iran. Daru. 2005;13:34–36. [Google Scholar]
- 10.Dodson C, Dunmire WW. Mountain Wildfowers of the Southern Rockies. University of New Mexico Press; 2007. [Google Scholar]
- 11.Honda G, Yesilada E, Tabata M, Sezik E, Fujita T, Takeda Y, Takaishi Y, Tanaka T. Traditional medicine in Turkey VI. Folk medicine in West Anatolia: Afyon, Kutahya, Denizli, Mugla, Aydin provinces. J Ethnopharm. 1996;53:75–87. doi: 10.1016/S0378-8741(96)01426-2. [DOI] [PubMed] [Google Scholar]
- 12.Ross J. Combining Western Herbs and Chinese Medicine: Principles, Practice, and Materia Medica. Seattle: Greenfields Press; 2003. pp. 165–181. [Google Scholar]
- 13.Skocibusic M, Bezic N, Dunkic V, Radonic A. Antibacterial activity of Achillea clavennae essential oil against respiratory tract pathogens. Fitoterapia. 2004;75:733. doi: 10.1016/j.fitote.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Falk A, J, Smolenski S, J, Bauer L, Bell C L. Isolation and identification of three new flavones from Achillea millefolium L. J Pharm Sci. 1975;64:1838–1842. doi: 10.1002/jps.2600641119. [DOI] [PubMed] [Google Scholar]
- 15.Cuong BN, Eszter GB, Lajos R, Jozsef T, Kalman U, Gizella VP. Achillicin. the first proazulene from Achillea millefolium . Phytochemistry. 1979;18:331. [Google Scholar]
- 16.Greger H. Alkamides: Structural relationship, distribution and biological activity. Planta Med. 1984;50:366–375. doi: 10.1055/s-2007-969741. [DOI] [PubMed] [Google Scholar]
- 17.Nemeth E. Essential oil composition of species in the genus Achillea . J Essent Oil Res. 2005;17:501–512. [Google Scholar]
- 18.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils-a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 19.Radusiene J, Gudaityte O. Distribution of proazulenes and productivity in Achillea millefolium L. spontaneous populations. Botucatu: Rev Bras Pl Med. 2006;8:155–158. [Google Scholar]
- 20.Goli SAH, Rahimmalek M, Tabatabaei BES. Physicochemical characteristics and fatty acid profile of yarrow (Achillea tenuifolia) seed oil. Int J Agric Biol. 2008;10:355–357. [Google Scholar]
- 21.Kiumarsi A, Abomahboub R, Rashedi SM, Parvinzadeh M. Achillea Millefolium, a new source of natural dye for wool dyeing. Prog. Color Colorants Coat. 2009;2:87–93. [Google Scholar]
- 22.Radulovic N, Stojanovic G, Asakawa Y. Hydroxycinnamoyl conjugates from the roots of Achillea holosericea Sibth. Et Sm. Biochem Syst Ecol. 2006;34:83–87. [Google Scholar]
- 23.Mehlfuhrer M, Troll K, Jurenitsch J, Auer H, Kubelka W. Betaines and free proline within the Achillea millefolium group. Phytochemistry. 1997;44:1067–1069. [Google Scholar]
- 24.Saeidnia S, Gohari AR, Yassa N. A search for betaine compounds within the Iranian Achillea . Int J Biol Biotech. 2004;1:719–720. [Google Scholar]
- 25.Wood KV, Bonham CC, Miles D, Rothwell AP, Peel G, Wood BC, Rhodes D. Characterization of betaines using electrospray MS/MS. Phytochemistry. 2002;59:759–765. doi: 10.1016/s0031-9422(02)00049-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Warskulat U, Haeussinger D. Modulation of tumor necrosis factor-( release by anisoosmolarity and betaine in rat liver macrophages (Kupffer cells) FEBS Lett. 1996;391:293–296. doi: 10.1016/0014-5793(96)00754-5. [DOI] [PubMed] [Google Scholar]
- 27.Hamidi H, Jahanian R, Pourreza J. Effect of dietary betaine on performance, immunocompetence and gut contents osmolarity of broilers challenged with a mixed coccidial infection. Asian J Anim Vet Adv. 2010;5:193–201. [Google Scholar]
- 28.Houghton PJ, Hylands PJ, Mensah AY, Hensel A, Deters AM. In vitro tests and ethnopharmacological investigations: wound healing as an example. J Ethnopharmacol. 2005;100:100–107. doi: 10.1016/j.jep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Priya KS, Gnanamani A, Radhakrishnan N, Babu M. Healing potential of Datura alba on burn wounds in albino rats. J Ethnopharm. 2002;83:193–199. doi: 10.1016/s0378-8741(02)00195-2. [DOI] [PubMed] [Google Scholar]
- 30.Pirbalouti AG, Koohpayeh A, Karimi I. The wound healing activity of flower extracts of Punica granatum and Achillea kellalensis in Wistar rats. Acta Pol Pharm. 2010;67:107–110. [PubMed] [Google Scholar]
- 31.Mantle D, Eddeb F, Pickering AT. Comparison of relative antioxidant activities of British medicinal plant species in vitro. J Ethnopharm. 2000;72:47–51. doi: 10.1016/s0378-8741(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 32.Konyalioglu S, Karamenderes C. The protective effects of Achillea L. species native in Turkey against H2O2-induced oxidative damage in human erythrocytes and leucocytes. J Ethnopharm. 2005;102:221–227. doi: 10.1016/j.jep.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Kundakovic T, Mimica Dukic N, Kovacevic N. Free radical scavenging activity of Achillea alexandriregis extracts. Fitoterapia. 2005;76:574–576. doi: 10.1016/j.fitote.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chemistry. 2007;104:21–29. [Google Scholar]
- 35.Tuberosoa CIG, Montoro P, Piacente S, Corona G, Deiana M, Assunta Dessi M, Pizza C, Cabras P. Flavonoid characterization and antioxidant activity of hydroalcoholic extracts from Achillea ligustica All. J Pharmaceut and Biomed Anal. 2009;50:440–448. doi: 10.1016/j.jpba.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 36.Giorgi A, Bombelli R, Luini A, Speranza G, Cosentino M, Lecchini S, Cocucci M. Antioxidant and cytoprotective properties of infusions from leaves and inflorescences of Achillea collina Becker ex Rchb. Phytother Res. 2009;23:540–545. doi: 10.1002/ptr.2679. [DOI] [PubMed] [Google Scholar]
- 37.Chandler RF, Hooper SN, Hooper DL, Jamieson WD, Flinn CG, Safe LM. Herbal remedies the maritime Indians: sterols and triterpenes of Achillea millefolium L. (yarrow) J Pharm Sci. 1982;71:690–693. doi: 10.1002/jps.2600710621. [DOI] [PubMed] [Google Scholar]
- 38.Schulz V, Hansel R, Tyler VE. Rational Phytotherapy: A Physician's guide to Herbal Medicine. Berlin: Springer; 2001. p. 294. [Google Scholar]
- 39.Innocentia G, Vegetob E, Dall-Acquaa S, Cianab P, Giorgettia M, Agradib E, Sozzib A, Ficoc G, Tomec F. In vitro estrogenic activity of Achillea millefolium L. Phytomedicine. 2007;14:147–152. doi: 10.1016/j.phymed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Yazdanparast R, Ardestani A, Jamshidi S. Experimental diabetes treated with Achillea santolina: Effect on pancreatic oxidative parameters. J Ethnopharm. 2007;112:13–18. doi: 10.1016/j.jep.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Montanari T, de Carvalho JE, Dolder H. Antispermatogenic Effect of Achillea millefolium L. in Mice. Contraception. 1998;58:309–313. doi: 10.1016/s0010-7824(98)00107-3. [DOI] [PubMed] [Google Scholar]
- 42.Cavalcanti AM, Baggio CH, Freitas CS, Rieck L, de Sousa RS, Da Silva-Santos JE, Mesia-Vela S. Marques MCA. Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J Ethnopharm. 2006;107:277–284. doi: 10.1016/j.jep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Potrich FB, Allemand A, da Silva LM, dos Santos AC, Baggio CH, Freitas CS, Mendes DAGB, Andre E, de Paula Werner MF, Marques MC. AAnnttiiuullcceerrooggeenniicc aaccttiivviittyy ooff hhyyddrrooaallccoohhoolliicc eexxttrraacctt ooff Achillea millefolium L. Involvement of the antioxidant system. J Ethnopharm. 2010;130:85–92. doi: 10.1016/j.jep.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Baggio CH, Freitas CS, Nhaducue PF, Rieck L, Marques MCA. Action of crude aqueous extract of leaves of Achillea millefolium L. (Compositae) on gastrointestinal tract. Rev Bras Farmacogn. 2002;12:31–33. [Google Scholar]
- 45.Niazmand S, Khooshnood E, Derakhshan M. Effects of Achillea wilhelmsii on rat's gastric acid output at basal, vagotomized, and vagal-stimulated conditions. Pharmacogn Mag. 2010;6:282–285. doi: 10.4103/0973-1296.71791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghantous A, Nasser N, Saab I, Darwiche N, Saliba NA. Structure-activity relationship of seco- tanapartholides isolated from Achillea falcata for inhibition of HaCaT cell growth. European J Med Chem. 2009;44:3794–3797. doi: 10.1016/j.ejmech.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 47.Trifunovic S, Vajs V, Juranic Z, Zizak Z, Tesevic V, Macura S, Milosavljevic S. Cytotoxic constituents of Achillea clavennae from Montenegro. Phytochemistry. 2006;68:887–893. doi: 10.1016/j.phytochem.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 48.Rezaeipoor R, Saeidnia S, Kamalinejad M. Immunosuppressive activity of Achillea talagonica on humoral immune responses in experimental animals. J Ethnopharm. 1999;65:273–276. doi: 10.1016/s0378-8741(98)00191-3. [DOI] [PubMed] [Google Scholar]
- 49.Saeidnia S, Yassa N, Rezaeipoor R, Shafiee A, Gohari AR, Kamalinejad M, Goodarzy S. Immunosuppressive principles from Achillea talagonica, An endemic species of Iran. Daru 2009. 17:37–41. [Google Scholar]
- 50.Yassa N, Saeidnia S, Pirouzi R, Akbaripour M, Shafiee A. Three phenolic glycosides and immunological properties of Achillea millefolium. Iran population of Golestan. Daru. 2007;15:49–52. [Google Scholar]
- 51.Saeidnia S, Yassa N, Rezaeipoor R. Comparative investigation of the essential oils of A talagonica Boiss and A millefolium L, Chemical composition and immunological studies. J Essent Oil Res ; : 262. 2004;16:264. [Google Scholar]
- 52.Saeidnia S, Moradi-Afrapoli F, Gohari AR, Malmir M. Cytotoxic Flavonoid from Achillea talagonica Bioss. J Med Plants. 2009;8:52–56. [Google Scholar]
- 53.Ozlem B, Gulluce M, Sahin F, Ozer H, Kilic H, Ozkan H, Sokmen M, Ozbek T. Biological activities of the essential oil and methanol extract of Achillea biebersteinii Afan. (Asteraceae) Turk J Biol. 2006;30:65–73. [Google Scholar]
- 54.Maggi F, Bramucci M, Cecchini C, Coman MM, Cresci A, Cristalli G, Lupidi G, Papa F, Quassinti L, Sagratini G, Vittori S. Composition and biological activity of essential oil of Achillea ligustica All. (Asteraceae) naturalized in central Italy: Ideal candidate for anti-cariogenic formulations. Fitoterapia. 2009;80:313–319. doi: 10.1016/j.fitote.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Stojanovic G, Radulovic N, Hashimoto T, Palic R. In vitro antimicrobial activity of extracts of four Achillea species. The composition of Achillea clavennae L. (Asteraceae) extract. J Ethnopharm. 2005;101:185–190. doi: 10.1016/j.jep.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 56.de Santanna JR, Franco CC, Miyamoto CT, Cunico MM, Miguel OG, Cocco LC, Yamamoto CI, Junior CC, de Castro-Prado MA. Genotoxicity of Achillea millefolium essential oil in diploid cells of Aspergillus nidulans . Phytother Res. 2009;23:231–235. doi: 10.1002/ptr.2596. [DOI] [PubMed] [Google Scholar]
- 57.Darwish RM, Aburjai TA. Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant inhibitors on Escherichia coli. BMC Complement Altern Med. 2010;10:9. doi: 10.1186/1472-6882-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holetz FB, Pessini GL, Sanches NR, Cortez DAG, Nakamura CV, Filho BPD. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz. 2002;97:1027–1031. doi: 10.1590/s0074-02762002000700017. [DOI] [PubMed] [Google Scholar]
- 59.Barbour EK, Al Sharif M, Sagherian VK, Habre AN, Talhouk RS, Talhouk SN. Screening of selected indigenous plants of Lebanon for antimicrobial activity. J Ethnopharm. 2004;93:1–7. doi: 10.1016/j.jep.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 60.Mahady GB, Pendland SL, Stoia A, Hamill FA, Fabricant D, Dietz BM, Chadwick LR. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother Res. 2005;19:988–991. doi: 10.1002/ptr.1776. [DOI] [PubMed] [Google Scholar]
- 61.Saeidnia S, Gohari AR, Kiuchi F, Honda G. In vitro anti-epimastigote activity of some Iranian medicinal plants. Iranian J Pharm Res. 2005;2:101–103. [Google Scholar]
- 62.Gohari AR, Saeidnia S, Hadjiakhoondi A, Naghinejad A., Yagura T. Trypanocidal activity of some medicinal plants against the epimastigotes of Trypanosoma cruzi . J Med Plants. 2008;7:44–48. [Google Scholar]
- 63.Soltan MM, Zaki AK. Antiviral screening of forty-two Egyptian medicinal plants. J Ethnopharm. 2009;126:102–107. doi: 10.1016/j.jep.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Mahmoud AA, Al-Shihry SS. A new ionone glucoside and terpenoid constituents from Achillea biebersteinii and their antifungal activity. Nat Prod Comm. 2006;1:697–703. [Google Scholar]
- 65.Karamenderes C, Apaydin S. Antispasmodic effect of Achillea nobilis L. subsp. sipylea (O. Schwarz) Bässler on the rat isolated duodenum. J Ethnopharmacol. 2003;84:175–9. doi: 10.1016/s0378-8741(02)00296-9. [DOI] [PubMed] [Google Scholar]
- 66.Hammad HM, Abdalla SS. Pharmacological effects of selected flavonoids on rat isolated ileum: Structure- activity relationship. General Pharmacol: The Vascular System. 1997;28:767–771. doi: 10.1016/s0306-3623(96)00299-6. [DOI] [PubMed] [Google Scholar]
- 67.Abu-Niaaj L, Abu-Zarga M, Abdalla SS. Isolation and inhibitory effects of eupatilin, a flavone isolated from Artemisia monosperma Del., on rat isolated smooth muscle. Pharmaceutical Biol. 1996;34:134–140. [Google Scholar]
- 68.Babaei M, Abarghoei ME, Akhavan MM, Ansari R, Vafaei AA, Taherian AA, Mousavi S, Toussy J. Antimotility effect of hydroalcoholic extract of yarrow (Achillea millefolium) on the guinea-pig ileum. Pak J Biol Sci. 2007;10:3673–3677. doi: 10.3923/pjbs.2007.3673.3677. [DOI] [PubMed] [Google Scholar]
- 69.Hegazy M-E F, Abdel-Lateff A, Gamal-Eldeen AM, Turky F, Hirata T, Pare PW, Karchesy JK. Anti-inflammatory activity of new guaiane acid derivatives from Achillea coarctata . Nat Prod Comm. 2008;3:851–856. [Google Scholar]
- 70.Werner I, Mucaji P, Presser A, Glasl S. Sesquiterpenes and phenolic compounds from Achillea clypeolata . Z Naturforsch B. 2007;62:267–271. [Google Scholar]
- 71.Jacobsson IJ, Anna K, Gerden B, Haegg S. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiology Drug Safety. 2009;18:1039–1047. doi: 10.1002/pds.1818. [DOI] [PubMed] [Google Scholar]
- 72.Cavalcanti AMaria, Baggio CH, Freitas CS, Rieck L, de Sousa RS, Da Silva-Santos JE, Mesia-Vela S, Marques MCA. Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J Ethnopharm. 2006;107:277–84. doi: 10.1016/j.jep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 73.Lane EM. Method and composition for dermatoses using antihistamines and NSAIDs and botanical medicinal compounds. PCT Int Appl 2009; CODEN: PIXXD2 WO 2009158144 A1 20091230. (US Patent) [Google Scholar]
- 74.Kukes VG, Kiseleva TL, Chauzova AV, Rebrov VG, Melnikova NN. A medicinal composition for the treatment of chronic colitis. Russ 1999; CODEN: RUXXE7 RU 2129006 C1 19990420. [Google Scholar]
- 75.Liu Y. A Chinese medicinal composition for relieving pain and inflammation. Faming Zhuanli Shenqing 2004; CODEN: CNXXEV CN 1480185 A 20040310. [Google Scholar]
- 76.Korsun VF, Kukharskii PS. A medicine for hysteromyoma treatment. Russ 2000; CODEN: RUXXE7 RU 214522C1 20000210. [Google Scholar]