Abstract
Purpose of the study
to determine the efficacy, adverse effects and safety of a new Iranian generic product of deferasirox (Osveral®) in Iranian transfusion dependent major thalassemic (TD-MT) patients.
Methods
In 9 main thalassemia treatment centers, all of TD-MT patients (aged ≥2 yrs) with serum ferritin (SF) levels≥1000 ng/ml, or >100 ml/kg of RBC transfusion,who could not tolerate parental iron chelating were recruited regardless of their previous iron chelation therapy. Periodical clinical and laboratory evaluations were conducted for adverse effects (AEs). Primary efficacy end point was Mean of Relative Change of Serum Ferritin (MRC-SF) from the baseline level during one year. Analysis of variance (ANOVA), t test, chi-square or Fisher exact test were used for statistic analysis appropriately (P values <0.05 were considered as statistical significant).
Results
In 407 cases the male/female ratio was 0.98. Mean age was 11.5±7.4 (2–58) years. The mean of initiating dose of Osveral® and mean usage dose during the study was 23.5±4.9 mg/kg and 24.9±4.9 mg/kg respectively. MRC-SF was −11.44% ±38.92 and it showed significant decline in SF (P value<0.001) one hundred and forty eight patients out of 407 patients experienced at least one. AE, the most common of them were transient increase in serum creatinin (97;24.1%) and>5 time increase in transaminases (24;5.89%).The causes of discontinuation of treatment were non-satisfactory treatment ( 24; 5.8%), poor or non-compliance of patients (21;5.1%), and adverse effects (13; 3.1%)
Conclusion
A detailed comparison with similar studies on deferasirox (Exjade®) shows a promising efficacy and safety for its Iranian generic product (Osveral ®).
Keywords: Thalassemia; Iron Chelation,; Osveral ®; Deferasirox; Safety; Efficacy.
INTRODUCTION
Comparatively iron overload is a more serious and lethal consequence of chronic blood transfusion than blood-borne infection. A unit of packed RBC contains 250–300mg iron (1 mg/ml). Therefore, patients receiving more than 100 units of RBC usually develop hemosiderosis. Due to lack of mechanisms for enhancing iron excretion, iron accumulates in chronically transfused patients, ferritin levels rise, followed by early endocrine dysfunction (glucose intolerance and delayed puberty) which will ultimately lead to cirrhosis and cardiomyopathy (1). Liver biopsy in these pateints shows both parenchymal and reticuloendothelial iron. Cardiac toxicity is often started with early development of pericarditis, followed by dysrhythmia and pump failure presaging death within few years (2).
The decision to start long-term transfusion support should also prompt one to institute therapy with iron-chelating agents. Deferoxamine (DFO) is a well known parenteral iron chelator which has been in use for more than four decades. Despite occasional occurance of cataracts, deafness, growth arrest and local skin reactions, including urticaria, DFO is a relatively nontoxic drug (3, 4). However, despite DFO's wide availability, some patients are unable to comply sufficiently with prescribed treatment due to side effects of variable severity, and the unpleasant and cumbersome nature of effective chelating regimens, which prolonged and daily subcutaneous or intravenous DFO administration is required (5). Therefore, in the absence of regular use of the chelator, iron steadily accumulates and may cause death due to iron-induced cardiac disease (6).
Deferiprone (DFP) was the first orally active iron chelator which was initially marketed in India in 1995 and subsequently in Europe in 1999 for patients whom treatment with DFO was inadequate. Studies have demonstrated its efficacy, and especially support a potential cardio protective role of DFP in patients is mostly dependent on transfusion (7–9). Although agranulocytosis has been mentioned as the most serious side effects of DFP, more common and problematic side effects are gastrointestinal symptoms (e.g., nausea, vomiting, gastric discomfort and arthralgia) (10). Interestingly, poor compliance with Deferiprone has been reported due to patient's negligence to take the drug 3 times daily for a prolonged period which has led to non-satisfactory response to treatment (11).
Deferasirox has shown promise in more than 45 registered clinical trials and other studies since 2003 (12). It was originally marketed by Novartis under brand name of Exjade® (13). Single daily doses of 10 up to 40 mg/kg deferasirox has shown to produce reductions in liver iron concentration comparable to deferoxamine in chronically transfused adult and pediatric patients in many clinical studies. Deferasirox is generally well tolerated in adults, adolescents, and children as young as 2 years of age (13–16).
Osveral® is a brand name for Deferasirox manufactured by Osvah, the Iranian Pharmaceutical Company (17). Osveral received its marketing authorization through National Food and Drug Department of Iran Ministry of Health (18) and is already on the list of drugs reimbursed by national health insurance scheme.
Despite presence of numerous studies conducted on efficacy and safety of Exjade®, it was deceided that safety and effciacy of Osvearl® to be evaluated in Iranian patients due to the possible genetical and environmental differences which might underline disease, theraputic behavior, diet, drug acceptence and complexity in pharmacokinetics of oral drugs. Therefore, a multicentral clinical trial was proposed and conducted to assess the efficacy and safety of a 52-weeks treatment with Osveral® in transfusion dependent thalasemic patients with evidence of transfusion induced iron overload.
METHODS
All of the transfusion-dependent major thalassemic patients (aged 2 yrs) in 9 main treatment centers in Iran, with serum ferritin (SF) levels 1000 ng/ml, or with a history of multiple transfusions (>10 transfusions or >100 ml/kg of RBCs) who could not tolerate parentral iron chelating, after acquiring primary clinical and lab examaminations according to the specified protocol, regardless of their previous iron chelation therapy were recruited with the observance of inclusion and exclusion criteria (Tables 1 and 2). Despite presence of previous marketing authorization of Osveral® in the Iranian market, each patient was comprehensively informed about the study and also their rights to withdraw from the study whenever they wished.
Table 1.
Inclusion Criteria.
| • Patients above 2 years of age and older; No gender limitation |
| • Patients diagnosed to have major thalassemia |
| • Ferritin level above 1000 mg/dl or if the patient had more than 10 times of blood transfusion or the volume of transfused blood was above 100 cc/kg |
| • Normal serum creatinine level |
| • No proteinuria in urine analysis |
| • Negative serologic tests for HCV, HBV and HIV |
| • Liver transaminases below 5-fold the normal upper limit |
| • Normal complete blood count (WBC>4500; AGC>1500; platelet>150000) |
| • No cardiac problem neither taking any cardiac drug |
| • No auditory or ophthalmologic problem |
| • Female patients who had reached menarche should be single or who are sexually active must use an effective method of contraception |
| • Not concomitant usage of hydroxyurea; interferon; or any investigational drugs |
Table 2.
Exclusion criteria and interruption of therapy at the beginning and during the course of treatment.
| • Pregnancy or breast-feeding |
| • Progressive or persistent increase in the creatinine level: |
| • Any cardiac, auditory or ophthalmic problem |
| • HCV, HBV or HIV infections |
| • Persistent liver transaminases above 5-fold of the normal level: |
| • Repeated unexplained cytopenia *(neutropenia<1500/ml and thrombocytopenia<150000) and, unexplained agranulocytosis <500/ml, |
| • Severe nausea and vomiting (not controlled within 24 hours diagnosed by physician) |
| • Hypersensitivity to deferasirox |
| • Occurrence of any sever adverse effects regardless of being drug related or not,diagnosed by physician |
| • Sever skin rashes (not controlled by corticosteroids diagnosed by physician) |
| • or progressive proteinuria |
| • Non-compliant or unreliable patients |
| • Any surgical or medical condition that might significantly alter the absorption, distribution, metabolism or excretion of any drug. |
| • Falling serum ferritin consistently below 500 µg/l, consideration should be given to temporarily interruption therapy |
| • if serum ferritin increasing in 30% of baseline level and persists for 3 months despite of proper dose scaling Osveral® should be interrupted |
Routine laboratory examinations were performed for each patient according to the specified protocols. Patient follow-up was performed in accordance with the therapeutic monitoring. Tablets were advised to be dispersed in a non-metallic glass of water (apple or orange juice if patient could not tolerate) until a fine suspension was obtained and it was taken on an empty stomach 30 min before a meal.
According to wide alternation in serum ferritin level in different clinical situations, in this study primary efficacy end-point was the mean of monthly relative changes in serum ferritin concentration during 52 weeks of treatment compared with baseline. Baseline serum ferritin was taken as the average of the available, reliable and stable measurements within 35 days prior to the start of treatment. Serum ferritin was assessed at the beginning (days 1–35) and then every 4–8 weeks.
Dose Adjustment
According to the serum ferritin level and the mean transfusional iron intake (considering the volume and rate of transfusion) dose adjustment might become necessary, and therefore a protocol was recommended to physicians (Table 3). They were allowed to modify doses according to the clinical or paraclinical assessments such as alteration in serum creatinine, ALT and AST, and of course other AEs which might occur during the study. Doses more than 30 mg/kg and up to 40 mg/kg were allowed in the middle of study according to FDA dose approval for Exjade® in 2008 (19).
Table 3.
Recommended dose adjustment Therapeutic Protocol.
| FERRITIN(mg/dl) | 500–1000 | 1000–1500 | 1500–2000 | 2000–3000 | 3000–4000 | >4000 |
|---|---|---|---|---|---|---|
| Rate of TX | ||||||
| Low (<2 units/month) or <7cc/kg/month | 10 mg/kg | 15 mg/kg | 20 mg/kg | 30 mg/kg | 35 mg/kg | 40 mg/kg |
| Medium(2–4U/month) or 7–14 cc/kg/month | 10 mg/kg | 20 mg/kg | 25 mg/kg | 30 mg/kg | 35 mg/kg | 40 mg/kg |
| High(>4 U/month) or>14 cc/kg/month | 10 mg/kg | 20 mg/kg | 30 mg/kg | 30 mg/kg | 35 mg/kg | 40 mg/kg |
Safety and Monitoring
Safety of the medicine was evaluated through the continuous monitoring and recording of AEs, as well as thorough monthly clinical visits by a trained physician during the study and routine laboratory assessments and physical examinations. The frequency of all observed AEs were reported as relative percent of the patients with defined AEs to total number of patients and according to this method the relative percentage of adverse effects could be categorized as below:
Very Common: >10%
Common: 1–10%
Uncommon: 0.1–1%
Rare:<0.1%
Physical examination and laboratory tests before enrolment of patients in the study were: complete blood count, serum creatinine, BUN, urine analysis, serum transaminases, and serum ferritin level (preferentially two times before initiation of iron chelating therapy). Monthly visits and monthly or at least bimonthly laboratory follow-ups profile (according to available facilities and physician judgment) were requested during the study. Serologic studies for HCV, HBV and HIV infection were conducted at the beginning of the study to exclude affected or suspicious cases from the study. Echocardiography or MRI T2*(for cases >10 years old), and assessment of auditory and ophthalmic system at the beginning of therapy were recommended but not obligatory for all asymptomatic and clinically normal cases according to available facilities and the physician's judgment. As a definition, if serum creatinine rose to more than 33% of baseline serum creatinine two consecutive times, it was considered to be “rising creatinine”. If it decreased to its normal level after dose adjustment it was mentioned as temporary, and if it persisted or progressed it was categorized as “progressive creatinine rise”. More than 5 fold increase above the normal serum transaminase level was considered as severe liver dysfunction.
Statistical analyses
Quantitative data are summarized as median or mean±standard deviation and categorical data as percentage. Analysis of variance (ANOVA) and t test were used to compare continuous variables between independent groups and repeated measure ANOVA was used to compare Mean of Relative Changes of serum ferritin during the study period. Comparison of categorical variables was performed with chi-square or Fisher exact test appropriately. P-values less than 0.05 were considered as statistically significant.
RESULTS
Out of 407 cases with completed data from 9 centers, 202 patients (49.5%) were male and 205 patients (50.5%) were female. Mean age was 11.5±7.4 (2–58) years and mean weight was 27.7±14.9 (8.5–75) kg. According to age groups, 93 patients (23%) were in the age group of 2–5 years, 206 patients (50.6%) in the age group 6–14 years, and 108 patients (26.4%) in the age group ≥17 years.
Dosage and changes in serum ferritin
Mean baseline serum ferritin (SF) was 2210.4±1175.0 (600–9076) ng/dl. Considering the baseline SF and recommended dose protocol, there were different starting doses of Osveral®. The mean initiating dose of Osveral® was 23.5±4.9 (10–35 mg/kg) and the mean dose of Osveral® prescribed during the course of the study was 24.9±4.9 (10–36.7) mg/kg. The number of patients in the centers in which their physicians did not accept to use doses more than 30 mg/kg (dose group A) was 191(46.9%) and those centers who used maximum dose of 40 mg/kg (dose group B) were 216(53.1%). The frequency of increase of dosage was significantly higher in dose group B in comparison to dose group A (P-value 0.0001).
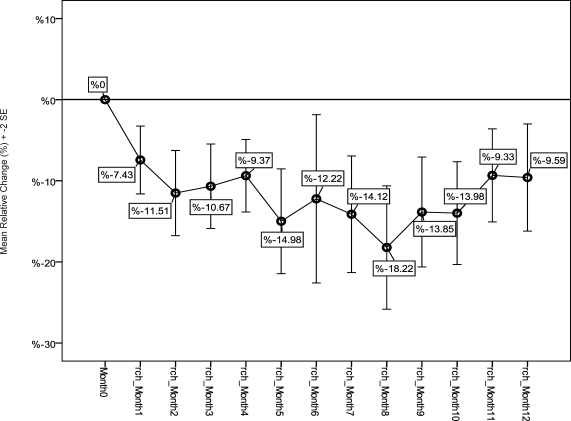
Mean of Relative Changes of Serum Ferritin (MRC-SF) was −11.4%±38.9 (+82.4% −197.4%) compared to baseline SF and it showed significant decline in SF during one year of iron chelating therapy with Osveral® (P value<0.001). The trend of MRC-SF (%)±2 Standard Errors during the study is shown in figure 3. This index in dose group B patients (−13.6%±42.1) was less than that in dose group A (−9%± 34.9). According to t test results there was no significant difference between dose group A and B in MRC-SF (P value=0.24). MRC-SF in patients older than 15 years age in their respective group was less than those in 6–14 and 2–5 years of age groups, but there was no significant difference among different age groups in MRC-SF (P value=0.14 and 0.26 respectively).
Figure 1.
Mean of Relative Changes of SF (%) in patients during study comparing baseline SF.
SF= Serum Ferritin ; Month 0 = starting month; rch_Month 1 (etc.)= relative change of SF (%) in first month comparing the baseline (etc.) ; 2SE = 2 Standard Errors of Mean
Safety
In this study 148 patients out of 407 patients experienced at least one form of AE.All AEs without any further investigation for the cause and effect relationship were recorded (Table 4).
Table 4.
Categorized frequency of AEs; number (%) of patients with AEs.
| Very common (>10%) | |
|---|---|
| Transient Increase in serum creatinin) 2 consecutive times) | |
| >33% of baseline level | 96 (23.58%) |
| >33% of baseline level AND >ULN* | 58 (14.2%) |
| Common (1–10%) | |
| More than 5 times increase in transaminases: | 24 (5.89%) |
| Skin rash | 6 (1.47%) |
| loss of appetite | 6 (1.47%) |
| Proteinuria | 5 (1.22%) |
| Uncommon (<1%)/Rare:<0.1% | |
| Diarrhea | 2 (0.49%) |
| Abdominal pain | 2 (0.49%) |
| Blurred vision;vomiting; Epistaxis; headache; flank pain; progressive creatinin rise; thrombocytopenia; prolonged fever | 1 (0.24%) |
ULN= Upper Limit of Normal
The median (range) of time span between commencing treatment with Osveral® and occurrence of all AEs, creatinine rise, and severe increase in transaminases were 4.1(1–11), 3(1–10), and 4.7(2–11) months, respectively. The median (range) time span between starting and interruption or discontinuation of treatment was 6.1(1–11) months which was 4.6(1–11) months due to patients’ incompliance and 6.4(1–11) months due to severe AEs. The main causes of treatment withdrawal were non-satisfactory response in 24 (5.8%), patients’ poor or non-compliance 21(5.1%), and AEs in 13(3.1%) cases.
Serum transaminases (ALT and AST) as indicator of liver function were elevated more than 5 fold of normal range in 24(5.89%) cases, which led to temporary or permanent discontinuation of drug. Only in 4 patients the drug was aborted by physician due to persistence or recurrence of severe liver function test (LFT) disturbance. There was no significant difference between dose groups A and B for occurrence of severe liver dysfunction (p-value=0.62). Although the rate of severe disturbance of LFTs in ≥15 years age group was less than other age groups, no statistically significant difference was observed (P value=0.90)
Rise in creatinine was observed in 97 out of 407 patients (24.1%). It was significantly higher in dose group B in comparison to dose group A patients (p-value=0.001). There was no significant difference among age groups in the case of rising creatinine (p-value=0.66–0.56). Fifty eight out of 407 (14.2%) cases had a rising creatinine not only greater than 33% of the baseline level but also more than upper limit of normal for age, 55 of them were children (<15 years old) and 3 of them were older than 15 years of age.
There was only one case of progressive creatinin rise that did not respond to dose reduction or transient drug interruption.
CONCLUSION
Although, phase 3 of clinical trial in evaluation of efficacy and safety of Exjade® had been preformed in other populations (14–16), this study on the Osveral® the Iranian brand of deferasirox has been designed to rule out the effects from differences due to variations in genetic; diet; complexity in absorption, metabolism and the pharmacokinetic characteristics of oral medications; and even cultures and beliefs regarding the imported or domestic products on clinical outcomes. In addition due to some published data about the diversity in efficacy and safety of some other drugs in Iranian thalassemic patients, there was a desire to evaluate efficacy and safety of this new generic drug Osveral® in Iranian patients (11, 20). On the other hand opponents of conducting a clinical study referred to this fact that there is no need to do clinical trial in chemical non biologic generic drugs after physical, chemical and bioequivalence comparison tests with the main drug (21). Finally this study, which was first proposed by Management Center for Transplantation and Special Disease of Health deputy of Iran Ministry of Health, was conducted by members of the Iranian Pediatric Hematology and Oncology Society in 2008 and ended on May 2010.
Among all enrolled patients, 407 had the complete and acceptable data. Lack of laboratory data at the time of enrollment and/or during the study; disregarding treatment protocol; and short treatment period of less than one year of using their medicine were among the main reasons for patients to drop out from the study.
In comparison to another multi centric non comparative single arm study on 237 thalassemic patients in the Middle East region, our study is further extended (15). Efficacy of Iron chelating agents were evaluated in many studies by measurement of dry liver iron concentration (LIC) and it was considered as a standard method for assessment of efficacy (14, 15).
On the other hand, significant correlations between changes in serum ferritin and liver iron concentration (LIC) have been identified across various underlying anemia (14, 22). In addition the measurement of serum ferritin is convenient and inexpensive, and serial measurements provide a relatively robust marker of iron burden. Accordingly current guidelines for assessment of iron burden and monitoring the efficacy of chelation therapy recommend the use of serum ferritin (23).
Therefore due to limitations in LIC measurement in different centers and non compliance of patients and parents especially for liver biopsy in children, in the present study serum ferritin level instead of LIC measurement was purposed as an indicator of body iron burden.
It seems that using end of study difference in serum ferritin level as an indicator of efficacy is more suitable for long term studies because of less significant influence of frequent changes in serum ferritin level in various clinical patterns such as infections, inflammations, and liver dysfunction (14–16, 24). In this study MRC-SF during one year as main index and primary endpoint of efficacy show a significant decline in SF. However, considering the increasing MRC-SF trend in the lasted 4 months, although statistically non-significant, the study seems should be extended longer for a better evaluation of efficacy.
In ESCALATOR study the reduction of serum ferritin at the end of study toward base line ferritin in children (−166 ng/ml) was not as high as in adults (−846 ng/ml); and this difference was statistically significant. This significant difference was explained by higher iron overload due to huge blood transfusion in children in comparison to adults (15). Discrepancy between results of this study and ESCALATOR study in the case of age groups may be due to the effect of chronic iron overload on liver in adults who lose the sensitivity of their serum ferritin level as an indicator of iron burden. EPIC study showed a median absolute reduction of serum ferritin of −163 ng/ml and −5.1% relative decline at the end of study but there was no comparison among different age groups (16).
Negative iron balance was not encountered in many clinical trials with 20 mg/kg and less, (14) but in several investigations especially in phase III clinical trails-due lack of enough data available about the drug- the initial dosage (≤20 mg/kg) was administered which might finally affect the efficacy of iron chelating therapy (14). For example in ESCALATOR study with initial median ferritin of 3326 ng/ml, the starting dose was 20 mg/kg in all patients and the increase of dosage after 26 weeks was detected in 78% of patients (15, 25). However in this study the starting dose and dosage adjustment was based on iron load (serum ferritin and/or LIC), iron intake, and the amount of transfusion. Therefore, the dosage increased and decreased in 44.7% and 4.7%, of patients under study respectively.
Frequencies of causes for discontinuation of treatment in this study compared with other studies are included in table 5. Although there was no definite description in other studies, unsatisfactory therapeutic effect (leads to drug discontinuation) are defined in our study as increase of serum ferritin more than 30% of baseline in more than 3 months despite dosage adjustment. Non compliance such as consent withdrawal and follow up discontinuation was mostly due to worriness about reported complications of deferasirox as a new medication which lead them to discontinue Osveral® even with presentation of first symptoms of any adverse effect.
Table 5.
Frequency of drug discontinuation in other studies.
| Current study | EPIC (16,28) | ESCALATOR(15,25) | Cappellini et al (26) | Piga et al (27) | |
|---|---|---|---|---|---|
| Non-satisfactory treatment* | 24 (5.8%) | 12(1.3%) | – | 39 (8.3%) | 8 (4.8%) |
| Poor or non-compliance of patients | 21(5.1%) | 29 (3.1%) | 9 (3.6%) | 52 (11%) | 5 (3%) |
| Adverse events | 13 (3.1%) | 31(3.3%) | 3 (1%) | 50 (10.6%) | 13 (7.7%) |
| Other(death, etc) | 17 (1.8%) | 3 (1%) | 13 (2.8%) | 4 (2.4%) | |
| Total | 58 (14.2%) | 89 (9.5%) | 12 (4.8%) | 159 (33%) | 31 (22.6%) |
Definition of this study: ferritin rise above 30% of baseline and not declining despite dosage adjustment for three consecutive months. There was not any definition in other studies.
Despite 153 reported adverse effects which led to drug discontinuation in EPIC study, there was no frequency reports about the type of AEs (16, 28). However in the present study among 13 patients whose AEs led to treatment termination the most prevalent complications by sequence are as below: proteinuria, 4 patients (31%); increase in serum liver transaminases more than 5 folds of normal,4 patients (31%); severe rash, 2 patients (15.5%);creatinine increase, blurred vision and diarrhea, each one in 1 patient (7.7%).
In other important trials the prevalence of AEs were 76 and 85 percent, however when those complications defined according to judgment of physicians, the frequencies were between 44–50 percent (15, 16).
In their studies, adverse effects without any investigation concerning the causes were reported in 152 patients (37.2%).
The most prevalent complications in this study were transient increase of serum creatinine and more than 5 folds of normal increase in serum liver transaminases. Skin rashes and proteinuria were less prevalent and the gastro-intestinal complications were rare.
The low rate of skin rashes and gastro-intestinal complications in comparison with other studies (Table 6) could be considered as mild complications which were not sensed by patients and not reported by the physician. For instance from 6 patients reported with rash, 2 cases discontinued the drug despite proper management. This can also explain the diversity in overall rate of complications reported in this study (37.2%) in comparison with other studies (76–85%). Mildness of majority of skin rashes and gastro-intestinal complications of Exjade® are reported in official documents (30).
Table 6.
Frequency of complications in other studies.
| EPIC (16,28) | ESCALATOR (15,25) | Cappellini et al (26) | Piga et al (27) | Cappellini et al (14,30) | |
|---|---|---|---|---|---|
| Rash | 129 (11.5%) | 19 (8%) | 23 (4.9%) | 9 (5.4%) | 25 (8.4%) |
| Diarrhea | 87 (7.8%) | 14 (6%) | 40 (8.5%) | 35 (11.8%) | |
| Abd. Pain | 77 (4.8%) | 14 (6%) | 62 (13%) | 12 (7.1%) | 23 (7.8%) |
| Nausea&Vomiting | 62 (5.6%) | 38 (16.1%) | 87 (22.5%) | 22(13.1%) | 61 (20.6%) |
| Increased in serum Cr >33% BL | 552 (31.7%)a | 73 (30.9%) | 113 (38.2%) | ||
| 9 (3.8%)c | |||||
| Increased in serum Cr>33% BL &>ULN | 37 (3.9%)b | 6 (2.5%) | 34 (7.2%) | 13 (7.7%) | |
| 175 (10%)a | |||||
| Increased serum ALT> 10×ULN | 7 (0.6%) | 13 (5.5%) | 29 (6.1%) | 11 (6.6%) | 25 (8.4%)d |
Transfusion dependent patients
major Thalasemia patients
This complication specified to drug in 9 patients out of 73 due to physician judgment.there was not any other explanation.
Definition of liver dysfunction in this study: ALT was 5 folds above normal
Diversity in statistics about transient increase of creatinine comes from different definitions about this index. In some studies the increase of creatinine level in two consecutive measures; was not only more than 33% of baseline, but also more than age specific limit especially in children; were considered as creatinine rise (26, 27) (29). On the other hand, other studies rely on definition of creatinine rise more than 33% of baseline or both of the definitions (14–16). With the first definition there were 58 cases (14.2%) with rising creatinine in our study, 55 of them were children and 3 were adults. On the other hand, this difference may be related to physician judgment in allocating complications to deferasirox in some studies. For example in ESCALATOR study, despite creatinine rise in 73 patients (31%) above 33% baseline and 6 patients (2.5%) above age specific limits, the complications which were allocated to drug by investigator, were reported just in 9 patients (3.8%) and there was not any drug interruption due to complication despite one case of renal failure (15).
In this study increase in serum liver transaminases (both ALT and AST) more than 5 folds of normal limits was considered as liver dysfunction, while other studies relied on more than10 folds of normal limits of ALT. Therefore, it seems that the prevalence of liver dysfunction in this study was less than other studies. Absence of complications such as hearing disorders, cataract, and cytopenia which were reported in other studies may be due to short duration of this study (1 year) compared with other studies.
In conclusion, the results of the Iranian generic oral iron chelating agent (Osveral®) both in iron chelating efficacy and safety was acceptable. But despite extended sample size in comparison to other studies, for more detailed results it is proposed that the study to be conducted for a more extended period of time with more patients. It seems that the patients satisfaction in different age groups would be the most required variable in future studies. Also it is advisable to include other methods of iron overload assessment liver tissue iron concentration and R2MRI in studies. Finally extension of ongoing generic medications in thalassemic patients emphasizes the importance of multi center clinical studies in Iran.
ACKNOWLEDGMENTS
We acknowledge the contribution of Dr. Abdolmajid Cheraghali-pharmacologist and Dr. Hasan Abolghasemi-chief president of Iranian Pediatric Hematology and Oncology Society-who supported us in all the stages of this great project.Also we should extend our thanks to Dr M. Aghighi-Director of Management Center for Transplantation and Special Disease of Health deputy of Iran Ministry of Health-and Dr R.Hantooshzadeh-person responsible and in charge of staff in the mentioned center for this study-for their supports and hard efforts during investigation.In addition, we appreciate the assistance of Mrs. Behnaz Habibpanah-special nurse of thalassemia and hemophilia in Mofid Children Hospital-for data gathering and multi center coordination. We also value consultation of Dr. Ahmadreza Shamshiri-epidemiologist in preparing final report.
CONFLICT OF INTEREST
This study was sponsored financially-without any supervision on design, data gathering and analysis-by Osvah Pharmaceutical Company.
REFERENCES
- 1.Britton RS, Leicester KL, Bacon BR. Iron toxicity and chelation therapy. Int J Hematol. 2002;76:219–228. doi: 10.1007/BF02982791. [DOI] [PubMed] [Google Scholar]
- 2.Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood. 2004;103:1934–1936. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 3.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farrell DE, Harris JW. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–573. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 4.Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematol. 1996;95:26–36. doi: 10.1159/000203853. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham MJ, Acklin EA, Neufeld EJ, Cohen R. Complications of beta-thalassemia major in North America. Blood. 2004;104:34–9. doi: 10.1182/blood-2003-09-3167. [DOI] [PubMed] [Google Scholar]
- 6.Arboretti R, Tognoni G, Alberti D. Pharmacosurveillance and quality of care of thalassaemic patients. Eur J Clin Pharmacol. 2001;56:915–922. doi: 10.1007/s002280000251. [DOI] [PubMed] [Google Scholar]
- 7.Piga A, Caglioti C, Fogliacco E, Tricta F. Comparative effects of deferiprone and deferoxamine on survival and cardiac disease in patients with thalassemia major: a retrospective analysis. Haematologica. 2003;88:489–496. [PubMed] [Google Scholar]
- 8.Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell D. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassemia. Lancet. 2002;360:516–520. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- 9.Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, Gotsis ED, Tanner MA, Smith GC, Westwood MA, Wonke B, Galanello R. Randomized controlled trial of deferiprone or deferoxamine in betathalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–3744. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AR, Galanello R, Piga A, Dipalma A, Vullo C, Tricta F. Safety profile of the oral iron chelator deferiprone: a multicenter study. Br. J Haematol. 2000;108:305–312. doi: 10.1046/j.1365-2141.2000.01866.x. [DOI] [PubMed] [Google Scholar]
- 11.Eshghi. P. Complications of Combined Treatment with Deferiprone and Desferrioxamine in Thalassemic Patients. IJMS. 2007;32:40–44. [Google Scholar]
- 12. http://clinicaltrials.gov/ct2/results?term=deferasirox/accessed on 6/17/2011.
- 13. http://www.exjade.com/index.jsp?lightbox=global-hcp/accessed on 6/17/2011.
- 14.Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, Aydinok Y, Kattamis A, Kilinc Y, Porter J, Capra M, Galanello R, Fattoum S, Drelichman G, Magnano C, Verissimo M, Athanassiou-Metaxa M, Giardina P, Kourakli-Symeonidis A, Janka-Schaub G, Coates T, Vermylen C, Olivieri N, Thuret I, Opitz H, Ressayre-Djaffer C, Marks P, Alberti D. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with b-thalassemia. Blood. 2006;107:3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 15.Taher A, El-Beshlawy A, Elalfy MS, Al Zir K, Daar S, Habr D, Kriemler-Krahn U, Hmissi A, Al Jefri A. Efficacy and safety of deferasirox, an oral iron chelator, in heavily iron-overloaded patients with beta-thalassaemia: the ESCALATOR study. Eur J Haematol. 2009;82:458–65. doi: 10.1111/j.1600-0609.2009.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cappellini MD, Porter J, El-Beshlawy A, Li CK, Seymour JF, Elalfy M, Gattermann N, Giraudier S, Lee JW, Chan LL, Lin KH, Rose C, Taher A, Thein SL, Viprakasit V, Habr D, Domokos G, Roubert B, Kattamis A. EPIC Study Investigators.Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. haematologica. 2010;95:557–566. doi: 10.3324/haematol.2009.014696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. http://www.osvahpharma.com/index.php?module=cdk&func=loadmodule&system=cdk&sismodule=user/content_view.php&sisOp=view&id=6&ctp_id=18&cnt_id=806www.fdo.ir/Drug/Fa/Downloads/ShowSection.aspx?fid=11/ accessed on 6/17/2011.
- 18. http://fdo.behdasht.gov.ir/ accessed on 6/17/2011.
- 19. www.accessdata.fda.gov/drugsatfda_docs/label/2008/021882s004lbl.pdf/ accessed on 6/17/2011.
- 20.Yavarian M, Karimi M, Bakker E, Harteveld CL, Giordano PC. Response to hydroxyurea treatment in Iranian transfusion-dependent beta-thalassemia patients. Haematologica. 2004;89:1172–1178. [PubMed] [Google Scholar]
- 21. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/QuestionsAnswers/ucm100100.htm/ accessed on 6/17/2011.
- 22.Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, Olivieri N, Piga A, Cunningham MJ, Soulières D, Gattermann N, Tchernia G, Maertens J, Giardina P, Kwiatkowski J, Quarta G, Jeng M, Forni GL, Stadler M, Cario H, Debusscher L, Della Porta M, Cazzola M, Greenberg P, Alimena G, Rabault B, Gathmann I, Ford JM, Alberti D, Rose C. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. European Journal of Haematology. 2008;80:168–176. doi: 10.1111/j.1600-0609.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappellini M-D, Cohen A, Eleftheriou A, Piga A, Porter J, Taher A. Thalassaemia International Federation publication. Nicosia,Team up Creation Ltd; 2008. Guidelines for the Clinical Management of Thalassaemia; pp. 35–40. 2nd Edition Revised. [PubMed] [Google Scholar]
- 24.Taher A, Cappellini MD, Vichinsky E, Galanello R, Piga A, Lawniczek T, Clark J, Habr D, Porter JB. Efficacy and safety of deferasirox doses of >30 mg/kg per d in patients with transfusion-dependent anaemia and iron overload. British Journal of Haematology. 2009;147:752–759. doi: 10.1111/j.1365-2141.2009.07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taher A, El-Beshlawy A, Elalfy MS, Al Zir K, Daar S, Damanhouri G, Habr D, Kriemler-Krahn U, Hmissi A, Al Jefri A. Efficacy and Safety of Once-Daily Oral Deferasirox (Exjade®) during a Median of 2.7 Years of Treatment in Heavily Iron-Overloaded Patients with β-Thalassemia Blood (ASH Annual Meeting Abstracts) 2008 Nov;112:5409. [Google Scholar]
- 26.Cappellini M-D, Galanello R, Piga A, Cohen A, Kattamis A, Aydinok Y, Williamson P, . Rojkjaer L, Porter J. Efficacy and Safety of Deferasirox (Exjade®) with up to 4.5 Years of Treatment in Patients with Thalassemia Major: A Pooled Analysis Blood (ASH Annual Meeting Abstracts) 2008 Nov;112:5411. [Google Scholar]
- 27.Piga A, Forni G-L, Kattamis A, Kattamis C, Aydinok Y, Rodriguez M, Rojkjaer L, Galanello R. Deferasirox (Exjade®) in Pediatric Patients with β-Thalassemia: Update of 4.7-Year Efficacy and Safety from Extension Studies Blood (ASH Annual Meeting Abstracts) 2008 Nov;112:3883. [Google Scholar]
- 28.Cappellini M-D, Elalfy MS, Kattamis A, Seymour J-F, Lee C-L, Porter J, El-Beshlawy A, Habr D, Domokos G, Hmissi A, Taher A. 2008 Nov;112:3878. [Google Scholar]
- 29. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021882s006lbl.pdf/ accessed on 6/17/2011.
- 30. http://www.exjade.com/exjade-safety/long-term-safety-profile.jsp?lightbox=global-hcp/ accessed on 6/17/2011.