Abstract
In this study, we systematically investigated fiber demography, based on function and distribution, from the periphery to their destinations in the various central (sub) nuclei in the trigeminal brainstem nuclear sensory complex. Conventional and novel compelling information is provided, demonstrating that the ratio and somatotopy of types A and C sensory fibers at the site of a lesion can elucidate important puzzles in TNP disorders. For instance, we explain how of a major shift in the fibers’ direction and ratio at the level of the trigeminal root entry zone (REZ) influences the pathophysiology of pre- and typical trigeminal neuralgia. As a result, there is a high A/C ratio of oral and peri-oral fibers in the supero-medial region of the REZ, which is mostly susceptible to vascular compression. However, this A/C ratio varies considerably at lower proportions in other areas along the peripheral trigeminal pathway, where an injury (viral, vessel compression, or trauma) can lead to a broader spectrum of fiber involvement and, consequently, pain outcome. In summary, we explain how fiber demography can influence pain quality, location, temporal features, progress, and treatment prognosis of TNP in those patients who develop it.
Keywords: pain, orofacial pain/TMD, oral medicine, nervous system, trigeminal neuralgia, pre-trigeminal neuralgia
Introduction
Trigeminal neuropathic pain (TNP) disorders, as typical, atypical, and post-herpetic trigeminal neuralgias, are commonly incapacitating conditions with pain that is either spontaneous or can be evoked by harmless but crucial activities, such as eating and talking, or by light touch to the facial skin. The puzzling and overly simplistic current view of the TNP disorders is mainly due to our limited understanding of their pathophysiology. Hitherto, TNP animal models noticeably do not duplicate all of the behavioral signs seen in the clinic. In addition, there are no objective tests available for patients. Therefore, except for imaging studies to detect possible lesions along the trigeminal sensory pathway, dentists and other health professionals depend almost entirely on clinical criteria for their diagnosis.
Here we discuss each of the most common TNP disorders based on fiber demography, including ratio and somatotopic distribution of myelinated and non-myelinated fibers affected by different types of injuries in the trigeminal system, whether viral (post-herpetic neuralgia), traumatic, tumors, or vessel compressions (atypical and typical-TN). Although peripheral injuries to the trigeminal system induce TNP in only a minority of patients—demonstrating the participation of pain modulation from multiple mechanisms, many outside of the scope of this paper—we present substantial data attesting to the fact that nuances in the quantitative and spatial organization of afferent fibers at the location of injury will mostly determine various unique features of TNP symptomatology. Even considering the effects of several peripheral and central mechanisms (e.g., inflammation, central sensitization), each type of afferent sensory fiber will supposedly respond to an insult in a different way, triggering a selected cascade of neurologic events and, consequently, pain outcomes. Nonetheless, the injuries commonly do not affect a single type of afferent fiber, but a wide-ranging combination of them. Hence, the study of fiber demography is a more tangible and physiologic way to understand their role in the final clinical presentation of TNP disorders.
For additional information regarding trigeminal sensory fibers, trigeminal neuropathic pain from dental origin, and the effects of different modalities of treatment on the function of sensory fibers, please refer to the Appendix.
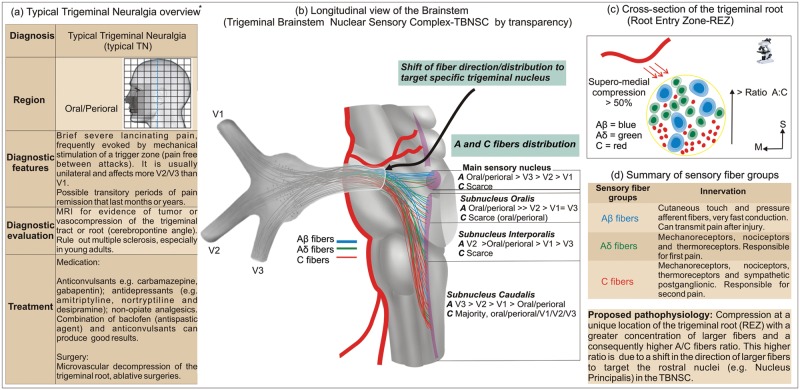
Typical Trigeminal Neuralgia (Typical-Tn)
Typical-TN is described as a disorder with an incidence of 3 to 5 cases per 100,000 persons, affecting, on average, elderly people. The pain is characterized as sharp and shooting. Severe brief paroxysmal pain is spontaneous or elicited when the trigger-point, usually located in the oral or peri-oral region, is stimulated by light touch. It is postulated that typical-TN is caused by compression of the trigeminal root by a blood vessel or tumors at the level of the root entry zone (REZ).
The Unique Ratio and Somatotopic Distribution of Rostral Fibers in the Trigeminal Root Entry Zone: Why Is It a Key Region for Typical-TN Pathophysiology?
The majority of nociceptive impulses from the orofacial region are mainly (but not exclusively) mediated by the trigeminal nerve. The action potentials are transmitted from its peripheral branches, ophthalmic (V1), maxillary (V2), and mandibular (V3), by pseudo-unipolar neurons with the cell bodies located in the trigeminal (gasserian or semilunar) ganglion. From the trigeminal ganglion, the central processes of these cells follow the trigeminal sensory root and enter the lateral portion of the pons in a region frequently referred to as the trigeminal root entry zone (REZ) (Shankland, 2000).
Although a somatotopic organization has been demonstrated in the trigeminal ganglion (Borsook et al., 2003), a functional segregation of fibers in the trigeminal root proposed by some authors is extremely controversial, having elicited many scientific debates in the past (Samii and Jannetta, 1981; Hussein et al., 1982). However, analysis of anatomical and clinical data supports our concept that at the level of the brainstem, when leaving the trigeminal root, specifically at the REZ, the sensory fibers change their direction to target their proper nuclei in the trigeminal brainstem nuclear sensory complex (TBNSC) (Crissman et al., 1996; Pajot et al., 2000; Devor et al., 2002b; Sindou et al., 2002, 2006; Miller et al., 2009). Consequently, many of the Aβ fibers transmitting tactile information from the orofacial region leave the trigeminal root rostrally in their trajectory to the main sensory nucleus, located at the level of the midpons (Samii and Jannetta, 1981; Sessle, 2000) (Fig. 1). Similar rostral convergence occurs with Aδ, in fact both A-fibers, when targeting other rostral nuclei in the TBNSC such as the subnucleus oralis and interporalis (Pajot et al., 2000). Conversely, only scarce C-fibers coming directly from the trigeminal root have terminals in the main sensory nucleus, oralis, and interporalis (Tashiro et al., 1984; Sugimoto et al., 1997; Woda, 2003). The majority of the C-fibers travel with a lower number of A-fibers (than in the rostral subnuclei) to the subnucleus caudalis (Samii and Jannetta, 1981; Tashiro et al., 1984; Pajot et al., 2000; Sessle, 2000), which is located inferiorly, extending from the level of the obex for approximately 15 mm to the C2 level, where it becomes continuous with the dorsal horn in the human spinal cord (Paxinos and Huang, 1995; Paxinos and Mai, 2004). Such C-fibers in the subnucleus caudalis terminate mainly in the superficial layers, and they can also modulate the activity of convergent neurons in the subnucleus oralis via interneurons (Dallel et al., 1998, 2003; Woda et al., 2001; Woda, 2003).
Figure 1.
Clinical characteristics and pathophysiology associated with typical trigeminal neuralgia (typical-TN). *All images are original, with the exception of the left table (a), which was reprinted, with small adaptations, from DaSilva and Acquadro (2005), with permission from SNELL Medical Communication Inc. **The A- and C-fiber distribution in the trigeminal brainstem nuclear sensory complex, located in the center table, is based on available animal studies.
This peculiar anatomical distribution of sensory fibers based on their function and TBNSC targets translates into a unique higher A/C fiber ratio in the most superior regions of the trigeminal root in the REZ. In contrast, this A/C fiber ratio decreases in more caudal parts of the REZ, due to the higher numbers of C-fibers going to the subnucleus caudalis. In fact, a recent study demonstrated that the ratio of myelinated to unmyelinated fibers is about 4:1 in the region adjacent to the trigeminal root compression in biopsy specimens taken from patients with trigeminal neuralgia (Marinkovic et al., 2009). Typical studies have described a reduced A/C fiber ratio in the spinal dorsal roots (up to 80% of unmyelinated fibers), when compared with the trigeminal sensory roots (40-50% of unmyelinated fibers), which could represent a greater proportion of C-fibers in lower segments of the body (Windle, 1926; Young and King, 1973; Samii and Jannetta, 1981).
In typical-TN, compressive injury occurs especially at the superior area of the REZ (actually supero-medial in more than 50% of cases) (Sindou et al., 2002), where the average diameter of myelinated fibers is larger compared with that in other regions of the trigeminal root (Crissman et al., 1996) (Fig. 1). Histological analysis from typical-TN patients showed that compressed trigeminal root specimens had axonopathies and axonal loss that could induce ectopic after-discharge and cross-excitation of neighboring fibers (Devor et al., 2002a,b). Those axonopathies are also evidenced by recent studies with diffusion tensor imaging (DTI) that demonstrated microstructural changes in the trigeminal roots of patients with trigeminal neuralgia (Fujiwara et al., 2010; Lutz et al., 2011). Furthermore, focal demyelination was demonstrated exactly at the REZ in cases of trigeminal neuralgia due to multiple sclerosis (Love et al., 2001). The main reason for the unique symptomatic features in typical-TN, with spontaneous but mostly evoked pain from tactile stimulation of the trigger zone, might be a high ratio of A-fibers in the root entry zone (REZ) area, as discussed above, given that they are critical for pain triggered by tactile stimulation (Devor, 2009). Although Aβ fibers are usually associated with innocuous sensations and respond to brush-touch stimuli, they can mediate pain after peripheral nerve injury. Some mechanisms have been proposed to explain this fact, including central sensitization, disinhibition, and central afferent sprouting. Furthermore, changes in the properties (“phenotype”) of low-threshold Aβ fibers, named “phenotypic switching”, have been recently suggested (Woolf and Mannion, 1999; Maihofner et al., 2003; Costigan et al., 2009; Devor, 2009). Notwithstanding, in addition to the Aβ fibers’ dysfunction, the impairment of Aδ fibers probably also contributes to the phenomenon of trigger zones in typical-TN (Cruccu et al., 2001; Obermann et al., 2007). Moreover, such massive activation of A-fibers is associated with another patent clinical phenomenon in typical-TN: the refractory period that follows the TN attacks, where immediate re-stimulation of the trigger zone will not elicit another bout of pain, at least for a few seconds or minutes. This is partially explained by the suppressive effect, at least in non-pathological states, of A-fiber stimulation (e.g., tactile, vibrotactile, pin-prick) on C-fiber responses (e.g., heat), and vice versa, via central inhibitory mechanisms (Watanabe et al., 1999; Hoshiyama and Kakigi, 2000; Nahra and Plaghki, 2003; Tran et al., 2008).
Why Is There a Higher Incidence of Trigger Zones in Maxillary and Mandibular Trigeminal Branches in Typical-TN Patients?
Another interesting characteristic concerning typical-TN is the main localization of the trigger zones in the oral and peri-oral regions (The International Classification of Headache Disorders, 2004; Truini et al., 2005), commonly affecting the maxillary (V2) and mandibular (V3) branches and sparing the ophthalmic branch (V1) (Wall et al., 2006). This fact is explained by the large oral/peri-oral somatotopic fiber distribution (Pajot et al., 2000; Truini et al., 2005) along with its high concentration of larger fibers (Aβ and Aδ fibers) when compared with the lateral zones of the face (Sugimoto et al., 1986, 1988). The same Aβ and Aδ fibers are located in a more superior position in the REZ and, consequently, are more vulnerable to compression, mostly by the superior cerebellar artery (SCA) (Sindou et al., 2002). Such fibers from oral and peri-oral structures are mainly projected to the higher regions of the TBNSC as a whole (Azerad et al., 1982; Broton and Rosenfeld, 1982; Dallel et al., 1987, 1988, 1990; Duale et al., 1996; Luccarini et al., 1998; Bae et al., 2004), and also in a pattern similar to each one of its subnuclei (Shigenaga et al., 1986a,b; Toratani et al., 2008). Conversely, fibers from the peripheral regions of the face, including V1, are primarily projected to the lower portions of the TBNSC (Azerad et al., 1982), especially the lower levels of the subnucleus caudalis, which constitutes the typical onionskin representation of the face at the TBNSC (DaSilva et al., 2002) (Fig. 1).
What Is Pre-trigeminal Neuralgia, and Why Do the Pain Quality and Location Differ from Those of Typical-TN?
Some individuals with typical trigeminal neuralgia have reported a prodromal pain termed ‘pre-trigeminal neuralgia’ (Symonds, 1949; Mitchell, 1980; Fromm et al., 1990; Evans et al., 2005).
As discussed above, although the majority of C-fibers project to lamina II and lamina I of the subnucleus caudalis (Kobayashi and Matsumura, 1996), there are reports of few and isolated nociceptive C-fibers arriving in the dorsal part of the main sensory nucleus, the medial edge of the interporalis, and the dorsal half of the oralis (Sugimoto et al., 1997). Those scarce C-fibers are almost exclusively from the oral region, especially the pulp (Azerad et al., 1982; Takemura et al., 1991; Kwan et al., 1993; Sessle, 2000). Interestingly, most pre-trigeminal neuralgia patients experience a toothache-like pain with continuous, aching, or burning quality resembling atypical-TN pain, which are symptoms associated with C-fiber input. Nonetheless, pre-TN is certainly not an exclusive contribution of unmyelinated fibers, since some patients also describe the pre-TN pain as sharp, and it is treated with the same array of medications used for typical-TN (Mitchell, 1980; Fromm et al., 1990; Evans et al., 2005). Hence, it is possible that the infrequent occurrence of pre-TN may, in some cases, represent the early stages of the REZ compression, where those few intra-oral C-fibers in the root targeting rostral nuclei are mostly affected initially. Later, with increased accommodation of the vessel on the superior portion of the root where A-fibers are in the majority, the symptoms shift to a more typical-TN scenario, with paroxysmal attacks.
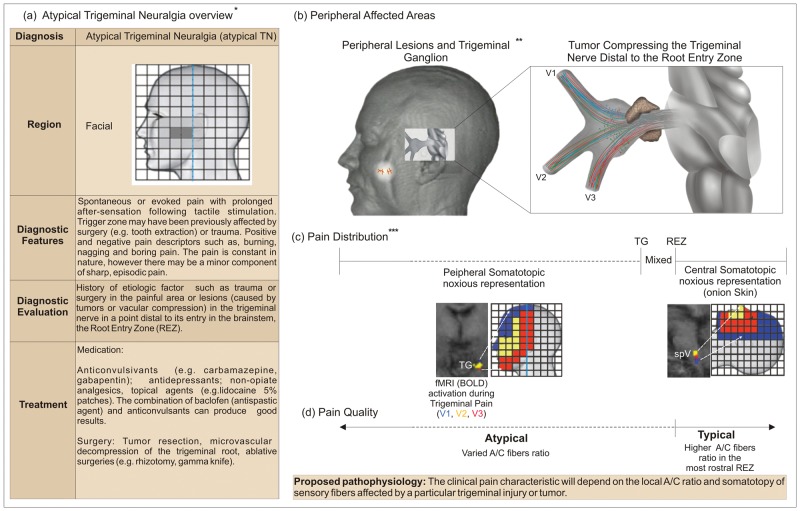
Atypical Trigeminal Neuralgia (Atypical-TN)
Atypical-TN affects a larger number of patients and is described in a wider variety of pain descriptors, including burning and throbbing. Nonetheless, patients have also reported, to a lesser degree, sharp pain as typical-TN (Eller et al., 2005). For those patients, the mild to severe pain is usually constant, with a characteristic after-sensation. This condition is caused by damage to the trigeminal system, usually peripheral, and is associated with tumors (Vassilakis et al., 1988; Nomura et al., 1994; Shankland, 2009), trauma (Shankland, 2009), or surgeries (Grigoryan Yu et al., 1994). Its classification is rather confusing and lacks agreement among different institutions; hence, in this article for the purpose of differentiation from typical-TN, and not taxonomy, we will use the term ‘atypical-TN’.
How Does the Local Ratio of Sensory Fibers Influence the Symptoms in Atypical-TN?
Orofacial Region
The ratio of sensory fibers at the location of injury is crucial to determine the clinical characteristics of atypical-TNP (e.g., more sharp or burning pain). For example, traumas to the oral/peri-oral areas of the face affect branches that contain a high A/C fiber ratio, albeit not as high as the superior portion of the REZ, and will translate into a more episodic and sharp pain, with some C-like descriptors of pain (e.g., burning). In contrast, superficial lesions to more lateral regions of the face, where the proportion of C-fibers is higher (Sugimoto et al., 1986, 1988), result in constant and burning pain, with a minor component of sharp pain.
Trigeminal Ganglion and Root
Following the same principle, the clinical characteristics of pain due to lesions at the level of the trigeminal ganglion or root will be influenced by the local A/C fiber ratio. For example, lesions in the trigeminal root closer to the ganglion will lead to more atypical descriptors, due to the sorted concentrations of A- and C-fibers, and lesions close to the root entry zone (REZ) from above will lead to more typical-TN symptomatology (Cusick, 1981; Tancioni et al., 1995; Turp and Gobetti, 1996) (Fig. 2). Likewise, the type of vessel compression and the extension of its area of contact along the trigeminal root can also influence symptoms. While arterial compressions, especially at the REZ, are strongly associated with typical-TN, venous compressions usually extend for a larger area and are firmly attached to the trigeminal root (Dandy, 1934); consequently, the latter do not spare any particular group of fibers, and the symptoms are more associated with atypical-TN than with typical-TN (Roski et al., 1982; Sekula et al., 2009). Similar principles apply to the clinical outcome of surgical treatments available for TNP, based on their selective or non-selective harmful effects upon the local groups of trigeminal fibers, sensory and motor.
Figure 2.
Clinical characteristics and pathophysiology associated with atypical trigeminal neuralgia (atypical-TN). *The left table (a) was reprinted, with small adaptations, from DaSilva and Acquadro (2005), with permission from SNELL Medical Communication Inc. **3-D image modified from DaSilva (2002). ***fMRI images reprinted from DaSilva et al. (2002), with permission from the Journal of Neuroscience, and from Borsook et al. (2003), also with permission from the Journal of Neuroscience.
Regarding pain location, if there is a lesion in the trigeminal ganglion, the pain will be restricted to the peripheral trigeminal somatotopy. Hence, injuries at the medial and anterior parts of the ganglion mostly lead to pain in V1, caudal and lateral injuries produce pain at V3, and intermediate injuries pain in V2 (Cusick, 1981; Bullitt et al., 1986; Nomura et al., 1994; Borsook et al., 2003; Eller et al., 2005). Conversely, lesions closer to and beyond the REZ will obey central pain somatotopy, which resembles the onionskin pattern. As a consequence, the trigeminal root area between the trigeminal ganglion and the REZ will serve as a transition zone between peripheral and central systems, which will translate into a less-defined arrangement of sensory fibers (Fig. 2). In fact, the different somatotopic dispositions of sensory fibers along the trigeminal somatosensory system may be a potential clinical tool to suggest whether a particular pain location and irradiation are associated with a peripheral or central lesion.
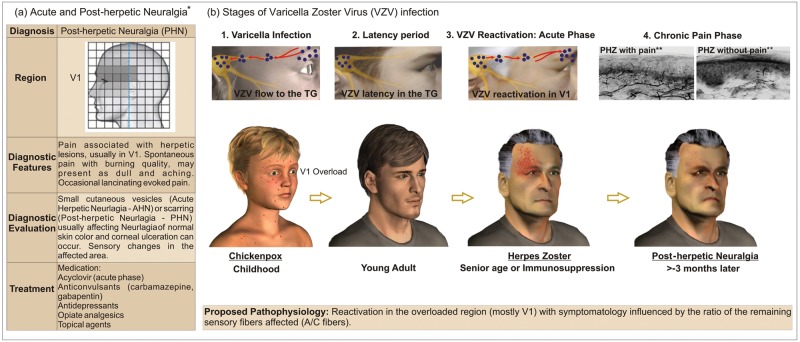
Post-herpetic Neuralgia (PHN)
Post-herpetic neuralgia (PHN) is one of the most common conditions of neuropathic pain disorders that affect the elderly population. PHN is often acquired after the reactivation of a latent varicella zoster virus (VZV) associated with an earlier infection during chickenpox (varicella) occurrence (Fig. 3). The later reactivation of varicella causes a clinical condition known as herpes zoster. Most patients recover completely from herpes zoster after some months. However, in some, the pain persists after the rash heals (Watson and Oaklander, 2002, 2006; Dworkin et al., 2008; Delaney et al., 2009).
Figure 3.
Clinical characteristics and stages of varicella zoster virus (VZV) infection related to the pathophysiology of post-herpetic neuralgia (PHN). All images are original with the exception of: *the left table (a), which was reprinted, with small adaptations, from DaSilva and Acquadro (2005), with permission from SNELL Medical Communication Inc.; and **the top images in illustration 4 (chronic pain phase), which were reprinted, with minimal adaptations, from Oaklander (2008), with permission from Elsevier, with special credit to Oaklander (2001), also reprinted with permission from Elsevier.
Why Is There a Predilection for the Trigeminal Ophthalmic Branch in PHN?
Despite the involvement of most of the body regions during a chickenpox event, a viral overload is reported in specific dermatomal areas (e.g., ophthalmic, mid and lower thoracic, and upper lumbar) (Kennedy and Steiner, 1994). Subsequent to the resolution of the primary varicella infection, residual provirus segments migrate through sensory nerve endings to sensory fibers. After colonization of the dorsal root or cranial ganglia (usually the trigeminal ganglia), VZV settles in neuronal or satellite cell nuclei, where it is protected from antibodies present in the circulation in response to the primary infection (Weinberg, 2007). As a consequence, the virus will be latent in great proportions in the cell bodies of sensory fibers associated with those overloaded regions (Kennedy and Steiner, 1994), notably at the ophthalmic division of the trigeminal nerve, which accounts for more than 75% of herpes zoster cases localized in the cranial region (de Leeuw, 2008). As previously mentioned, this region (V1) has a large concentration of unmyelinated fibers and consequently, a lower A/C fiber ratio when compared with V2 and V3 (Sugimoto et al., 1986, 1988). This ratio of fibers is strongly related to the symptoms described by patients with PHN affecting the ophthalmic region.
It has been well-established that VZV recurrence is usually a single event that occurs only after the immunological system is compromised. One speculation for this is the VZV DNA and RNA tissue amounts present in the peripheral sensory ganglia (PSG) during latent infection. Therefore, VZV is much less capable of reactivation than HSV-1, and only the most affected dermatomes (such as the ophthalmic) are able to supply the PSG with a heavy load of viral particles. This explains the high incidence of herpes zoster as well as PHN for the ophthalmic division at the trigeminal nerve (Hope-Simpson, 1965; Kennedy and Steiner, 1994).
How Do the Virus Spread and Ratio of Affected Sensory Fibers Influence the Symptomatic Progression in Acute Herpes Zoster (AHZ) and Post-herpetic Neuralgia (PHN)?
Following its reactivation, the VZV spreads by a neuronal/ non-neuronal (i.e., satellite cells) pathway (Kennedy and Steiner, 1994; Arvin and Gershon, 2000). As a consequence, active replication will release infectious particles that will extend to most of the branch in a cell-to-cell manner, infecting many neurons and glial cells, culminating in a peripheral disease within the distribution of the nerve affected. This fact will result in a destruction of many neurons and surrounding satellite cells, affecting an extensive region and producing a transient or permanent sensory loss. Actually, the permanent neurological damage, such as post-herpetic neuralgia, that occurs following herpes zoster may be due to extensive neuronal destruction.
There is scientific evidence indicating that the varicella zoster virus (VZV) migrates from the dorsal root or trigeminal ganglia to the periphery mostly via myelinated fibers. This concept is based on the initial spread of the VZV to the skin via the isthmus of hair follicles, the same place where myelinated fibers predominantly end (Kennedy and Steiner, 1994; Muraki et al., 1996; Iwasaki et al., 2001; Walsh et al., 2005). This means of peripheral transportation used by VZV might determine symptoms during the acute phase of herpes zoster (AHZ). Patients at this initial phase appear to report sharp, stabbing, as well as lancinating pain (Dworkin et al., 2008; Oaklander, 2008), generally related to damage to A-fibers. Following infection of the follicular and sebaceous epithelium, the virus spreads to the neighboring epidermis (Boer et al., 2006). During a subsequent phase, burning pain is more often reported, which is a common characteristic of post-herpetic neuralgia (PHN) (Filadora et al., 1999; Dworkin et al., 2008; Oaklander, 2008). This later symptom could partly reflect the damage by VZV to smaller diameter sensory fibers such as the C-type in the adjacent epidermis. Ultimately, most patients with PHN describe multiple types of pain, such as constant, deep, burning, paroxysmal, and lancinating. However, it is important to mention the possible contributions of C-low-threshold mechanoreceptors for tactile allodynia, reported in a recent publication (Seal et al., 2009). In addition, other sensory disturbances frequently associated with PHN are paresthesia, dysesthesia, hyperalgesia, and itching (Dworkin et al., 2008; Truini et al., 2008). Many hypotheses have been proposed to explain this diversity in symptomatology during PHN. Initially, it was proposed that there was a preferential destruction of larger myelinated fibers (Aβ fibers), leaving an excess of the small myelinated (Aδ) and unmyelinated (C) fibers interpreted as the cause of sensory dysfunction in PHN (Noordenbos, 1959; Watson et al., 1988). Recent studies have stated that all sets of sensory fibers may be affected in this condition (Truini et al., 2008; Delaney et al., 2009), and data from neurophysiological-clinical correlations suggest a relationship between constant pain and loss of Aδ and C fibers and between paroxysmal pain and Aβ fiber demyelinization (Truini et al., 2008). Furthermore, the density of epidermal innervation has been associated with the occurrence of PHN, since samples from skin biopsies, when immunolabeled with PGP 9.5 (an axonal marker), have shown a greater amount of neuritis/mm2 in individuals without PHN than in those with PHN (Oaklander, 2001). By the same technique, an inverse correlation was demonstrated between the loss of cutaneous innervation and allodynia in patients with PHN (Rowbotham et al., 1996). According to this scenario, it is possible to establish that the progression of the disease and the remaining A/C ratio of injured fibers in the peripheral skin following herpes zoster determine the symptoms frequently reported by PHN patients. In addition, it explains the great variety of pain descriptors (Dworkin et al., 2008) usually related to PHN.
Conclusions
Pain research advanced dramatically with the novel neuroimaging tools that became largely available in the past decade, providing non-invasive access to human brain function and dysfunction. This opportunity has opened new frontiers in the study of central neuropathic pain mechanisms, complementing findings associated with other crucial sectors in animal pain models and genetics. Nonetheless, the field has recently refocused its attention on the peripheral nervous system in TNP, with a more ample description of the molecular, anatomical, and functional properties of afferent sensory fibers and their subclasses. Independent of the intermingled contributions of other, also important, central and even peripheral mechanisms, sensory fibers are the first gateway in the neuronal system for inputs that will later lead to pain perception. The role of the ratio and somatotopic distribution of injured sensory fibers in trigeminal neuropathic pain is a notable component of its pathophysiology and can immensely influence clinical pain characteristics, including its quality descriptors, location and irradiation, temporal features, progress, and treatment prognosis.
Supplementary Material
Acknowledgments
We thank Dr. Kenneth L. Casey, University of Michigan, and Dr. Jianren Mao, Harvard University, for their extraordinary assistance during the writing of this paper.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by the following grants: Dr. DaSilva was supported by NIH K23 NS062946, DANA Foundation’s Brain and Immuno-imaging award, and MICHR Clinical Trial Planning Program/CTSA high-tech funding UL1RR024986, University of Michigan. Dr. Santos was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, and by the University of Michigan, Ann Arbor, USA.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Arvin AM, Gershon AA. (2000). Varicella-zoster virus: virology and clinical management. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Azerad J, Woda A, Albe-Fessard D. (1982). Physiological-properties of neurons in different parts of the cat trigeminal sensory complex. Brain Res 246:7-21. [DOI] [PubMed] [Google Scholar]
- Bae YC, Oh JM, Hwang SJ, Shigenaga Y, Valtschanoff JG. (2004). Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol 478:62-71. [DOI] [PubMed] [Google Scholar]
- Boer A, Herder N, Blodorn-Schlicht N, Falk T. (2006). Herpes incognito most commonly is herpes zoster and its histopathologic pattern is distinctive! Am J Dermatopathol 28:181-186. [DOI] [PubMed] [Google Scholar]
- Borsook D, DaSilva AF, Ploghaus A, Becerra L. (2003). Specific and somatotopic functional magnetic resonance imaging activation in the trigeminal ganglion by brush and noxious heat. J Neurosci 23:7897-7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broton JG, Rosenfeld JP. (1982). Rostral trigeminal projections signal perioral facial-pain. Brain Res 243:395-400. [DOI] [PubMed] [Google Scholar]
- Bullitt E, Tew JM, Boyd J. (1986). Intracranial tumors in patients with facial-pain. J Neurosurg 64:865-871. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. (2009). Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman RS, Sodeman T, Denton AM, Warden RJ, Siciliano DA, Rhoades RW. (1996). Organization of primary afferent axons in the trigeminal sensory root and tract of the rat. J Comp Neurol 364:169-183. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Leandri M, Iannetti GD, Mascia A, Romaniello A, Truini A, et al. (2001). Small-fiber dysfunction in trigeminal neuralgia: carbamazepine effect on laser-evoked potentials. Neurology 56:1722-1726. [DOI] [PubMed] [Google Scholar]
- Cusick JF. (1981). Atypical trigeminal neuralgia. J Am Med Assoc 245:2328-2329. [PubMed] [Google Scholar]
- Dallel R, Raboisson P, Auroy P, Woda A. (1987). Participation of the rostral portion of the spinal nucleus of the trigeminal sensory complex in nociception. C R Acad Sci III 304:269-274. [PubMed] [Google Scholar]
- Dallel R, Raboisson P, Auroy P, Woda A. (1988). The rostral part of the trigeminal sensory complex is involved in orofacial nociception. Brain Res 448:7-19. [DOI] [PubMed] [Google Scholar]
- Dallel R, Raboisson P, Woda A, Sessle BJ. (1990). Properties of nociceptive and nonnociceptive neurons in trigeminal subnucleus oralis of the rat. Brain Res 521:95-106. [DOI] [PubMed] [Google Scholar]
- Dallel R, Villanueva L, Woda A, Voisin D. (2003). Neurobiology of trigeminal pain. Med Sci (Paris) 19:567-574. [DOI] [PubMed] [Google Scholar]
- Dandy WE. (1934). Concerning the cause of trigeminal neuralgia. Am J Surg 24:447-455. [Google Scholar]
- DaSilva AF. (2002). Somatotopic activation in the human trigeminal pain pathway. Boston, MA: Harvard University. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Acquadro MA. (2005). Orofacial pain. Pain Management Rounds 2(1):1-6. [Google Scholar]
- DaSilva AF, Becerra L, Makris N, Strassman A, Gonzalez R, Geatrakis N, et al. (2002). Somatotopic activation in the human trigeminal pain pathway. J Neurosci 22:8183-8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw R, American Academy of Orofacial Pain (2008). Orofacial pain: guidelines for assessment, diagnosis, and management. Chicago, IL: Quintessence. [Google Scholar]
- Delaney A, Colvin LA, Fallon MT, Dalziel RG, Mitchell R, Fleetwood-Walker SM. (2009). Postherpetic neuralgia: from preclinical models to the clinic. Neurotherapeutics 6:630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M. (2009). Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 196:115-128. [DOI] [PubMed] [Google Scholar]
- Devor M, Amir R, Rappaport ZH. (2002a). Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain 18:4-13. [DOI] [PubMed] [Google Scholar]
- Devor M, Govrin-Lippmann R, Rappaport ZH. (2002b). Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg 96:532-543. [DOI] [PubMed] [Google Scholar]
- Duale C, Luccarini P, Cadet R, Woda A. (1996). Effects of morphine microinjections into the trigeminal sensory complex on the formalin test in the rat. Exp Neurol 142:331-339. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Gnann JW, Oaklander AL, Raja SN, Schmader KE, Whitley RJ. (2008). Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain 9(Suppl 1):37-44. [DOI] [PubMed] [Google Scholar]
- Eller JL, Raslan AM, Burchiel KJ. (2005). Trigeminal neuralgia: definition and classification. Neurosurg Focus 18:E3. [DOI] [PubMed] [Google Scholar]
- Evans RW, Graff-Radford SB, Bassiur JP. (2005). Pretrigeminal neuralgia. Headache 45:242-244. [DOI] [PubMed] [Google Scholar]
- Filadora VA, 2nd, Sist TC, Lema MJ. (1999). Acute herpetic neuralgia and postherpetic neuralgia in the head and neck: response to gabapentin in five cases. Reg Anesth Pain Med 24:170-174. [DOI] [PubMed] [Google Scholar]
- Fromm GH, Graff-Radford SB, Terrence CF, Sweet WH. (1990). Pretrigeminal Neuralgia. Neurology 40:1493-1495. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Sasaki M, Wada T, Kudo K, Hirooka R, Ishigaki D, et al. (2010). High-resolution diffusion tensor imaging for the detection of diffusion abnormalities in the trigeminal nerves of patients with trigeminal neuralgia caused by neurovascular compression. J Neuroimaging 21: e102-e108. [DOI] [PubMed] [Google Scholar]
- Grigoryan Yu A, Slavin KV, Ogleznev K. (1994). Ultrasonic lesion of the trigeminal nucleus caudalis for deafferentation facial pain. Acta Neurochir (Wien) 131:229-235. [DOI] [PubMed] [Google Scholar]
- Hope-Simpson RE. (1965). The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med 58:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R. (2000). After-effect of transcutaneous electrical nerve stimulation (TENS) on pain-related evoked potentials and magnetic fields in normal subjects. Clin Neurophysiol 111:717-724. [DOI] [PubMed] [Google Scholar]
- Hussein M, Wilson LA, Illingworth R. (1982). Patterns of sensory loss following fractional posterior-fossa Vth nerve-section for trigeminal neuralgia. J Neurol Neurosurg Psychiatry 45:786-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki T, Muraki R, Kasahara T, Sato Y, Sata T, Kurata T. (2001). Pathway of viral spread in herpes zoster: detection of the protein encoded by open reading frame 63 of varicella-zoster virus in biopsy specimens. Arch Virol (Suppl 17):109-119. [DOI] [PubMed] [Google Scholar]
- Kennedy PG, Steiner I. (1994). A molecular and cellular model to explain the differences in reactivation from latency by herpes simplex and varicella-zoster viruses. Neuropathol Appl Neurobiol 20:368-374. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Matsumura G. (1996). Central projections of primary afferent fibers from the rat trigeminal nerve labeled with isolectin B4-HRP. Neurosci Lett 217:89-92. [PubMed] [Google Scholar]
- Kwan CL, Hu JW, Sessle BJ. (1993). Effects of tooth pulp deafferentation on brainstem neurons of the rat trigeminal subnucleus oralis. Somatosens Mot Res 10:115-131. [DOI] [PubMed] [Google Scholar]
- Love S, Gradidge T, Coakham HB. (2001). Trigeminal neuralgia due to multiple sclerosis: ultrastructural findings in trigeminal rhizotomy specimens. Neuropathol Appl Neurobiol 27:238-244. [DOI] [PubMed] [Google Scholar]
- Luccarini P, Cadet R, Duale C, Woda A. (1998). Effects of’ lesions in the trigeminal oralis and caudalis subnuclei on different orofacial nociceptive responses in the rat. Brain Res 803:79-85. [DOI] [PubMed] [Google Scholar]
- Lutz J, Linn J, Mehrkens JH, Thon N, Stahl R, Seelos K, et al. (2011). Trigeminal neuralgia due to neurovascular compression: high-spatial-resolution diffusion-tensor imaging reveals microstructural neural changes. Radiology 258:524-530. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Neundorfer B, Stefan H, Handwerker HO. (2003). Cortical processing of brush-evoked allodynia. Neuroreport 14:785-789. [DOI] [PubMed] [Google Scholar]
- Marinkovic S, Gibo H, Todorovic V, Antic B, Kovacevic D, Milisavljevic M, et al. (2009). Ultrastructure and immunohistochemistry of the trigeminal peripheral myelinated axons in patients with neuralgia. Clin Neurol Neurosurg 111:795-800. [DOI] [PubMed] [Google Scholar]
- Miller JP, Acar F, Hamilton BE, Burchiel KJ. (2009). Radiographic evaluation of trigeminal neurovascular compression in patients with and without trigeminal neuralgia. Clinical article. J Neurosurg 110: 627-632. [DOI] [PubMed] [Google Scholar]
- Mitchell RG. (1980). Pre-trigeminal neuralgia. Br Dent J 149:167-170. [DOI] [PubMed] [Google Scholar]
- Muraki R, Iwasaki T, Sata T, Sato Y, Kurata T. (1996). Hair follicle involvement in herpes zoster: pathway of viral spread from ganglia to skin. Virchows Arch 428:275-280. [DOI] [PubMed] [Google Scholar]
- Nahra H, Plaghki L. (2003). Modulation of perception and neurophysiological correlates of brief CO2 laser stimuli in humans using concurrent large fiber stimulation. Somatosens Mot Res 20:139-147. [DOI] [PubMed] [Google Scholar]
- No Authors Listed (2004). The international classification of headache disorders. 2nd ed. Cephalalgia 24(Suppl 1):9-160. [DOI] [PubMed] [Google Scholar]
- Nomura T, Ikezaki K, Matsushima T, Fukui M. (1994). Trigeminal neuralgia: differentiation between intracranial mass lesions and ordinary vascular compression as causative lesions. Neurosurg Rev 17:51-57. [DOI] [PubMed] [Google Scholar]
- Noordenbos W. (1959). Pain: problems pertaining to the transmission of nerve impulses which give rise to pain; preliminary statement. Amsterdam: Elsevier Publ. Co. [Google Scholar]
- Oaklander AL. (2001). The density of remaining nerve endings in human skin with and without postherpetic neuralgia after shingles. Pain 92:139-145. [DOI] [PubMed] [Google Scholar]
- Oaklander AL. (2008). Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain 9(Suppl 1):10-18. [DOI] [PubMed] [Google Scholar]
- Obermann M, Yoon MS, Ese D, Maschke M, Kaube H, Diener HC, et al. (2007). Impaired trigeminal nociceptive processing in patients with trigeminal neuralgia. Neurology 69:835-841. [DOI] [PubMed] [Google Scholar]
- Pajot J, Pelissier T, Sierralta F, Raboisson P, Dallel R. (2000). Differential effects of trigeminal tractotomy on A delta- and C-fiber-mediated nociceptive responses. Brain Res 863:289-292. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF. (1995). Atlas of the human brainstem. San Diego, CA: Academic Press. [Google Scholar]
- Paxinos G, Mai JK, editors (2004). The human nervous system. 3rd ed. San Diego, CA: Elsevier Academic Press. [Google Scholar]
- Roski RA, Horwitz SJ, Spetzler RF. (1982). Atypical trigeminal neuralgia in a 6-year-old boy. Case report. J Neurosurg 56:424-425. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Yosipovitch G, Connolly MK, Finlay D, Forde G, Fields HL. (1996). Cutaneous innervation density in the allodynic form of postherpetic neuralgia. Neurobiol Dis 3:205-214. [DOI] [PubMed] [Google Scholar]
- Samii M, Jannetta PJ, editors (1981). The cranial nerves: anatomy, pathology, pathophysiology, diagnosis, treatment. Berlin: Springer-Verlag. [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, et al. (2009). Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462:651-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekula RF, Frederickson AM, Jannetta PJ, Bhatia S, Quigley MR, Abdel Aziz KM. (2009). Microvascular decompression in patients with isolated maxillary division trigeminal neuralgia, with particular attention to venous pathology. Neurosurg Focus 27:E10. [DOI] [PubMed] [Google Scholar]
- Sessle BJ. (2000). Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med 11:57-91. [DOI] [PubMed] [Google Scholar]
- Shankland WE. (2000). The trigeminal nerve. Part I: An over-view. Cranio 18:238-248. [DOI] [PubMed] [Google Scholar]
- Shankland WE., 2nd (2009). Atypical trigeminal neuralgia of the mental nerve: a case study. Cranio 27:19-23. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Chen IC, Suemune S, Nishimori T, Nasution ID, Yoshida A, et al. (1986a). Oral and facial representation within the medullary and upper cervical dorsal horns in the cat. J Comp Neurol 243:388-408. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Okamoto T, Nishimori T, Suemune S, Nasution ID, Chen IC, et al. (1986b). Oral and facial representation in the trigeminal principal and rostral spinal nuclei of the cat. J Comp Neurol 244:1-18. [DOI] [PubMed] [Google Scholar]
- Sindou M, Howeidy T, Acevedo G. (2002). Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia (with correlations between topography of pain and site of the neurovascular conflict). Prospective study in a series of 579 patients. Acta Neurochir (Wein) 144:1-13. [DOI] [PubMed] [Google Scholar]
- Sindou M, Leston J, Howeidy T, Decullier E, Chapuis F. (2006). Micro-vascular decompression for primary Trigeminal Neuralgia (typical or atypical). Long-term effectiveness on pain; prospective study with survival analysis in a consecutive series of 362 patients. Acta Neurochir (Wein) 148:1235-1245. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Takemura M, Sakai A, Ishimaru M. (1986). Cell size analysis of trigeminal primary afferent neurons comprizing [sic] individual peripheral branches of the rat mandibular nerve. Exp Neurol 93:565-573. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Takemura M, Wakisaka S. (1988). Cell size analysis of primary neurons innervating the cornea and tooth pulp of the rat. Pain 32:375-381. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Fujiyoshi Y, He YF, Xiao C, Ichikawa H. (1997). Trigeminal primary projection to the rat brain stem sensory trigeminal nuclear complex and surrounding structures revealed by anterograde transport of cholera toxin B subunit-conjugated and Bandeiraea simplicifolia isolectin B4-conjugated horseradish peroxidase. Neurosci Res 28:361-371. [DOI] [PubMed] [Google Scholar]
- Symonds C. (1949). Facial pain [lecture delivered at the Royal College of Surgeons of England, on March 1, 1949]. Ann R Coll Surg Engl 4:206-212. [PMC free article] [PubMed] [Google Scholar]
- Takemura M, Sugimoto T, Shigenaga Y. (1991). Difference in central projection of primary afferents innervating facial and intraoral structures in the rat. Exp Neurol 111:324-331. [DOI] [PubMed] [Google Scholar]
- Tancioni F, Gaetani P, Villani L, Zappoli F, Rodriguez Y, Baena R. (1995). Neurinoma of the trigeminal root and atypical trigeminal neuralgia: case report and review of the literature. Surg Neurol 44:36-42. [DOI] [PubMed] [Google Scholar]
- Tashiro T, Higo S, Matsuyama T. (1984). Soma size comparison of the trigeminal ganglion cells giving rise to the ascending and descending tracts: a horseradish peroxidase study in the cat. Exp Neurol 84:37-46. [DOI] [PubMed] [Google Scholar]
- Toratani N, Moriwaki H, Hyon B, Naritomi H. (2008). Isolated hemifacial sensory impairment with onion skin distribution caused by small pontine hemorrhage. Eur Neurol 59:192-194. [DOI] [PubMed] [Google Scholar]
- Tran TD, Matre D, Casey KL. (2008). An inhibitory interaction of human cortical responses to stimuli preferentially exciting Adelta or C fibers. Neuroscience 152:798-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A, Galeotti F, Cruccu G. (2005). New insight into trigeminal neuralgia. J Headache Pain 6:237-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A, Galeotti F, Haanpaa M, Zucchi R, Albanesi A, Biasiotta A, et al. (2008). Pathophysiology of pain in postherpetic neuralgia: a clinical and neurophysiological study. Pain 140:405-410. [DOI] [PubMed] [Google Scholar]
- Turp JC, Gobetti JP. (1996). Trigeminal neuralgia versus atypical facial pain. A review of the literature and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81:424-432. [DOI] [PubMed] [Google Scholar]
- Vassilakis D, Phylaktakis M, Selviaridis P, Karavelis A, Sirmos C, Vlaikidis N. (1988). Symptomatic trigeminal neuralgia. J Neurosurg Sci 32: 117-120. [PubMed] [Google Scholar]
- Wall PD, McMahon SB, Koltzenburg M. (2006). Wall and Melzack’s textbook of pain. Philadelphia, PA: Elsevier/Churchill Livingstone. [Google Scholar]
- Walsh N, Boutilier R, Glasgow D, Shaffelburg M. (2005). Exclusive involvement of folliculosebaceous units by herpes: a reflection of early herpes zoster. Am J Dermatopathol 27:189-194. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Svensson P, Arendt-Nielsen L. (1999). Influence of segmental and extra-segmental conditioning, stimuli on cortical potentials evoked by painful electrical stimulation. Somatosens Mot Res 16:243-250. [DOI] [PubMed] [Google Scholar]
- Watson CP, Oaklander AL. (2002). Postherpetic neuralgia. Pain Pract 2:295-307. [DOI] [PubMed] [Google Scholar]
- Watson CP, Oaklander AL. (2006). Chapter 44. Postherpetic neuralgia. Handb Clin Neurol 81:661-677. [DOI] [PubMed] [Google Scholar]
- Watson CP, Morshead C, Van der Kooy D, Deck J, Evans RJ. (1988). Post-herpetic neuralgia: post-mortem analysis of a case. Pain 34: 129-138. [DOI] [PubMed] [Google Scholar]
- Weinberg JM. (2007). Herpes zoster: epidemiology, natural history, and common complications. J Am Acad Dermatol 57(6 Suppl):130S-135S. [DOI] [PubMed] [Google Scholar]
- Windle WF. (1926). The distribution and probable significance of unmyelinated nerve fibers in the trigeminal nerve of the cat. J Comp Neurol 41:453-477. [Google Scholar]
- Woda A. (2003). Pain in the trigeminal system: from orofacial nociception to neural network modeling. J Dent Res 82:764-768. [DOI] [PubMed] [Google Scholar]
- Woda A, Molat JL, Luccarini P. (2001). Low doses of N-methyl-D-aspartate antagonists in superficial laminae of medulla oblongata facilitate wind-up of convergent neurones. Neuroscience 107:317-327. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. (1999). Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 353:1959-1964. [DOI] [PubMed] [Google Scholar]
- Young RF, King RB. (1973). Fiber spectrum of trigeminal sensory root of baboon determined by electron-microscopy. J Neurosurg 38:65-72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.