Abstract
Using the Bmp2 floxed/3.6Col1a1-Cre (Bmp2-cKOod) mouse model, we have observed severe defects in odontogenesis and dentin formation with the removal of the Bmp2 gene in early-polarizing odontoblasts. The odontoblasts in the Bmp2-cKOod do not mature properly and fail to form proper dentin with normal dentinal tubules and activate terminal differentiation, as reflected by decreased Osterix, Col1a1, and Dspp expression. There is less dentin, and the dentin is hypomineralized and patchy. We also describe an indirect effect of the Bmp2 gene in odontoblasts on formation of the vascular bed and associated pericytes in the pulp. This vascular niche and numbers of CD146+ pericytes are likely controlled by odontogenic and Bmp2-dependent VegfA production in odontoblasts. The complex roles of Bmp2, postulated to be both direct and indirect, lead to permanent defects in the teeth throughout life, and result in teeth with low quantities of dentin and dentin of poor quality.
Keywords: bone morphogenetic protein 2, blood vessels, dentinogenesis, dental pulp stem cells, odontogenesis, pericytes
Introduction
The Bmp2 gene is expressed in post-natal odontoblasts and ameloblasts during tooth cytodifferentiation from birth to approximately 20 days after birth (Aberg et al., 1997). Previous studies have determined key roles for Bmp2 and Bmp4 during embryonic tooth development, but little is known about the specific role of Bmp2 during post-natal tooth cytodifferentiation (Maas and Bei, 1997). Several genes have been linked to dentinogenesis imperfecta (DGI) in humans and several mouse models (Sreenath et al., 2003). One of the most highly expressed odontoblast-specific terminal differentiation genes is dentin sialophosphoprotein (Dspp). Mutations in this gene have been linked to DGI type III. Studies have shown that Dspp in odontoblasts is regulated by Bmp signaling in vitro and in vivo (Chen et al., 2008, 2009; Cho et al., 2010). Defects in odontogenic collagen type 1a (Col1a1) production are also linked to dentin defects of various degrees in both humans and mice (Hart and Hart, 2007). It is known that dentin-derived Bmp2 drives dental stem cells from exfoliated deciduous teeth (SHED) into mature dentin-producing odontoblasts (Huang et al., 2009; Casagrande et al., 2010). CD146+ and Stro-1 perivascular cells have the capability of differentiating into odontoblasts in vivo (Shi and Gronthos, 2003). These types of cells in human bone marrow have been shown to have the characteristics of skeletal stem cells and organize around blood vessels (Sacchetti et al., 2007). Analysis of recent data, with a lineage-tracing method for NG2-marked pericytes, demonstrated that as much as 15% of the pericytes can become odontoblasts in an incisor model of tissue repair (Feng et al., 2011). One other likely region for a dental stem cell niche is the apical papilla at the base of the root (Huang et al., 2008).
We demonstrate how the Bmp2 gene controls the terminal differentiation of odontoblasts in post-natal animals and, at the same time, the formation of the vascular bed in the dental pulp and associated pericytes on the pulp blood vessels.
Materials & Methods
Animals
All mice were used in this study in compliance with the UTHSCSA institutional Animal Care and Committee guidelines. Mice deficient in Bmp2 in early odontoblasts and osteoblasts were generated by crossing Bmp2 floxed (Bmp2-fx/fx) with a 3.6Col1a1-Cre mouse (Liu et al., 2004; Singh et al., 2008). These mice are referred to as Bmp2-cKOod. Specific deletion of Bmp4 in teeth (odontoblasts), in the 3.6Col1a1-Cre model, has recently been described and demonstrates odontoblast specificity with a Rosa 26-GFP reporter in which the GFP cassette is activated after Cre recombination (Gluhak-Heinrich et al., 2010).
In this study, we used a total of 86 animals: 29 Bmp2-cKOod, 26 heterozygotes (Hets) (Bmp2 fx/+), and 31 wild types (Wt) with the following ages: 1, 5, and 14 days, 1 mo, 4 mos, and 8 mos. At least 3 independent littermates were used in each of these studies. Control mice, representing heterozygotes and wild types, did not show significant differences in tooth or bone phenotype and were pooled for statistical evaluation. For initial x-ray studies, all mice were evaluated with age and group size 4-20. For µCT, 3 animals per group (genotype) were used for statistical evaluation by a simple unpaired t test. For quantitation of the CD146+ cells, the cells were counted in 2 independent littermates from multiple sections, and significance was determined by simple unpaired t test. For most in situ hybridization and immunological studies, multiple sections from a given mouse were first evaluated and then from 2 or 3 independent littermates, and data from the 2-3 different animals were combined for statistical evaluation with a simple unpaired t test for significance. ANOVA analysis was also carried out for most studies with the 2 groups.
X-ray Analysis and µCT
Radiographic images of mandibles and associated teeth were obtained with image capture in a digital Faxitron radiograph unit (Model 8050-020, Field Emission Corporation, Inc. Tokyo, Japan) and analyzed with AnalySIS software measuring tooth parameters of the 1st and 2nd molars, as described in Gluhak-Heinrich et al. (2010). µCT analysis of 1st and 2nd molars, by Numira, Inc. (www.numirabio.com), shows volumetric evaluation of the enamel, crown dentin, crown pulp, root dentin, root pulp, and the periodontium with at least 2 independent litters of 1 and 4 mos of age. Male mice were used.
Tissue Preparation and Analysis
Tissues were fixed, demineralized, and processed RNase-free for in situ hybridization as described previously (Gluhak-Heinrich et al., 2008). [A detailed description of the in situ hybridization protocol and our method for numerical estimate of expression signal (blue) and corrected background signals is given in the Appendix.] For dentin formation rates, mice injected with alizarin red at 14 days and calcein at 22 days were sacrificed at 25 days. We used plastic-embedded sections after double-labeling as described previously (Ye et al., 2004). We used Col1a1, Dspp (Gluhak-Heinrich et al., 2010), Bmp2 exon3 (region deleted by Cre), Osterix-Sp7, and VegfA probes. For numerical evaluation of expression signal hybridization, several parameters must be carefully followed. First, the mandibles are fixed with fresh DEPC-treated cold 4% paraformaldehyde for 48 hrs at 4°C with rotation at 30 rpm. The tissue is washed with RNase-free PBS and decalcified for 6 wks at 4°C, rotating 30 rpm with 15% EDTA–RNase-free solution and changed 2× per wk. This procedure ensures complete decalcification; leaving the fixative in during the 6-week decalcification is neither desirable nor necessary. Next, the digoxigenin probes are checked for an approximate linear relationship of RNA and digoxigenin signal (see Appendix). The in situ hybridization signal with the BCIP/NBT is blue to blue-purple, and the counterstain is light green. Consecutive sections were used for histological hematoxylin-eosin (H&E), immunocytochemistry, and Goldner staining (Intini et al., 2007). Immunocytochemistry was carried out as previously described (Gluhak-Heinrich et al., 2010) to detect Phospho-Smad1/5/8 (Cell Signaling Technology, Inc. #9511S, Boston, MA USA), CD31 (Abcam#28364, San Francisco, CA, USA), CD146 (Abcam#75769), and VegfA (Abcam#46154). With the Fast Red substrate for alkaline phosphatase used in the immunocytochemistry, the signal is always red, and a good contrast with the light green counterstain is achieved.
A full list of the ages of animals used in each method applied and the numbers of animals used for each method is given in the Appendix Table.
Results
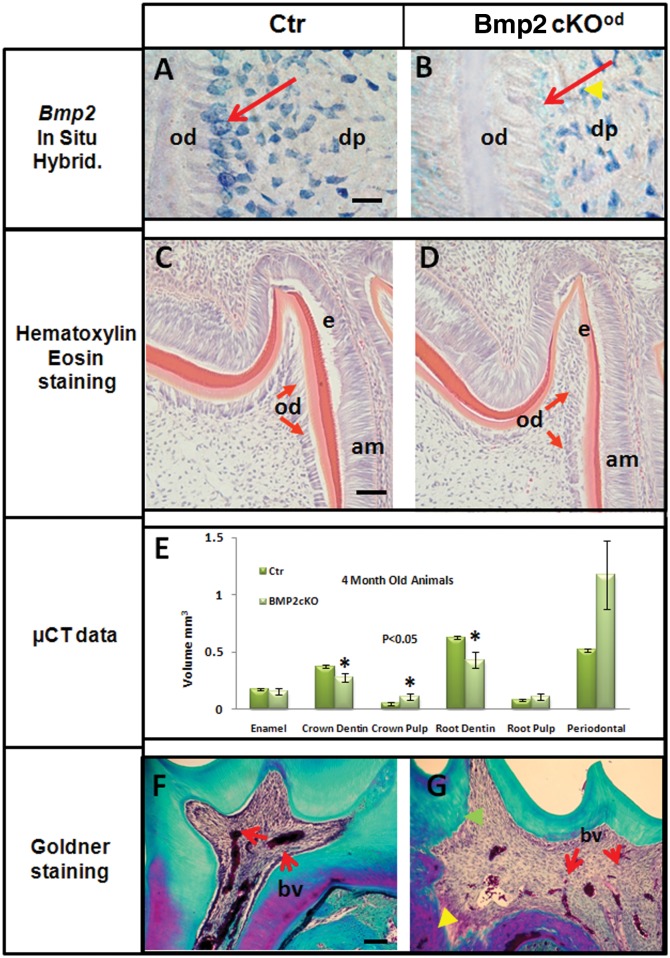
We determined that the 3.6Col1a1-Cre model deleted the Bmp2 gene in odontoblasts (Figs. 1A, 1B). The Bmp2 (Exon3) signal was reduced 90% in Bmp2-cKOod odontoblasts (red arrow) of 1st molar teeth at 5 days of age (n = 3 animals per group). The level of Bmp2 Exon3 expression in select cells in the dental pulp was not affected (yellow arrowhead) in Bmp2-cKOod. The odontoblasts in the Bmp2-cKOod were dysmorphic and failed to form a well-organized polarized structure, as noted by the red arrows in Figs. 1C and 1D. The thickness of the predentin at this early stage was also reduced (light red). The ameloblasts appeared morphologically normal, although there was a delay in the formation of pre-enamel, as noted by the darker red staining (Figs. 1C and 1D). By µCT of the 1st and 2nd molars of 4-month-old animals (Fig. 1E) and 1-month-old animals (Appendix Figs. 1A, 1B), volumetric measurements were carried out on the mineralized regions in enamel, crown dentin, crown pulp, root dentin, root pulp, and periodontium. We observed a 30 to 50% decrease in dentin volume of both crown and roots. By x-ray analysis, dentin thickness decreased 50% (Appendix Fig. 1C). This pronounced decreased dentin thickness was still observed in 8-month-old Bmp2-cKOod animals compared with controls (data not shown). When the sections from 4-month-old molars (Figs. 1F and 1G) were stained with Goldner (Intini et al., 2007), the dentin of the Bmp2-cKOod showed a patchy disorganized structure with disorganized dentinal tubules and reduced blood vessel size and number in the pulp. No significant change in the enamel or periodontal volume was noted, although there was a trend of increased periodontal volume in Bmp2-cKOod, possibly due to the overall smaller teeth relative to growth of the jaw at 4 mos. These defects were permanent in the teeth as evaluated at 4 to 8 mos.
Figure 1.
Characterization of tooth phenotype after Bmp2 deletion in a 3.6Col1a1Bmp2Cre-cKO (Bmp2-cKOod) model. (Panel A) Control, Ctr. (Panel B) Bmp2-cKOod. Bmp2 mRNA Exon3 (region deleted by Cre recombination) in situ hybridization (blue) of 5-day-old Bmp2-cKOod mice was reduced about 90% in odontoblasts compared with control-WT mice (red arrows). Little change of Bmp2 Exon3 signal in the pulp was noted (yellow arrowhead). Bar = 20 µm. (Panel C) Control, Ctr. (Panel D) Bmp2-cKOod. Hematoxylin & eosin staining of 5-day-old Bmp2-cKOod mice. 1st molar shows 50% (P < 0.001, n = 3) thinner pre-dentin compared with that in control-Wt. Note the disorganized odontoblasts (red arrows) in the Bmp2-cKOod animals, compared with the well-organized and polarized odontoblasts in the Ctr. Bar = 100 µm. (Panel E) µCT analysis: Four-month-old Bmp2-cKOod mice displayed a significant (P < 0.05) decrease in crown dentin and root dentin volume and also displayed a significant (P < 0.05) increase in crown pulp volume (n = 3 in each group). (Panel F) Control, Ctr. (Panel G) Bmp2-cKOod. Goldner staining of 1st molars of 4-month-old control and Bmp2-cKOod animals demonstrated permanent post-natal quantitative and structural changes in the dentin, with extensive poorly mineralized and “patchy” dentin (purple and noted by yellow arrowhead in Panel G, Bmp2-cKOod), as well as disorganized tubules (green arrowhead) with fewer and smaller blood vessels in the dental pulp (marked bv and red arrows). Bar = 40 µm. Ameloblasts (am), odontoblasts (od), dentin-pulp (dp), and blood vessels (bv).
We first determined changes in the Bmp-dependent signaling pathway for possible mechanisms. There was a decrease of over 80% in the level of phospho-Smad1/5/8 immunoreactivity in odontoblasts of Bmp2-cKOod compared with control teeth at 5 post-natal days during active molar tooth growth and formation (n = 3 animals per group) (Figs. 2A and 2B). We noted a low level of Bmp signaling in the dental pulp in the control, and less in Bmp2-cKOod. We also found decreased phospho-Smad1/5/8 levels in the ameloblasts, suggesting an indirect role of odontogenesis in amelogenesis. However, we noted no significant permanent decrease in enamel volume at 1 or 4 mos (Fig. 1E; Appendix Fig. 1B).
Figure 2.
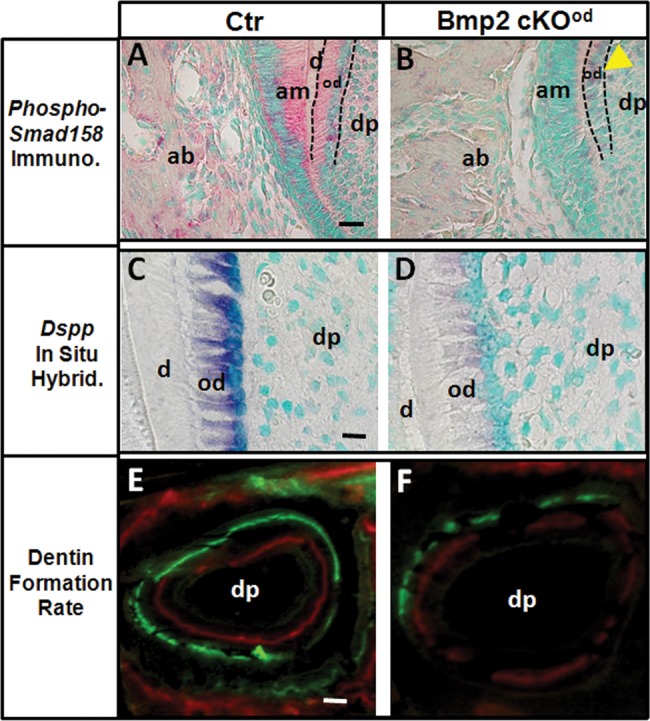
Bmp Signaling, Dspp expression, and dentin formation rate (DFR) of the post-natal teeth in the Bmp2-cKOod model. (Panel A) Control, Ctr. (Panel B) Bmp2-cKOod. Phospho-Smad 1/5/8 imm-unoreactivity was reduced 80% in odontoblasts (yellow arrowhead) and in the dental pulp and ameloblasts of the 1-day-old Bmp2-cKOod compared with Ctr. Bar = 20 µm. (Panels C, D) In situ hybridization with Dspp probe shows a 90% reduced hybridization numerical estimated signal in odontoblasts of Bmp2-cKOod compared with Ctr, 5-day-old animals. Bar = 20 µm. DFR analysis shows a 45% reduced dynamic dentin formation rate, as measured at 25 days, in Bmp2-cKOod mice compared with Ctr (Panels E, F) (n = 3 animals per group, P < 0.05). Bar = 100 µm. Alveolar bone (ab), ameloblasts (am), odontoblasts (od), dental pulp (dp).
A key transcription factor that regulates odontogenesis as well as osteoblastogenesis is the gene Osterix/Sp7 (Chen et al., 2009; Zhou et al., 2010). Osterix, a Zn2+ finger transcription factor, directly co-regulates many genes, including Col1a1, Dmp1 (Zhou et al., 2010), and Dspp. Osterix expression was reduced 60% in odontoblasts of 5-day molar odontoblasts (n = 3) (Appendix Figs. 2D, 2E, 2F). The major structural protein for dentin is type 1 collagen (Col1a1), which is decreased 70% during early tooth cytodifferentiation (data not shown). Even in the continuously erupting incisors (4 mos), we observed a 75% decrease in Col1a1 expression (Appendix Figs. 2A, 2B, 2C). Osterix was also reduced 60% in the 4-month-old continuously growing incisors (data not shown).
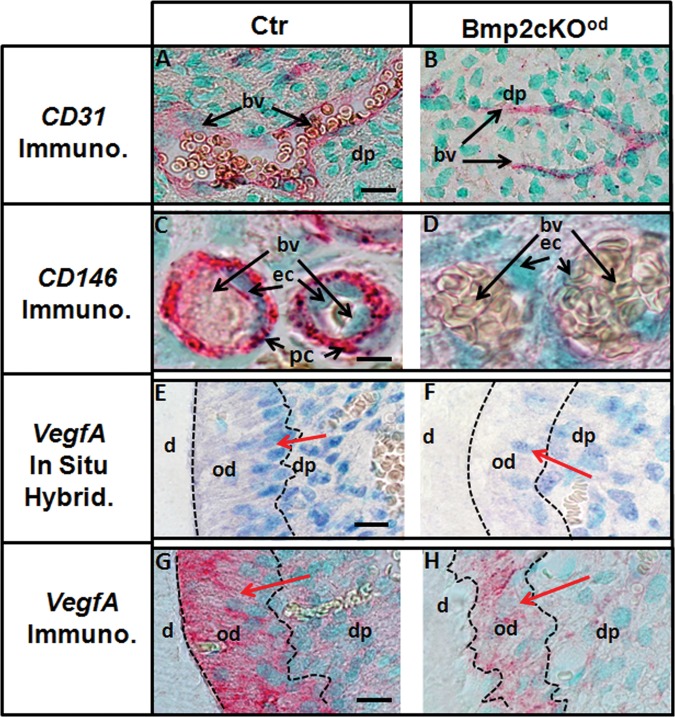
Dspp expression was reduced over 90% in the Bmp2-cKOod compared with control, 5-day 1st molars, indicating a failure in terminal differentiation (Figs. 2C, 2D) (n = 3). There was 60% reduced dynamic Dentin Formation Rate (DFR) as measured between 14 and 22 days, in Bmp2-cKOod mice, compared with control (Figs. 2E, 2F) (n = 2). A decrease in blood vessels in dental pulp of the Bmp2-cKOod animals was initially observed (Figs. 1F, 1G). The blood vessels in dental pulp provide at least one niche for dental pulp stem cells, and those in bone marrow do the same for skeletal stem cells (Gronthos et al., 2002; Shi and Gronthos, 2003; Sacchetti et al., 2007; Feng et al., 2011). Osteoblast stem cells also move into developing bone along with invading blood vessels (Maes et al., 2010a). CD146 expression is a marker for vascular-associated pericyte/stem cells, including bone marrow and muscle and neural tissue (Sacchetti et al., 2007; Tormin et al., 2011). CD146+ vascular-associated cells show all the required properties of a self-renewing stem cell population (Sacchetti et al., 2007). CD146+ cells have also been proposed as dental pulp stem cells, or DPSC (Gronthos et al., 2002; Shi and Gronthos, 2003). Using antibody to CD31, we observed a 50% reduction of blood vessels in the dental pulp of 5-day molars (Figs. 3A, 3B). Low magnification is shown in Appendix Figs. 3A and 3B. There was 90% reduction of total CD146+ cells in pulp in the Bmp2-cKOod compared with control and 70% actual reduction of CD146+ cells on blood vessels. In many cases, in the Bmp2-cKOod blood vessels, we saw no positive CD146+ pericytes (Fig. 3D) compared with control (Fig. 3C) (n = 3 animals per group). Low magnification of these CD146+ results is shown in Appendix Figs. 3C and 3D.
Figure 3.
Blood vessels (CD31+) and associated CD146+ pericytes plus VegfA expression were reduced in the Bmp2-cKOod compared with control. (Panels A, B) The number of BV (CD31+ regions) in the Bmp2-cKOod dental pulp region was decreased 50% compared with Ctr in 5-day-old animals. Bar = 20 µm. (Panel C) Control, Ctr. (Panel D) Bmp2-cKOod. CD146+ cells/pericytes associated with BV. Total CD146+ immunoreactivity was reduced 90% in the Bmp2-cKOod mice in the dental pulp as shown for the 1st molars of 1-month-old animals (n = 3 per group). (See Appendix Figs. 3C and 3D for low magnification.) The numbers of CD146+ cells directly associated with a BV are also reduced by 70%, as shown by an extreme example in panels C and D. Bar = 10 µm. VegfA mRNA expression in incisors of 4-month-old control and Bmp2-cKOod is shown in Panels E and F (blue). VegfA was expressed in more odontoblasts and showed a stronger signal per odontoblast in control compared with Bmp2-cKOod animals (red arrows). The VegfA hybridization signal in the odontoblast layer, on average, was reduced over 60% in the Bmp2-cKOod compared with Ctr. Bar = 10 µm. VegfA immunocytochemistry (red) signal in incisors of 4-month-old animals was reduced 80% in Bmp2-cKOod compared with Ctr (Panels G, H, red arrows). See Appendix Figs. 3G and 3H for low magnifications, with reduced VegfA protein associated with blood vessels in the pulp and the odontoblast layer in the Bmp2-cKOod animals. Bar = 10 µm. Dental pulp (dp), blood vessels (bv), Pericyte (pc), endothelial cells (ec), odontoblasts (od), and dentin (d).
VegfA (vascular endothelial cell growth factor A), a Bmp2-regulated protein, is a key factor in angiogenesis and blood vessel formation in bone marrow osteoblasts and dental pulp region (Deckers et al., 2002; Gonçalves et al., 2007; Zhang et al., 2009; Maes et al., 2010b). Bmp2 has been shown to promote proliferation and migration of endothelial cells through the VegfA/VegfR2 signaling pathway (Suzuki et al., 2008). We observed a 65% reduction in VegfA mRNA expression signal (blue) in odontoblasts, as well as in a subset of dental pulp cells in the Bmp2-cKOob (n = 3) (Figs. 3E, 3F). Low magnifications are given in Appendix Figs. 3E and 3F. By immunocytochemistry with a VegfA antibody (Figs. 3G and 3H; Appendix Figs. 3G and 3H, low magnification), we observed an 80% reduction of VegfA protein in the odontoblasts of the Bmp2-cKOod (n = 3).
Hif1α transcription factor expression is a sensitive read-out of hypoxic conditions, and the decreased blood vessels in dental pulp could potentially lead to hypoxia, which may affect terminal differentiation of the odontoblasts, as in bone (Araldi and Schipani, 2010) and in teeth (Amemiya et al., 2003). VegfA is a direct transcriptional target for Hif1α that is activated by hypoxia. In the Bmp2-cKOod model, VegfA was actually decreased 60 to 70% in the odontoblasts, suggesting that hypoxia is not a major issue. We confirmed this hypothesis and, in fact, found Hif1α levels decreased in the pulp region associated with blood vessels and near the base of the pulp chamber in the Bmp2-cKOod, with little or slightly decreased Hif1a levels in the odontoblast upper layer (Figs. 4A and 4B, overall low magnification; Figs. 4C and 4D, red immunostain in pulp and associated blood vessels; Figs. 4E and 4F, Hif1a immunostain associated with odontoblasts).
Figure 4.
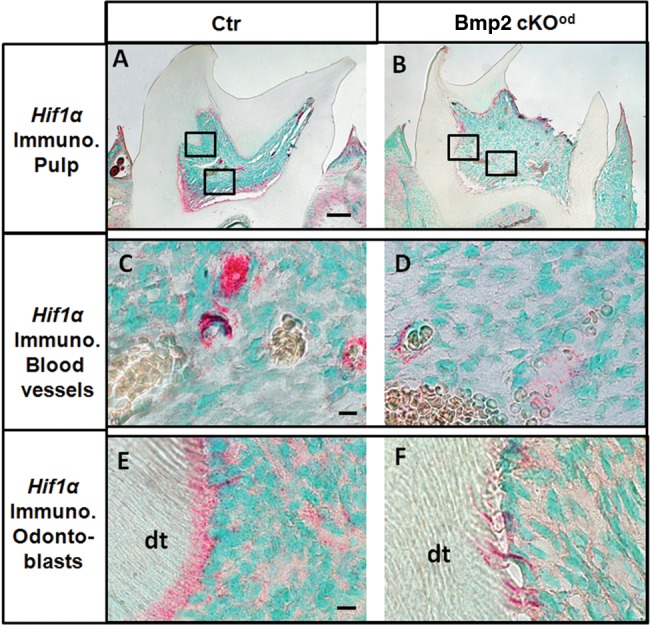
Hif1α immunocytochemistry of Ctr and Bmp2-cKOod animals, 1 mo old. (Panel A) Control, Ctr. (Panel B) Bmp2-cKOob. Low magnification (bar = 100 µm) of 1st molars, showing overall Hif1α levels. (Panel C) Control, Ctr. (Panel D) Bmp2-cKOob. High magnification (bar = 20 µm) of Hif1α levels in the pulp associated with the blood vessels. (Panel E) Control, Ctr. (Panel F) Bmp2-cKOod. High magnification (bar = 20 µm) showing Hif1α levels associated with the odontoblast layer. Note the disorganized odontoblasts in the Bmp2-cKOod and the permanent disorganized dentinal tubules (dt) in the Bmp2-cKOob.
Discussion
Deletion of the Bmp2 gene in early odontoblasts results in a permanent tooth phenotype in both the molars and incisors, with a decrease of dentin in the crown, and shows a more pronounced effect on root dentin. The quality of dentin is altered, with patchy unmineralized areas and dysmorphic dentinal tubules. There is a delay in amelogenesis in the absence of odontoblast Bmp2, but no overt change in the quantity of enamel. In the dental pulp, there are reduced blood vessels and associated pericytes. There appear to be no hypoxic conditions. The dentin and pulp phenotypes may be independent, but we hypothesize that the VegfA from terminal odontoblasts is required to link the terminal differentiation program to generation of new blood vessels and associated pericytes. These pericytes are at least one class of dental pulp stem cells, but are by no means the major niche in the context of incisor function (Feng et al., 2011). The lack of blood vessels and associated pericytes most likely does not directly contribute to the major defects in dentin formation seen in the Bmp2-cKOod. There may be an overall link in a cycle of terminal differentiation and production of VegfA and other unknown factors that play a role in blood vessel and pericyte formation. We summarize our results and model in Appendix Fig. 4, as 2 separate Bmp2-dependent pathways, linked by VegfA.
Why do Bmp2 and Bmp-4 differ in their individual roles in tooth cytodifferentiation? Previously, we reported that deletion of Bmp4 gene also leads to decreased terminal differentiation of odontoblasts (Gluhak-Heinrich et al., 2010). However, the phenotype was milder in the Bmp4-cKOod. In Bmp2-cKOod, we see no change in Bmp4 expression, suggesting that they are not linked and may signal through different receptor combinations (Cheifetz, 1999; Little and Mullins, 2009). The extent of interaction of Bmp4 and Bmp2 with extracellular heparin-sulfate proteoglycan and noggin-like inhibitor molecules may also play a role in the difference between how Bmp2 and Bmp4 function (Dutko and Mullins, 2011). Investigations with Bmp2 and Bmp4-cKO in bone and teeth are now being carried out to resolve some of these issues.
Thus, the primary mechanism for the decreased quantity and quality of dentin in the Bmp2-cKOod resides in the failure of functional odontoblasts to differentiate and to produce the necessary terminal products such as collagen type I and expression of Dspp and its products. At a morphological level, the odontoblasts in Bmp2-cKOod are not elongated and do not form well-polarized odontoblasts. Bmp2 in an autocrine fashion, produced by odontoblasts, directly signals to themselves and adjacent odontoblasts, drives differentiation, and activates the genetic program for proper dentin formation. A secondary consequence of this failure in function is the lack of the Bmp2-regulated gene product, VegfA. VegfA overexpression in bone marrow leads not only to new blood vessels and associated skeletal stem cells but also to massive new bone formation (Maes et al., 2010b). We hypothesize that the lack of VegfA from odontoblasts leads to the loss of blood vessels in the pulp and associated pericytes. How much this vascular phenotype contributes to overall changes in quantity and quality of dentin is not known, but is worthy of further study.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by the National Institutes of Health research grants NIDCR DE16949 and DE018865 to JGH and WY, and by NIAMSAR054616 and AR46798 grants to SEH. This work was submitted to the graduate faculty, UTHSCSA, in partial fulfillment of the requirements for the PhD degree to Wuchen Yang, DDS, MS, PhD.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aberg T, Wozney J, Thesleff I. (1997). Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn 210:383-396. [DOI] [PubMed] [Google Scholar]
- Amemiya K, Kaneko Y, Muramatsu T, Shimono M, Inoue T. (2003). Pulp cell responses during hypoxia and reoxygenation in vitro. Eur J Oral Sci 111:332-338. [DOI] [PubMed] [Google Scholar]
- Araldi E, Schipani E. (2010). Hypoxia, HIFs and bone development. Bone 47:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. (2010). Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res 89:603-608. [DOI] [PubMed] [Google Scholar]
- Cheifetz S. (1999). BMP receptors in limb and tooth formation. Crit Rev Oral Biol Med 10:182-198. [DOI] [PubMed] [Google Scholar]
- Chen S, Gluhak-Heinrich J, Martinez M, Li T, Wu Y, Chuang H, et al. (2008). Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling. J Biol Chem 283:19359-19370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Gluhak-Heinrich J, Wang YH, Wu YM, Chuang HH, Chen L, et al. (2009). Runx2, Osx and Dspp expression in tooth development. J Dent Res 88:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YD, Yoon WJ, Woo KM, Baek JH, Park JC, Ryoo HM. (2010). The canonical BMP signaling pathway plays a crucial part in stimulation of dentin sialophosphoprotein expression by BMP-2. J Biol Chem 285:36369-36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, et al. (2002). Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 143:1545-1553. [DOI] [PubMed] [Google Scholar]
- Dutko JA, Mullins MC. (2011). SnapShot: BMP signaling in development. Cell 145:636. [DOI] [PubMed] [Google Scholar]
- Feng J, Mantesso A, DeBari C, Nishiyama A, Sharpe PT. (2011). Dual origin of mesenchymal stem cells contributing organ growth and repair. Proc Natl Acad Sci USA 108:6503-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluhak-Heinrich J, Yang W, Harris M, Harris SE. (2008). Quantitative in situ hybridization with enhanced sensitivity in soft, bone and tooth tissue using digoxigenin tagged RNA probes. Biochem Med 18:59-80. [Google Scholar]
- Gluhak-Heinrich J, Guo D, Yang W, Harris MA, Lichtler A, Kream B, et al. (2010). New roles and mechanism of action of BMP4 in postnatal tooth cytodifferentiation. Bone 46:1533-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves SB, Dong Z, Bramante CM, Holland GR, Smith AJ, Nör JE. (2007). Tooth slice-based models for the study of human dental pulp angiogenesis. J Endod 33:811-814. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. (2002). Stem cell properties of human dental pulp stem cells. J Dent Res 81:531-535. [DOI] [PubMed] [Google Scholar]
- Hart PS, Hart TC. (2007). Disorders of human dentin. Cells Tissues Organs 186:70-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. (2008). The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and BioRoot engineering. J Endod 34:645-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Gronthos S, Shi S. (2009). Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intini G, Andreana S, Intini FE, Buhite RJ, Bobek LA. (2007). Calcium sulfate and platelet-rich plasma make a novel osteoinductive biomaterial for bone regeneration. J Transl Med 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SC, Mullins MC. (2009). Bone morphogenetic protein heterodimers assemble heteromeric type l receptor complexes to pattern the dorsoventral axis. Nat Cell Biol 11:637-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Woitge HW, Braut A, Kronenberg MS, Lichtler AC, Mina M, et al. (2004). Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int J Dev Biol 48:645-653. [DOI] [PubMed] [Google Scholar]
- Maas R, Bei M. (1997). The genetic control of early tooth development. Crit Rev Oral Biol Med 8:4-39. [DOI] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, et al. (2010a). Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 19:329-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Goossens S, Bartunkova S, Drogat B, Coenegrachts L, Stockmans I, et al. (2010b). Increased skeletal Vegf enhances beta-catenin activity and results in excessively ossified bones. EMBO J 29:424-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131:324-336. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. (2003). Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18:696-704. [DOI] [PubMed] [Google Scholar]
- Singh AP, Castranio T, Scott G, Guo D, Harris MA, Ray M, et al. (2008). Influences of reduced expression of maternal bone morphogenetic protein 2 on mouse embryonic development. Sex Dev 2:134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, et al. (2003). Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem 278:24874-24880. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Montagne K, Nishihara A, Watabe T, Miyazono K. (2008). BMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signalling. J Biochem 143:199-206. [DOI] [PubMed] [Google Scholar]
- Tormin A, Li O, Brune JC, Walsh S, Schötz B, Ehinger M, et al. (2011). CD146 expression on primary non-hematopoietic bone marrow stem cells correlates to in situ localization. Blood 117:5067-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, et al. (2004). Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem 279:19141-19148. [DOI] [PubMed] [Google Scholar]
- Zhang F, Qiu T, Wu X, Wan C, Shi W, Wang Y, et al. (2009). Sustained BMP signaling in osteoblasts stimulates bone formation by promoting angiogenesis and osteoblast differentiation. J Bone Miner Res 24:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhang Z, Feng JQ, Dusevich VM, Sinha K, Zhang H, et al. (2010). Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci USA 107:12919-12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.