S. mutans dextranase was crystallized by the sitting-drop vapour-diffusion method. The crystals diffracted to a resolution of 1.6 Å and belonged to space group P21.
Keywords: dextran, dextranases, glycoside hydrolase family 66, isomaltooligosaccharides, Streptococcus mutans
Abstract
Streptococcus mutans dextranase hydrolyzes the internal α-1,6-linkages of dextran and belongs to glycoside hydrolase family 66. An N- and C-terminal deletion mutant of S. mutans dextranase was crystallized by the sitting-drop vapour-diffusion method. The crystals diffracted to a resolution of 1.6 Å and belonged to space group P21, with unit-cell parameters a = 53.2, b = 89.7, c = 63.3 Å, β = 102.3°. Assuming that the asymmetric unit of the crystal contained one molecule, the Matthews coefficient was calculated to be 4.07 Å3 Da−1; assuming the presence of two molecules in the asymmetric unit it was calculated to be 2.03 Å3 Da−1.
1. Introduction
Dextranases (EC 3.2.1.11) hydrolyze the internal α-1,6-linkages of dextran in an endo reaction mode, producing isomaltooligosaccharides of various sizes. Based on the similarities in their amino-acid sequences, dextranases are mainly classified into glycoside hydrolase family (GH) 49 and GH 66 in the CAZY database (Henrissat & Bairoch, 1996 ▶; Cantarel et al., 2009 ▶; http://www.cazy.org/). Dextranases belonging to GH 49 are found in bacteria and fungi, and a crystallographic study has revealed that the catalytic domain of Dex49A from Penicillium minioluteum is composed of a right-handed parallel β-helix (Larsson et al., 2003 ▶). GH 66 enzymes consist of dextranases and cycloisomaltooligosaccharide glucanotransferases (CITases; EC 2.4.1.248) and these enzymes are mainly found in bacteria. Both enzymes utilize dextran as a substrate, but CITases synthesize cycloisomaltooligosaccharide (a cyclic saccharide linked by α-1,6-glucosyl units) from dextran by intramolecular transglycosylation (Oguma et al., 1993 ▶, 1994 ▶; Funane et al., 2011 ▶). Amino-acid sequence comparison among GH 66 enzymes revealed that they are composed of four regions: an N-terminal variable region, a conserved region, a glucan-binding region and a C-terminal variable region (Igarashi et al., 2002 ▶, 2004 ▶; Morisaki et al., 2002 ▶), together with a CITase-specific region which is only observed in CITases (Funane et al., 2011 ▶). Although no three-dimensional structures of GH 66 enzymes have been determined, the similarity of the amino-acid sequence of the conserved region to members of GH 13, GH 27, GH 31 and GH 36 implies that GH 66 enzymes share a common (β/α)8-barrel core with these families (Rigden, 2002 ▶). GH 49 enzymes act with an inverting catalytic mechanism, but GH 66 enzymes, as well as GH 13, GH 27, GH 31 and GH 36 enzymes, are known to employ a retaining mechanism.
Dextranase from Streptococcus mutans (SmDex), a bacterium found in human dental plaque, is a well studied enzyme in GH 66. S. mutans synthesizes extracellular α-glucans from sucrose using glucosyltransferases and hydrolyzes the α-1,6-linkages in the glucans using SmDex to produce isomaltooligosaccharides for metabolic utilization (Walker et al., 1981 ▶; Colby et al., 1995 ▶). Several isoforms of SmDex, as well as other dextranases belonging to GH 66, have been detected (Igarashi et al., 1995 ▶; Khalikova et al., 2005 ▶) and our recent work has also shown that recombinant full-length SmDex (Ser25–Asp850; 95 kDa) expressed in Escherichia coli is susceptible to protease digestion after long storage (Kim et al., 2011 ▶). Since the production of stable enzymes is a prerequisite for further biochemical studies and industrial applications, we have constructed a truncation mutant tolerant to protease digestion which is devoid of the N- and C-terminal variable regions (SmDexTM; Gln100–Ile732).
In biochemical studies of SmDex and two other GH 66 proteins, Asp385 of SmDex (Igarashi et al., 2002 ▶), Asp270 of CITase from Bacillus circulans T3040 (Yamamoto et al., 2006 ▶) and Asp243 of endodextranase from Thermotoga lettingae TMO (Kim & Kim, 2010 ▶) have been implicated as catalytic residues. However, the detailed catalytic mechanism of GH 66 enzymes is as yet unclear owing to the lack of three-dimensional structures. We recently reported that ω-epoxyalkyl α-glucopyranosides inactivate SmDex irreversibly, but the mechanism of this suicide inhibitor needs to be further elucidated (Kang et al., 2010 ▶). In order to address these questions and to obtain a structural basis for protein engineering applicable for use in isomaltooligosaccharide production, we attempted the crystallization of SmDexTM.
2. Materials and methods
SmDexTM was overexpressed with the pET-28 expression system (Novagen, Madison, Wisconsin, USA) using E. coli BL21 (DE3) cells in LB medium by induction with 0.1 mM isopropyl β-d-1-thiogalactopyranoside overnight at 298 K as reported previously (Kim et al., 2011 ▶). The protein was purified from the soluble fraction of the bacterial cell lysate by Ni2+-charged HiTrap chelating HP column chromatography (GE Healthcare, Buckinghamshire, England) in a buffer consisting of 50 mM sodium phosphate pH 6.8, 300 mM NaCl, 200 mM imidazole. After desalting the solution using PD-10 column chromatography (GE Healthcare) into a buffer consisting of 20 mM Tris–HCl pH 8.0, the protein was further purified by anion-exchange chromatography using HiTrap Q-Sepharose HP (GE Healthcare) using a buffer consisting of 1 M NaCl, 20 mM Tris–HCl pH 8.0.
The protein solution was desalted and concentrated to 9.8 mg ml−1 by ultrafiltration using a YM-30 membrane (Millipore, Billerica, Massachusetts, USA) and filtered through a 0.1 µm membrane (Millipore). The pooled solution without buffer reagents was used in crystallization trials. Sparse-matrix crystal screening was performed using Crystal Screen HT, Index HT (Hampton Research, Aliso Viejo, California, USA), Wizard I and II and Cryo I and II (Emerald BioSystems, Bainbridge Island, Washington, USA). Sitting-drop vapour-diffusion trials were set up using the Thermo Scientific Matrix Hydra II (Thermo Fisher Scientific Inc., Hudson, New Hampshire, USA) automated liquid-handling system in 96-well Intelli-Plates (Art Robbins Instruments, Sunnyvale, California, USA) at 293 K using 50 µl reservoir solution; each drop consisted of 0.3 µl protein solution and 0.3 µl reservoir solution. Within two weeks, several tiny plate-shaped crystals were observed under several conditions using polyethylene glycol 600 or polyethylene glycol monomethyl ether 2000 as a precipitant. Crystallization conditions were optimized manually by refinement of the protein and/or precipitant concentrations and buffer solution using CrystalClear Strips 96-well sitting-drop plates (Douglas Instruments, Berkshire, England).
Native diffraction data were collected from a single crystal on beamline BL-17A of the Photon Factory (PF), High Energy Accelerator Research Organization, Tsukuba, Japan. The crystal was scooped in a nylon CryoLoop (Hampton Research) and then flash-cooled in a nitrogen-gas stream at 95 K. Diffraction data were collected with 5 s exposures for 1° oscillations over a total of 360° at a wavelength of 0.970 Å with a Quantum 270 CCD detector (ADSC, Poway, California, USA). Data were integrated and scaled using the programs DENZO and SCALEPACK from the HKL-2000 program suite (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
We optimized the crystallization condition for SmDexTM, which was composed of 30%(w/v) polyethylene glycol monomethyl ether 2000, 100 mM phosphate–citrate buffer pH 4.2. The plate-shaped crystals were obtained in a drop consisting of 2 µl protein solution with a protein concentration of 3.6 mg ml−1 and 2 µl reservoir solution equilibrated against 50 µl reservoir solution at 293 K. The largest crystal grew to dimensions of 300 × 150 × 30 µm within one month (Fig. 1 ▶).
Figure 1.
The crystal of SmDexTM. The scale bar represents 100 µm.
The crystal diffracted to a maximum resolution of 1.6 Å using synchrotron radiation at PF. The crystal belonged to space group P21, with unit-cell parameters a = 53.2, b = 89.7, c = 63.3 Å, β = 102.3°. The processing statistics of the collected data are summarized in Table 1 ▶. Assuming that the asymmetric unit of the crystal contained one SmDexTM molecule, the Matthews coefficient was calculated to be 4.07 Å3 Da−1 (Matthews, 1968 ▶); assuming two molecules to be present in the asymmetric unit it was calculated to be 2.03 Å3 Da−1.
Table 1. Data-collection statistics for the SmDexTM crystal.
Values in parentheses are for the highest resolution shell.
| Beamline | PF BL-17 |
| Detector | ADSC Quantum 270 |
| Wavelength (Å) | 0.970 |
| Space group | P21 |
| Unit-cell parameters (Å, °) | a = 53.2, b = 89.7, c = 63.3, β = 102.3 |
| Resolution range (Å) | 50.0–1.6 (1.66–1.60) |
| Rmerge† | 0.063 (0.175) |
| Completeness (%) | 100.0 (100.0) |
| Multiplicity | 7.5 (7.5) |
| Average I/σ(I) | 39.2 (11.5) |
| Unique reflections | 76683 (7641) |
| Total reflections | 571898 |
| Mosaicity (°) | 0.77 |
| Overall B factor from Wilson plot (Å2) | 13.5 |
R
merge = 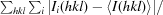
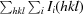 , where Ii(hkl) is the intensity of the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all i observations of reflection hkl.
, where Ii(hkl) is the intensity of the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all i observations of reflection hkl.
Although three-dimensional structures of GH 66 enzymes have not been reported, several crystal structures of enzymes belonging to GH 13, GH 27 and GH 31, which share a common catalytic (β/α)8-barrel, are available. We performed molecular-replacement calculations with the program MOLREP (Vagin & Teplyakov, 2010 ▶; Winn et al., 2011 ▶) using polyalanine structures of the conserved regions, including those of two GH 13 α-amylases from B. subtilis (PDB entry 1bag; Fujimoto et al., 1998 ▶) and from Geobacillus stearothermophilus (PDB entry 1hvx; Suvd et al., 2001 ▶), two GH 27 α-galactosidases from rice (PDB entry 1uas; Fujimoto et al., 2003 ▶) and from Umbelopsis vinacea (PDB entry 3a5v; Fujimoto et al., 2009 ▶) and GH 31 α-xylosidase (PDB entry 1we5; Kitamura et al., 2005 ▶). All of the trials gave a single solution, indicating that the crystal contains one SmDexTM molecule in the asymmetric unit, with a Matthews coefficient of 4.07 Å3 Da−1 corresponding to 69.8% solvent content. However, the solutions were insufficient to solve the structure because of their low amino-acid sequence identities and the lack in these structures of the extra domains possessed by SmDexTM. We are currently preparing a selenomethionine-substituted protein crystal (17 methionines per molecule) for phase determination using the multiple anomalous dispersion technique.
Acknowledgments
We would like to thank the beamline researchers and staff at PF. This work was partially supported by the Program for Promotion of Basic and Applied Research for Innovations in Bio-oriented Industry (BRAIN) and a grant-in-aid from JSPS (Japan Society for the Promotion of Science) Fellows relating to a JSPS Postdoctoral Fellowship for Foreign Researchers (17-05467).
References
- Cantarel, B. L., Coutinho, P. M., Rancurel, C., Bernard, T., Lombard, V. & Henrissat, B. (2009). Nucleic Acids Res. 37, D233–D238. [DOI] [PMC free article] [PubMed]
- Colby, S. M., Whiting, G. C., Tao, L. & Russell, R. R. (1995). Microbiology, 141, 2929–2936. [DOI] [PubMed]
- Fujimoto, Z., Kaneko, S., Kim, W.-D., Park, G.-G., Momma, M. & Kobayashi, H. (2009). Biosci. Biotechnol. Biochem. 73, 2360–2364. [DOI] [PubMed]
- Fujimoto, Z., Kaneko, S., Momma, M., Kobayashi, H. & Mizuno, H. (2003). J. Biol. Chem. 278, 20313–20318. [DOI] [PubMed]
- Fujimoto, Z., Takase, K., Doui, N., Momma, M., Matsumoto, T. & Mizuno, H. (1998). J. Mol. Biol. 277, 393–407. [DOI] [PubMed]
- Funane, K., Kawabata, Y., Suzuki, R., Kim, Y. M., Kang, H.-K., Suzuki, N., Fujimoto, Z., Kimura, A. & Kobayashi, M. (2011). Biochim. Biophys. Acta, 1814, 428–434. [DOI] [PubMed]
- Henrissat, B. & Bairoch, A. (1996). Biochem. J. 316, 695–696. [DOI] [PMC free article] [PubMed]
- Igarashi, T., Morisaki, H. & Goto, N. (2004). Microbiol. Immunol. 48, 155–162. [DOI] [PubMed]
- Igarashi, T., Morisaki, H., Yamamoto, A. & Goto, N. (2002). Oral Microbiol. Immunol. 17, 193–196. [DOI] [PubMed]
- Igarashi, T., Yamamoto, A. & Goto, N. (1995). Microbiol. Immunol. 39, 387–391. [DOI] [PubMed]
- Kang, H.-K., Kim, Y.-M., Nakai, H., Kang, M.-S., Hakamada, W., Okuyama, M., Mori, H., Nishio, T. & Kimura, A. (2010). J. Appl. Glycosci. 57, 269–272.
- Khalikova, E., Susi, P. & Korpela, T. (2005). Microbiol. Mol. Biol. Rev. 69, 306–325. [DOI] [PMC free article] [PubMed]
- Kim, Y.-M. & Kim, D. (2010). Appl. Microbiol. Biotechnol. 85, 581–587. [DOI] [PubMed]
- Kim, Y.-M., Shimizu, R., Nakai, H., Mori, H., Okuyama, M., Kang, M.-S., Fujimoto, Z., Funane, K., Kim, D. & Kimura, A. (2011). Appl. Microbiol. Biotechnol. 91, 329–339. [DOI] [PubMed]
- Kitamura, M., Ose, T., Okuyama, M., Watanabe, H., Yao, M., Mori, H., Kimura, A. & Tanaka, I. (2005). Acta Cryst. F61, 178–179. [DOI] [PMC free article] [PubMed]
- Larsson, A. M., Andersson, R., Ståhlberg, J., Kenne, L. & Jones, T. A. (2003). Structure, 11, 1111–1121. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Morisaki, H., Igarashi, T., Yamamoto, A. & Goto, N. (2002). Lett. Appl. Microbiol. 35, 223–227. [DOI] [PubMed]
- Oguma, T., Horiuchi, T. & Kobayashi, M. (1993). Biosci. Biotechnol. Biochem. 57, 1225–1227. [DOI] [PubMed]
- Oguma, T., Tobe, K. & Kobayashi, M. (1994). FEBS Lett. 345, 135–138. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Rigden, D. J. (2002). FEBS Lett. 523, 17–22. [DOI] [PubMed]
- Suvd, D., Fujimoto, Z., Takase, K., Matsumura, M. & Mizuno, H. (2001). J. Biochem. 129, 461–468. [DOI] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Walker, G. J., Pulkownik, A. & Morrey-Jones, J. G. (1981). J. Gen. Microbiol. 127, 201–208. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Yamamoto, T., Terasawa, K., Kim, Y.-M., Kimura, A., Kitamura, Y., Kobayashi, M. & Funane, K. (2006). Biosci. Biotechnol. Biochem. 70, 1947–1953. [DOI] [PubMed]



