In this study, a putative glucokinase/hexokinase from T. thermophilus was purified and crystallized. Diffraction data were collected and processed to 2.02 Å resolution.
Keywords: glucokinase/hexokinase, Thermus thermophilus
Abstract
Glucokinase/hexokinase catalyzes the phosphorylation of glucose to glucose 6-phosphate, which is the first step of glycolysis. The open reading frame TTHA0299 of the extreme thermophile Thermus thermophilus encodes a putative glucokinase/hexokinase which contains the consensus sequence for proteins from the repressors, open reading frames and sugar kinases family. In this study, the glucokinase/hexokinase from T. thermophilus was purified and crystallized using polyethylene glycol 8000 as a precipitant. Diffraction data were collected and processed to 2.02 Å resolution. The crystal belonged to space group P21, with unit-cell parameters a = 70.93, b = 138.14, c = 75.16 Å, β = 95.41°.
1. Introduction
Glycolysis is an important pathway for both energy metabolism and biosynthesis in cells. There are two major glycolytic pathways: the Embden–Meyerhof (EM) pathway and the Entner–Doudoroff (ED) pathway. In both pathways the first step of glycolysis is the phosphorylation of glucose to glucose 6-phosphate, which is catalyzed by either glucokinase (GK; EC 2.7.1.2) or hexokinase (HK; EC 2.7.1.1). These enzymes are homologous, yet functionally distinct: GK exhibits a narrow substrate specificity for glucose, whereas HK exhibits a broad substrate specificity for hexoses.
Microbial GKs/HKs have been classified into three groups. Group I includes ATP-dependent and ADP-dependent GKs (EC 2.7.1.147) from archaea such as Pyrococcus furiosus and Thermococcus litoralis (Sakuraba et al., 2004 ▶) and a bifunctional ADP-dependent glucokinase/phosphofructokinase from Methanococcus jannaschii (Sakuraba et al., 2002 ▶). Group II members include ATP-dependent GKs such as those from Escherichia coli (Lunin et al., 2004 ▶). Group III includes ATP-dependent GKs and HKs (from both archaea and bacteria) that contain the ROK [repressors, open reading frames (ORFs) and sugar kinases] signature sequence [(L/I/V/M)X 2G(L/I/V/M/F/C/T)GX(G/A)(L/I/V/M/F/A)X 8GX 3–5(G/A/T/P)X 2G(R/K/H); consensus sequence 1; Hofmann et al., 1999 ▶] and a CXCGX 2GCXE motif (consensus sequence 2; Mesak et al., 2004 ▶). The ROK-type enzymes vary in their sugar specificity. The ROK GK from the hyperthermophilic bacterium Thermotoga maritima (Hansen & Schönheit, 2003 ▶) is highly specific for glucose, whereas the ROK enzymes from the hyperthermophilic archaea Aeropyrum pernix (Hansen et al., 2002 ▶) and Thermoproteus tenax (Dörr et al., 2003 ▶) and those from the thermophilic bacterium Thermus caldophilus (Bae et al., 2005 ▶) have a broad substrate specificity. Crystallographic studies would help in understanding the factors that determine the substrate specificity of ROK-type GKs and HKs.
Thermus thermophilus is a model organism for thermophilic bacteria and has been the subject of extensive genomic and proteomic studies. ORFs for all of the enzymes involved in the ED glycolytic pathway are found in the genome sequence of T. thermophilus. The ORF TTHA0299 encodes a ROK-type kinase (TthGK/HK; Fig. 1 ▶). Because this enzyme is highly homologous to HK from T. caldophilus (Bae et al., 2005 ▶), with only one residue mismatch (Fig. 1 ▶), TthGK/HK can exhibit hexokinase activity. Although the tertiary structures of several group I thermophilic ADP-dependent GKs from thermophilic archaea (Ito et al., 2001 ▶, 2003 ▶; Tsuge et al., 2002 ▶) are available, the tertiary structures of thermophilic ROK-type GKs/HKs have not been reported to date. In this paper, we describe the purification and crystallization of TthGK/HK. This is a pioneering study of an ATP-dependent GK/HK from a thermophilic organism.
Figure 1.
Sequence alignment of TthGK/HK and homologous proteins. The white and black bars indicate consensus sequences 1 and 2, respectively, of TthGK/HK. Multiple alignments were performed by ClustalW (Higgins et al., 1996 ▶). Tth, TthGK/HK (AAS82030); Tca, HK from Thermus caldophilus (AAV66920); Tma, GK from Thermotoga maritima (AAD36537); Ape, HK from Aeropyrum pernix (BAA81102); Tte, HK from Thermoproteus tenax (CAD52839); Eco, GK from E. coli (ACI80495); Pfu, GK from Pyrococcus furiosus (AAL80436); Pho; GK from P. horikoshii (BAA29678); Tli, GK from Thermococcus litoralis (Q7M537). GenBank/Swiss-Prot accession numbers are given in parentheses.
2. Materials and methods
2.1. Expression and purification of TthGK/HK
The coding sequence of TthGK/HK was constructed from the amino-acid sequence (Fig. 1 ▶), with codon optimization of the E. coli genes. The synthesized gene was cloned into the NdeI and EcoRI sites of plasmid vector pET-21a(+). E. coli BL21 (DE3) cells harbouring the expression plasmid were cultivated to an A 600 of ∼0.6 in LB medium supplemented with 100 µg ml−1 ampicillin at 310 K and expression of the recombinant protein was induced by the addition of 20 µM (final concentration) isopropyl β-d-1-thiogalactopyranoside and incubation for a further 22 h at 310 K.
The cells were disrupted by sonication in buffer A (20 mM Tris–HCl pH 8.0) and a soluble fraction was obtained by centrifugation at 31 000g for 30 min. The soluble materials were heat-treated at 348 K for 60 min and then centrifuged. The supernatant was dialyzed against buffer A and then loaded onto a HiTrap Q column (GE Healthcare). The bound protein was eluted with a linear gradient of 0–1 M NaCl in buffer A. The fraction containing TthGK/HK was subjected to gel filtration on a Superdex 200 pg column (GE Healthcare) with buffer A containing 150 mM NaCl. The protein concentration was determined with a Protein Assay Kit (Bio-Rad) with bovine serum albumin as the standard (Bradford, 1976 ▶).
2.2. Crystallization
TthGK/HK was crystallized using the hanging-drop vapour-diffusion method with reservoir solution comprising 0.1 M Tris–HCl pH 8.5 and 8%(w/v) polyethylene glycol 8000. 1.5 µl protein sample (20 mg ml−1 in 20 mM Tris–HCl pH 7.5) was mixed with an equal volume of reservoir solution and left to equilibrate against 400 µl reservoir solution at 293 K.
2.3. Data collection and processing
A single crystal was dipped into reservoir solution supplemented with 25% glycerol for a few seconds and cooled to 100 K in a stream of nitrogen gas. Diffraction data were collected on BL38B1, SPring-8, Hyogo, Japan using an ADSC Quantum 315 detector. The crystal-to-detector distance was 290 mm. The oscillation range was 1° per frame. 240 frames of diffraction data were integrated and scaled using the HKL-2000 suite (Otwinowski & Minor, 1997 ▶). The self-rotation function was calculated using the MOLREP program (Vagin & Teplyakov, 2010 ▶) from the CCP4 suite (Winn et al., 2011 ▶).
2.4. Gel filtration
Analytical gel-filtration chromatography was performed using a Superdex 200 GL (10/30) column (GE Healthcare) with a buffer consisting of 20 mM Tris–HCl pH 8.0 and 150 mM NaCl. The flow rate was 0.5 ml min−1.
3. Results and discussion
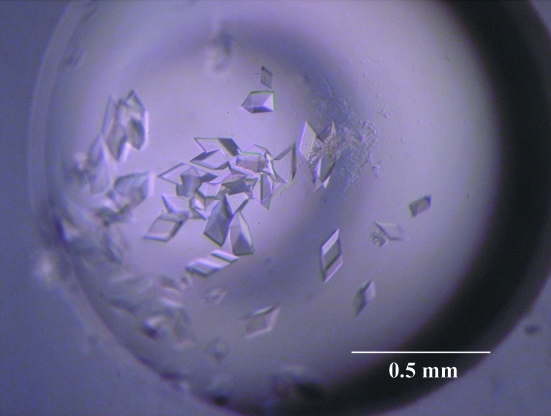
100 mg of TthGK/HK was obtained from 1 l of culture. Crystals of dimensions 0.1 × 0.1 × 0.1 mm were obtained by the vapour-diffusion method within a week (Fig. 2 ▶). An X-ray diffraction data set was collected to 2.02 Å resolution with an overall completeness of 99.7% (Fig. 3 ▶). The space group of the crystals was P21. The detailed conditions and measurements are summarized in Table 1 ▶.
Figure 2.
Crystals of TthGK/HK.
Figure 3.
Diffraction image of TthGK/HK. Circles corresponding to 2.0 and 2.5 Å resolution are shown.
Table 1. Crystallographic data-collection statistics.
Values in parentheses are for the highest resolution shell.
| X-ray source | BL38B1, SPring-8 |
| Wavelength (Å) | 1.0000 |
| Space group | P21 |
| Unit-cell parameters (Å, °) | a = 70.93, b = 138.14, c = 75.16, β = 95.41 |
| Resolution range (Å) | 50.0–2.02 (2.09–2.02) |
| Z | 8 |
| VM (Å3 Da−1) | 2.91 |
| Rmerge† (%) | 7.1 (36.3) |
| Completeness (%) | 99.7 (99.3) |
| Total reflections | 415123 |
| Unique reflections | 93772 |
| Multiplicity | 4.4 (3.8) |
| 〈I/σ(I)〉 | 11.6 (3.22) |
| Wilson plot B factor (Å2) | 26.3 |
R
merge = 
 , where I
i(hkl) is the intensity of the ith observation of reflection hkl and 〈I(hkl)〉 is the average of symmetry-related observations of a unique reflection.
, where I
i(hkl) is the intensity of the ith observation of reflection hkl and 〈I(hkl)〉 is the average of symmetry-related observations of a unique reflection.
Assuming the presence of four polypeptides in the asymmetric unit, the crystal volume per unit protein weight (V M) and solvent content were calculated to be 2.91 Å3 Da−1 and 57.8%, respectively. Fig. 4 ▶ shows the results of the self-rotation function in the χ = 180° and χ = 90° sections. While three twofold axes were clearly observed, no fourfold axis was found. These results support a tetrameric assembly of TthGK/HK molecules in the crystal; these molecules have 222 symmetry. To confirm the molecular assembly in solution, gel-filtration chromatography was performed (Fig. 5 ▶). The results showed a tetrameric assembly of TthGK/HK in solution (see legend to Fig. 5 ▶). However, structure solution requires phase determination and this is currently in progress.
Figure 4.
Self-rotation function calculated in the (a) χ = 180° and (b) χ = 90° sections.
Figure 5.
Gel-filtration chromatogram of TthGK/HK and calibration curve (inset). The standard proteins are (1) catalase, (2) aldolase, (3) albumin and (4) ovalbumin. The estimated molecular mass of TthGK/HK is 131.9 kDa, while the calculated monomer mass is 31 479 Da.
Acknowledgments
The diffraction data were collected on BL38B1 at SPring-8, Hyogo, Japan with the approval of the Japan Synchrotron Radiation Research Institute (approval No. 2011A1986).
References
- Bae, J., Kim, D., Choi, Y., Koh, S., Park, J. E., Kim, J. S., Moon, S. H., Park, B.-H., Park, M., Song, H.-E., Hong, S.-I. & Lee, D.-S. (2005). Biochem. Biophys. Res. Commun. 334, 754–763. [DOI] [PubMed]
- Bradford, M. M. (1976). Anal. Biochem. 72, 248–254. [DOI] [PubMed]
- Dörr, C., Zaparty, M., Tjaden, B., Brinkmann, H. & Siebers, B. (2003). J. Biol. Chem. 278, 18744–18753. [DOI] [PubMed]
- Hansen, T., Reichstein, B., Schmid, R. & Schönheit, P. (2002). J. Bacteriol. 184, 5955–5965. [DOI] [PMC free article] [PubMed]
- Hansen, T. & Schönheit, P. (2003). FEMS Microbiol. Lett. 226, 405–411. [DOI] [PubMed]
- Higgins, D. G., Thompson, J. D. & Gibson, T. J. (1996). Methods Enzymol. 266, 383–402. [DOI] [PubMed]
- Hofmann, K., Bucher, P., Falquet, L. & Bairoch, A. (1999). Nucleic Acids Res. 27, 215–219. [DOI] [PMC free article] [PubMed]
- Ito, S., Fushinobu, S., Jeong, J.-J., Yoshioka, I., Koga, S., Shoun, H. & Wakagi, T. (2003). J. Mol. Biol. 331, 871–883. [DOI] [PubMed]
- Ito, S., Fushinobu, S., Yoshioka, I., Koga, S., Matsuzawa, H. & Wakagi, T. (2001). Structure, 9, 205–214. [DOI] [PubMed]
- Lunin, V. V., Li, Y., Schrag, J. D., Iannuzzi, P., Cygler, M. & Matte, A. (2004). J. Bacteriol. 186, 6915–6927. [DOI] [PMC free article] [PubMed]
- Mesak, L. R., Mesak, F. M. & Dahl, M. K. (2004). BMC Microbiol. 4, 6. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Sakuraba, H., Goda, S. & Ohshima, T. (2004). Chem. Rec. 3, 281–287. [DOI] [PubMed]
- Sakuraba, H., Yoshioka, I., Koga, S., Takahashi, M., Kitahama, Y., Satomura, T., Kawakami, R. & Ohshima, T. (2002). J. Biol. Chem. 277, 12495–12498. [DOI] [PubMed]
- Tsuge, H., Sakuraba, H., Kobe, T., Kujime, A., Katunuma, N. & Ohshima, T. (2002). Protein Sci. 11, 2456–2463. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.