A thermostable multicopper oxidase from Thermus thermophilus HB27 (Tth-MCO) has been successfully crystallized using the sitting-drop and hanging-drop vapour-diffusion methods.
Keywords: laccases, multicopper oxidases, Thermus thermophilus
Abstract
A thermostable multicopper oxidase from Thermus thermophilus HB27 (Tth-MCO) was successfully crystallized using the sitting-drop and hanging-drop vapour-diffusion methods. Crystallization conditions and preliminary X-ray diffraction data to 1.5 Å resolution obtained using synchrotron radiation at 100 K are reported. The crystals belonged to space group C2221, with unit-cell parameters a = 93.6, b = 110.3, c = 96.3 Å. A monomer in the asymmetric unit yielded a Matthews coefficient (V M) of 2.60 Å3 Da−1 and a solvent content of 53%. An inactive enzyme form, apo-Tth-MCO, was also crystallized and diffraction data were collected to 1.7 Å resolution. In addition, a second inactive form of the enzyme, Hg-Tth-MCO, was obtained by soaking apo-Tth-MCO crystals with mercury(II) chloride and data were collected to a resolution of 1.7 Å.
1. Introduction
Multicopper oxidases (MCOs) catalyze four one-electron oxidations of reducing substrates coupled to the four-electron reduction of molecular oxygen to water. MCOs are a family of enzymes comprising laccases (EC 1.10.3.2), ferroxidases (EC 1.16.3.1), ascorbate oxidase (EC 1.10.3.3) and ceruloplasmin (Hoegger et al., 2006 ▶). The catalytic motif in these proteins includes at least four Cu atoms, which are classified into three types of sites: type 1 copper (T1Cu), type 2 copper (T2Cu) and the type 3 binuclear T3′Cu–T3Cu cluster. The type 2 and type 3 sites form a trinuclear Cu cluster (TNC) where molecular oxygen is reduced to water (Quintanar et al., 2007 ▶). These enzymes have been found and described in several fungi, plants and bacteria (Mayer & Staples, 2002 ▶; Valderrama et al., 2003 ▶; Sharma et al., 2007 ▶); however, in the case of thermophilic bacterial MCOs little is known about their functional properties (Hildén et al., 2009 ▶). MCOs present low substrate specificity and have been used in a variety of biotechnological applications, including organic synthesis (Bernini et al., 2011 ▶), biofuel cells (Service, 2002 ▶; Vincent et al., 2005 ▶), textile dye bleaching (Rodríguez Couto & Toca Herrera, 2006 ▶) and bioremediation (Gullotto et al., 2008 ▶). The most thoroughly studied thermoactive bacterial MCO is CotA from Bacillus subtilis. CotA is an enzyme with optimum ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] oxidation activity at 348 K and a T 1/2 of almost 2 h at 353 K (Martins et al., 2002 ▶). Thermus thermophilus strain HB27 locus TT_C1370 encodes the only MCO in this organism (Henne et al., 2004 ▶). The protein encoded by this gene has been isolated and found to present oxidase activity with an optimal temperature for ABTS oxidation at 365 K and a T 1/2 of over 14 h at 353 K (Miyazaki, 2005 ▶). Owing to this remarkable resistance to thermal denaturation, T. thermophilus HB27 multicopper oxidase (Tth-MCO), also named T. thermophilus HB27 laccase (EC 1.10.3.2; Tth-laccase; Miyazaki, 2005 ▶), may have potential in biotechnological applications and is an attractive target for protein engineering. Additionally, the presence of Cu atoms in the active sites of MCOs concentrates radiation damage and/or radiolysis effects in the vicinity, making them targets for radiation-dose X-ray-induced reduction studies.
When a crystal of a copper-containing protein is exposed to X-ray radiation, one of the first events to occur is photoreduction of the metal atoms. This effect can be seen in the final crystallographic structure as a mixture of copper oxidation states (Macedo et al., 2009 ▶). In addition, it has been suggested that the electrons generated during X-ray data collection are able to lead to the reduction of bound molecular oxygen to water in MCOs (Hakulinen et al., 2006 ▶). Therefore, structural study of T. thermophilus multicopper oxidase in its apo, holo and apo loaded with mercury forms (Tth-MCO, apo-Tth-MCO and Hg-Tth-MCO, respectively) offers an opportunity to obtain insights into the redox mechanism of this particular family of enzymes guided by their crystalline structures and the interaction of metal atoms with X-ray radiation.
2. Materials and methods
2.1. Gene cloning, protein expression and purification
T. thermophilus strain HB27 (Oshima & Imahori, 1974 ▶; DSM 7039, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH) was grown as described elsewhere (Domínguez et al., 2010 ▶) and genomic DNA was isolated using an UltraClean Microbial DNA-isolation kit (MO BIO). The gene encoded by locus TT_C1370 was obtained by amplification of genomic DNA using the primers 5′-CATATGCAAGGCCCTTCCTTCCCC-3′ and 5′-CGAATTCCCCACCTCGAGGACTCCCAT-3′. The amplified fragment was cloned into pGEM-T Easy vector (Promega) for sequence confirmation. One of the plasmids with the correct sequence was digested with NdeI and EcoRI and the released fragment of ∼1.5 kb was cloned into the corresponding sites of pET32a(+) (Novagen), resulting in plasmid pTth1. Electrocompetent Escherichia coli BL21 (DE3) cells were transformed with pTth1 and grown on Luria–Bertani (LB) [1%(w/v) tryptone, 0.5%(w/v) yeast extract, 1%(w/v) NaCl] agar plates containing 200 µg ml−1 ampicillin and 20 µg ml−1 tetracycline at 310 K. A single colony was picked and grown for plasmid isolation and insert verification by sequencing. Once validated, a sample of a liquid culture of exponentially growing BL21 (DE3)/pTth1 was collected by centrifugation, resuspended in a sterile solution of 50% glycerol and maintained at 193 K. Aliquots of the frozen cell stock were thawed and inoculated into 30 ml LB medium containing 200 µg ml−1 ampicillin and 20 µg ml−1 tetracycline and incubated for 12 h at 303 K. Part of the culture (10 ml) was used to inoculate 1 l LB medium with 200 µg ml−1 ampicillin and 20 µg ml−1 tetracycline at 303 K until the optical density at 600 nm reached 0.5. Expression of Tth-MCO was then induced by adding IPTG (final concentration 0.1 mM) to the medium and incubating for 24 h at 303 K. Cells were concentrated by centrifugation (7500g, 30 min, 277 K) and resuspended in 50 ml 20 mM Tris pH 8.0 containing Complete protease-inhibitor cocktail (Roche Molecular Biochemicals) and DNase (1 µg ml−1). The cell suspension was sonicated on ice and the resulting lysate was heated at 338 K for 20 min. Cell debris was removed by centrifugation (15 300g, 35 min, 277 K) and the supernatant was dialyzed five times at 278 K against ten volumes of 20 mM Tris pH 8.0 with 0.1 mM CuSO4 to obtain Tth-MCO and without CuSO4 to obtain apo-Tth-MCO. Subsequent protein purifications were performed at room temperature and under the same conditions for the apoenzyme and the holoenzyme unless stated otherwise. The supernatant was loaded onto 25 ml SP Sepharose Fast Flow (GE Healthcare) pre-equilibrated with 20 mM Tris pH 8.0 and washed with several volumes of the same buffer. Bound proteins were eluted with a linear gradient of NaCl (0–1 M) in the equilibration buffer at a flow rate of 2.0 ml min−1. Fractions with oxidase activity were pooled and concentrated in an ultrafiltration cell (Amicon cellulose filter, 30 kDa molecular-weight cutoff). This pool was loaded onto a Sephacryl 300-HR column (140 ml, 25–75 µm, Sigma) pre-equilibrated with 20 mM Tris pH 8.0, 50 mM NaCl, and Tth-MCO was eluted with the same buffer at a flow rate of 1 ml min−1. Fractions with oxidase activity were concentrated and dialyzed by ultrafiltration. Purified recombinant Tth-MCO showed a molecular mass of ∼50 kDa on SDS–PAGE. Protein concentration was determined by the Bradford assay (Bradford, 1976 ▶) using bovine serum albumin as a standard. The purification yield was approximately 6 mg purified protein from 1 l culture.
2.2. Protein crystallization
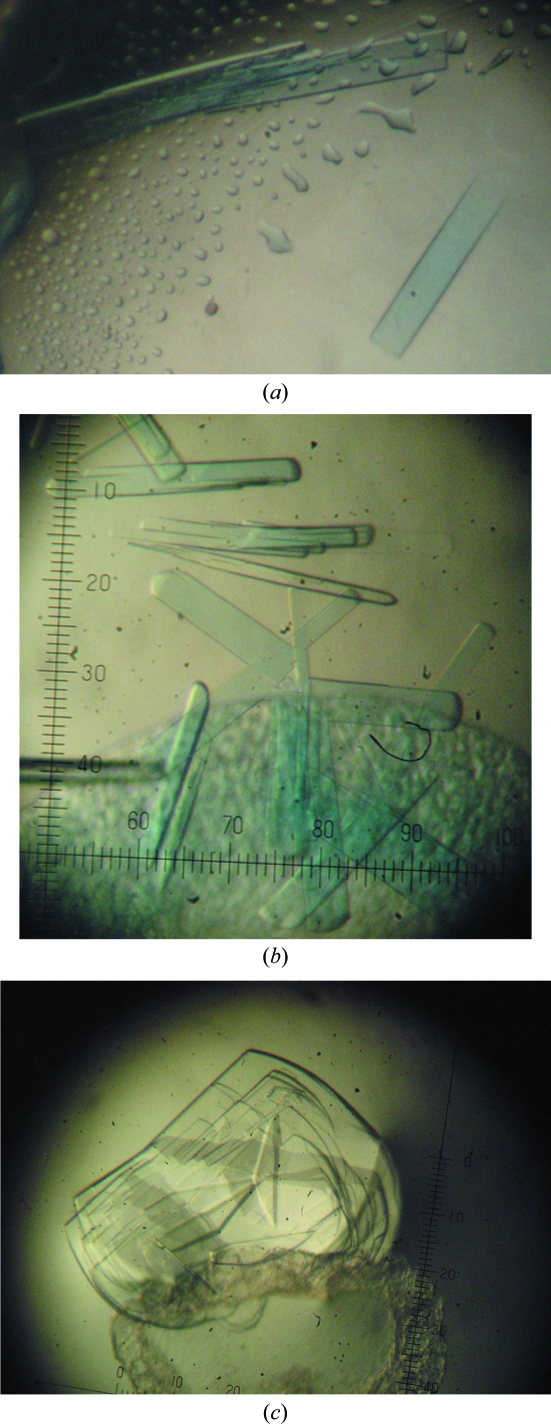
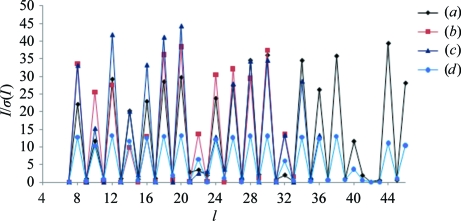
Initial crystallization conditions were obtained by the hanging-drop vapour-diffusion method using Crystal Screen and Crystal Screen 2 (Hampton Research). Protein droplets consisting of 1 µl Tth-MCO (19.5 mg ml−1 in 20 mM Tris pH 8.0) and 1 µl reservoir solution (0.1 M HEPES pH 7.5, 70% MPD; condition No. 35 of Crystal Screen 2) were equilibrated against 1 ml reservoir solution at 278 K. Plate-like crystals appeared and grew to approximate dimensions of around 0.025 × 0.1 × 0.25 mm after ten months. After optimization, protein crystals with approximate dimensions of 0.025 × 0.05 × 0.1 mm were obtained by the sitting-drop vapour-diffusion method with a protein concentration of 30 mg ml−1 in 20 mM Tris pH 8.0 using 1 + 1 µl drops and 0.5 ml reservoir solution (0.1 M HEPES pH 7.5, 70% MPD) at a temperature of 278 K, with a growth time of two to three months (Fig. 1 ▶ a). These crystals were suitable for X-ray analysis; however, they were also used as seeds. Using the microseeding technique, a large number of crystal nuclei were transferred using a horse hair into droplets consisting of 1 µl protein solution (30 mg ml−1 in 20 mM Tris pH 8.0) and 1 µl reservoir solution (0.1 M HEPES pH 7.5, 70% MPD), which were equilibrated against 0.5 ml reservoir solution using the sitting-drop vapour-diffusion method or against 1 ml reservoir solution using the hanging-drop vapour-diffusion method. After a week small crystals of Tth-MCO were observed, but they required one to two months to reach approximate dimensions of 0.025 × 0.05 × 0.1 mm (Fig. 1 ▶ b). Apo-Tth-MCO crystals were obtained with a protein concentration of 30 mg ml−1 in 20 mM Tris pH 8.0 using a reservoir solution consisting of 0.1 M HEPES pH 7.5, 70% MPD. Plate-like crystals appeared and grew to approximate dimensions of around 0.025 × 0.1 × 0.25 mm within three months (Fig. 1 ▶ c). These apo-Tth-MCO crystals were separated using an acupuncture needle before mounting.
Figure 1.
Crystals of Tth-MCO obtained by (a) the sitting-drop vapour-diffusion method and (b) the hanging-drop vapour-diffusion method (microseeding technique); (c) crystals of apo-Tth-MCO obtained by the hanging-drop vapour-diffusion method. All photographs were taken without a polarizer in order to obtain real colour images and all of the images were obtained at the same magnification. In the case of the plate-like crystals observed in (c) an acupuncture needle was used to separate and cut the crystals before mounting.
2.3. Data collection and crystallographic analysis
Data collection from the Tth-MCO, apo-Tth-MCO and Hg-Tth-MCO crystals was performed on beamline X6A of the National Synchrotron Light Source (NSLS), Brookhaven National Laboratory (BNL), USA using an ADSC Quantum 270 detector. X-ray diffraction data for Tth-MCO were collected from a single crystal at two different wavelengths, λ = 0.9795 and 1.3767 Å, with the latter being used for the single-wavelength anomalous dispersion (SAD) technique (Tth-MCO-SAD data set). Before choosing the wavelengths, an energy scan was performed on the sample in order to identify the energy of the anomalous peak for Cu in Tth-MCO (9.003 keV, 1.3767 Å). The crystal-to-detector distance was maintained at 180 mm with an oscillation range per image of 0.5°. For data collection under cryogenic conditions, crystals were soaked for a few seconds in a solution consisting of 0.1 M HEPES pH 7.5, 45% MPD. Crystals were loop-mounted and flash-cooled in a 100 K dry nitrogen stream. Diffraction images were integrated using XDS (Kabsch, 2010 ▶) and scaling was performed with SCALA from the CCP4 suite (Winn et al., 2011 ▶). All of the Tth-MCO crystals belonged to a C-centred orthorhombic space group, with unit-cell parameters a = 93.6, b = 110.3, c = 96.3 Å, α = β = γ = 90°. A total of 899 414 reflections were integrated to a resolution of 1.5 Å and were merged to obtain 78 896 unique reflections with an overall R merge of 0.086 and a completeness of 94.7%. Matthews coefficient calculations suggested that there was one molecule per asymmetric unit (V M = 2.60 Å3 Da−1, 53% solvent content). A data set (λ = 1.3747 Å) was collected to a resolution of 1.7 Å for the apo-Tth-MCO crystal; its unit-cell parameters were a = 93.0, b = 110.1, c = 96.3 Å, α = β = γ = 90°. The derivative Hg-Tth-MCO was obtained by soaking an apo-Tth-MCO crystal for 5 min in 5 mM HgCl2 at 278 K and a data set (λ = 0.8321 Å) was collected to a resolution of 1.7 Å; the unit-cell parameters of the crystal were a = 93.5, b = 110.2, c = 96.3 Å, α = β = γ = 90°. Data-collection statistics are summarized in Table 1 ▶.
Table 1. Summary of crystallographic data.
Values in parentheses are for the highest resolution shell.
| Tth-MCO | Tth-MCO-SAD | Apo-Tth-MCO | Hg-Tth-MCO | |
|---|---|---|---|---|
| Source | BNL NSLS beamline X6A | BNL NSLS beamline X6A | BNL NSLS beamline X6A | BNL NSLS beamline X6A |
| Wavelength (Å) | 0.9795 | 1.3767 | 1.3747 | 0.8321 |
| Space group | C2221 | C2221 | C2221 | C2221 |
| Unit-cell parameters (Å) | a = 93.6, b = 110.3, c = 96.3 | a = 93.6, b = 110.3, c = 96.7 | a = 93.0, b = 110.1, c = 96.3 | a = 93.5, b = 110.2, c = 96.3 |
| Resolution (Å) | 23.0–1.50 (1.60–1.50) | 35.0–2.00 (2.10–2.00) | 26.0–1.70 (1.80–1.70) | 20.0–1.70 (1.80–1.70) |
| No. of observed reflections | 899414 | 493015 | 435926 | 542926 |
| No. of unique reflections | 78896 (10654) | 34001 (4848) | 53818 (7154) | 54841 (8585) |
| Completeness (%) | 94.7 (83.9) | 99.6 (98.6) | 98.9 (82.1) | 94.6 (91.4) |
| Multiplicity | 11.4 (9.9) | 14.5 (14.1) | 8.1 (6.6) | 9.9 (10.3) |
| 〈I/σ(I)〉 | 19.4 (4.1) | 23.6 (8.5) | 18.5 (3.8) | 9.1 (3.7) |
| Rmerge† (%) | 8.6 (39.5) | 8.1 (34.7) | 9.0 (45.0) | 13.3 (35.3) |
R
merge = 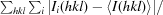
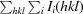 , where Ii(hkl) and 〈I(hkl)〉 represent the diffraction-intensity values of the individual measurements and the corresponding mean values. The summation is over all unique measurements.
, where Ii(hkl) and 〈I(hkl)〉 represent the diffraction-intensity values of the individual measurements and the corresponding mean values. The summation is over all unique measurements.
The most probable space group for all of the crystals analyzed was a C-centred orthorhombic space group. Assuming space group C222, the overall R merge for the Tth-MCO data set (resolution limits 23.0–1.5 Å) was 0.093 (0.668 in the highest resolution bin). However, when the same data set with the same resolution limits was integrated in space group C2221 the corresponding value of R merge was 0.086 (0.395 in the highest resolution bin). When all data sets were integrated in space group C222, one screw-rotation axis was clearly present in the I/σ(I) versus axial reflection plot on l (00l: l = 2n); this general reflection condition is indicative of space group C2221 (Fig. 2 ▶). Additionally, POINTLESS (Evans, 2006 ▶) clearly chose space group C2221 with a probability of 97.5%.
Figure 2.
I/σ(I) versus 00l reflection plots for the (a) Tth-MCO, (b) Tth-MCO-SAD, (c) apo-Tth-MCO and (d) Hg-Tth-MCO data sets. All data sets were integrated in space group C222 in order to test the presence of axial reflections in l (00l: l = 2n). The presence of a 21 screw-rotation axis is clearly visible in the plot, supporting space group C2221.
The structure of Tth-MCO was determined by a combination of molecular replacement and single-wavelength anomalous dispersion (SAD) techniques using the Cu signal and assuming space group C2221. Molecular-replacement trials were performed in Phaser (McCoy et al., 2007 ▶) using the structure of CueO from E. coli (Roberts et al., 2002 ▶; PDB entry 1kv7) as a model; this structure has a sequence identity of 31.5% to Tth-MCO. The phases obtained from molecular replacement were not sufficient for structure determination, but an attempt to solve the phase problem using a combination of molecular replacement and single-wavelength anomalous dispersion (SAD) was successful and two fully occupied and one partially occupied copper-ion positions were found using Phaser in MR-SAD mode (McCoy et al., 2007 ▶). Initial construction of the protein model was carried out using ARP/wARP (Langer et al., 2008 ▶). The apo-Tth-MCO and Hg-Tth-MCO structures were determined by molecular replacement using Phaser (McCoy et al., 2007 ▶) with the Tth-MCO atomic coordinates as a template. Refinement is currently under way using the programs PHENIX (Adams et al., 2010 ▶) and Coot (Emsley et al., 2010 ▶).
Acknowledgments
HSP was supported by a PhD fellowship from CONACyT. ERP and BV acknowledge financial support from CONACyT projects 102370 and 128156, respectively. ERP gratefully acknowledges financial support of PAPIIT project IN204611. We thank the staff at BNL NSLS beamline X6A for data-collection facilities. Beamline X6A is funded by NIGMS (GM-0080) and the US Department of Energy (No. DE-AC02-98CH10886). The authors thank Biol. Sonia P. Rojas-Trejo, Biol. Guadalupe Paredes-Valdéz and Dr Hector Ayala-Castro for technical assistance.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Bernini, R., Crisante, F., Gentili, P., Morana, F., Pierini, M. & Piras, M. (2011). J. Org. Chem. 76, 820–832. [DOI] [PubMed]
- Bradford, M. M. (1976). Anal. Biochem. 72, 248–254. [DOI] [PubMed]
- Domínguez, A., Deive, F. J., Pastrana, L., Rúa, M. L., Longo, M. A. & Sanroman, M. A. (2010). Bioprocess Biosyst. Eng. 33, 347–354. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- Gullotto, A., Branciamore, S., Duchi, I., Caño, M. F., Randazzo, D., Tilli, S., Giardina, P., Sannia, G., Scozzafava, A. & Briganti, F. (2008). Bioresour. Technol. 99, 8353–8359. [DOI] [PubMed]
- Hakulinen, N., Kruus, K., Koivula, A. & Rouvinen, J. (2006). Biochem. Biophys. Res. Commun. 350, 929–934. [DOI] [PubMed]
- Henne, A. et al. (2004). Nature Biotechnol. 22, 547–553. [DOI] [PubMed]
- Hildén, K., Hakala, T. K. & Lundell, T. (2009). Biotechnol. Lett. 31, 1117–1128. [DOI] [PubMed]
- Hoegger, P. J., Kilaru, S., James, T. Y., Thacker, J. R. & Kües, U. (2006). FEBS J. 273, 2308–2326. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Langer, G., Cohen, S. X., Lamzin, V. S. & Perrakis, A. (2008). Nature Protoc. 3, 1171–1179. [DOI] [PMC free article] [PubMed]
- Macedo, S., Pechlaner, M., Schmid, W., Weik, M., Sato, K., Dennison, C. & Djinović-Carugo, K. (2009). J. Synchrotron Rad. 16, 191–204. [DOI] [PubMed]
- Martins, L. O., Soares, C. M., Pereira, M. M., Teixeira, M., Costa, T., Jones, G. H. & Henriques, A. O. (2002). J. Biol. Chem. 277, 18849–18859. [DOI] [PubMed]
- Mayer, A. M. & Staples, R. C. (2002). Phytochemistry, 60, 551–565. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Miyazaki, K. (2005). Extremophiles, 9, 415–425. [DOI] [PubMed]
- Oshima, T. & Imahori, K. (1974). Int. J. Syst. Evol. Microbiol. 24, 102–112.
- Quintanar, L., Stoj, C., Taylor, A. B., Hart, P. J., Kosman, D. J. & Solomon, E. I. (2007). Acc. Chem. Res. 40, 445–452. [DOI] [PubMed]
- Roberts, S. A., Weichsel, A., Grass, G., Thakali, K., Hazzard, J. T., Tollin, G., Rensing, C. & Montfort, W. R. (2002). Proc. Natl Acad. Sci. USA, 99, 2766–2771. [DOI] [PMC free article] [PubMed]
- Rodríguez Couto, S. & Toca Herrera, J. L. (2006). Biotechnol. Adv. 24, 500–513. [DOI] [PubMed]
- Service, R. F. (2002). Science, 296, 1223. [DOI] [PubMed]
- Sharma, P., Goel, R. & Capalash, N. (2007). World J. Microbiol. Biotechnol. 23, 823–832.
- Valderrama, B., Oliver, P., Medrano-Soto, A. & Vazquez-Duhalt, R. (2003). Antonie Van Leeuwenhoek, 84, 289–299. [DOI] [PubMed]
- Vincent, K. A., Cracknell, J. A., Lenz, O., Zebger, I., Friedrich, B. & Armstrong, F. A. (2005). Proc. Natl Acad. Sci. USA, 102, 16951–16954. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.