Abstract
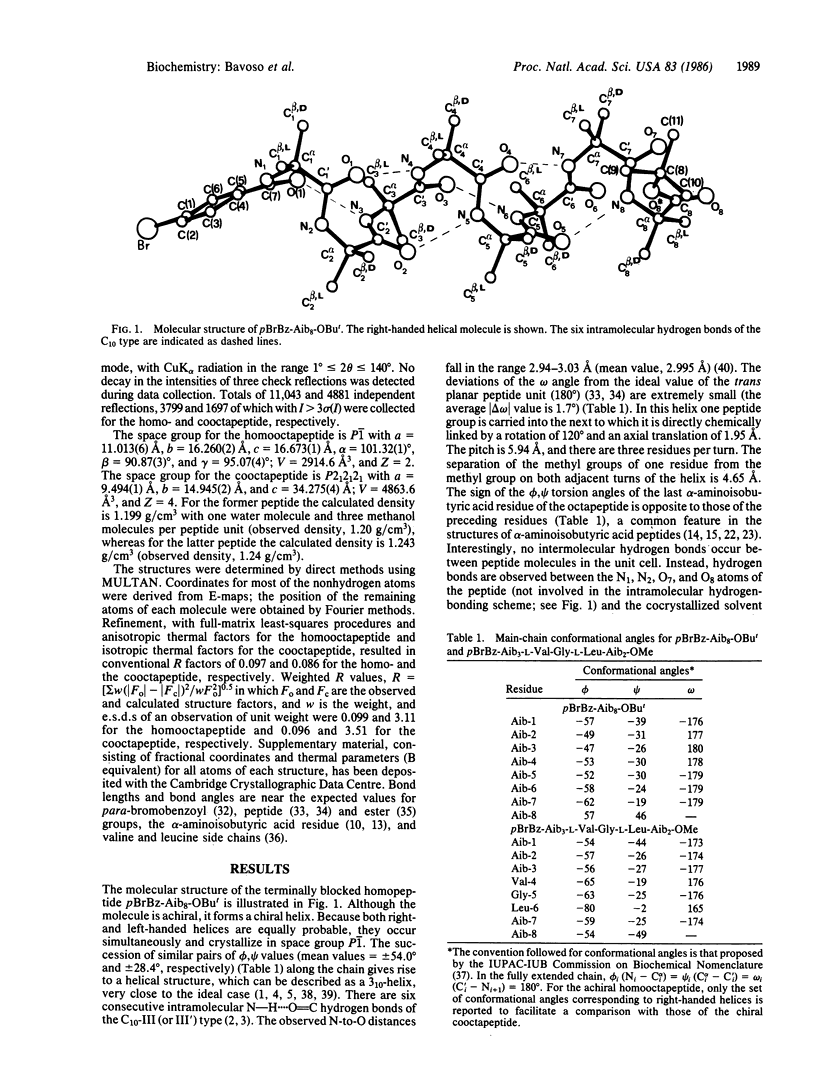
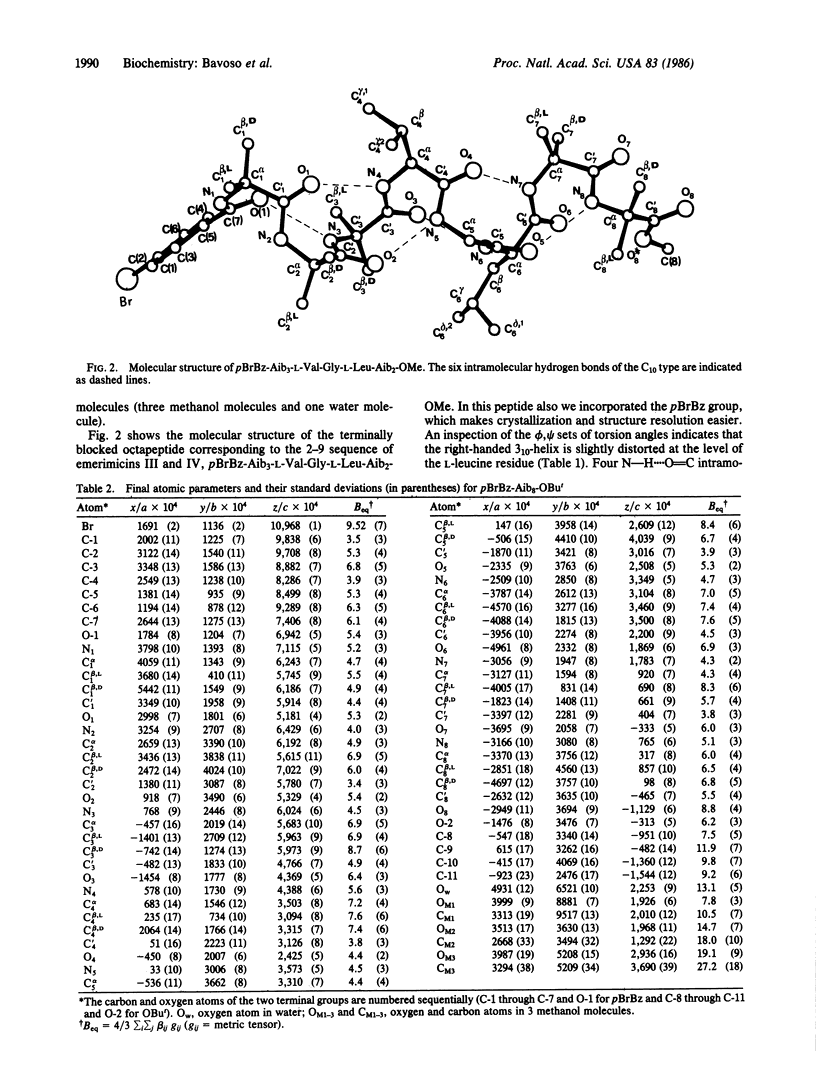
The crystal-state preferred conformation of the terminally blocked homooctapeptide from the Cα,α-dimethylated α-aminoisobutyric acid (Aib) residue, pBrBz-(Aib)8-OBut, in which pBrBz is para-bromobenzoyl and OBut is tert-butoxy, determined by x-ray diffraction analysis using direct methods, was found to be a 310-helix stabilized by six consecutive intramolecular N—H....O=C hydrogen bonds of the C10-III (or III′) type. This is the first observation at atomic resolution of a regular 310-helix longer than two complete turns. The solid-state structural analysis was extended to the terminally blocked, α-aminoisobutyric acid-rich octapeptide corresponding to the 2-9 sequence of the peptaibol antibiotics emerimicins III and IV, pBrBz-Aib3-L-Val-Gly-L-Leu-Aib2-OMe. Again, this peptide adopts a (right-handed) 310-helical structure, although slightly distorted at the level of the L-leucine residue. The role of specific amino acid sequence and peptide main-chain length in stabilizing either the 310- or the α-helical conformation and their possible implications on the nature of the channel formed by peptaibol antibiotics in the membrane are also briefly discussed.
Keywords: crystal structure, conformation, peptaibol antibiotics, emerimicins
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argoudelis A. D., Johnson L. E. Emerimicins II, 3 and IV, antibiotics produced by Emericellopsis microspora in media supplemented with trans-4-n-propyl-L-proline. J Antibiot (Tokyo) 1974 Apr;27(4):274–282. doi: 10.7164/antibiotics.27.274. [DOI] [PubMed] [Google Scholar]
- Benedetti E., Bavoso A., Di Blasio B., Pavone V., Pedone C., Toniolo C., Bonora G. M. Peptaibol antibiotics: a study on the helical structure of the 2-9 sequence of emerimicins III and IV. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7951–7954. doi: 10.1073/pnas.79.24.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti E., Morelli G., Némethy G., Scheraga H. A. Statistical and energetic analysis of side-chain conformations in oligopeptides. Int J Pept Protein Res. 1983 Jul;22(1):1–15. doi: 10.1111/j.1399-3011.1983.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Brückner H., Graf H., Bokel M. Paracelsin; characterization by NMR spectroscopy and circular dichroism, and hemolytic properties of a peptaibol antibiotic from the cellulolytically active mold Trichoderma reesei. Part B. Experientia. 1984 Nov 15;40(11):1189–1197. doi: 10.1007/BF01946646. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Leach S. J. An obligatory alpha-helical amino acid residue. Biopolymers. 1973 Nov;12(11):2599–2605. doi: 10.1002/bip.1973.360121112. [DOI] [PubMed] [Google Scholar]
- Donohue J. Hydrogen Bonded Helical Configurations of the Polypeptide Chain. Proc Natl Acad Sci U S A. 1953 Jun;39(6):470–478. doi: 10.1073/pnas.39.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flippen J. L., Karle I. L. Conformation of the cyclic tetrapeptide dihydrochlamydocin. Iabu-L-Phe D-Pro-LX, and experimental values for 3 leads to 1 intramolecular hydrogen bonds by X-ray diffraction. Biopolymers. 1976 Jun;15(6):1081–1092. doi: 10.1002/bip.1976.360150605. [DOI] [PubMed] [Google Scholar]
- Fox R. O., Jr, Richards F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 1982 Nov 25;300(5890):325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- Némethy G., Scheraga H. A. Stereochemical requirements for the existence of hydrogen bonds in beta-bends. Biochem Biophys Res Commun. 1980 Jul 16;95(1):320–327. doi: 10.1016/0006-291x(80)90741-x. [DOI] [PubMed] [Google Scholar]
- Pandey R. C., Cook J. C., Jr, Rinehart K. L., Jr Structures of the peptide antibiotics. Emerimicins III and IV 1,2. J Am Chem Soc. 1977 Jul 20;99(15):5205–5206. doi: 10.1021/ja00457a064. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Balaram P. The stereochemistry of peptides containing alpha-aminoisobutyric acid. CRC Crit Rev Biochem. 1984;16(4):307–348. doi: 10.3109/10409238409108718. [DOI] [PubMed] [Google Scholar]
- Ramarkrishnan C., Prasad N. Study of hydrogen bonds in amino acids and peptides. Int J Protein Res. 1971;3(4):209–231. doi: 10.1111/j.1399-3011.1971.tb01714.x. [DOI] [PubMed] [Google Scholar]
- Rao C. P., Shamala N., Nagaraj R., Rao C. N., Balaram P. Hydrophobic channels in crystals of an alpha-aminoisobutyric acid pentapeptide. Biochem Biophys Res Commun. 1981 Dec 15;103(3):898–904. doi: 10.1016/0006-291x(81)90895-0. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Toniolo C. Intramolecularly hydrogen-bonded peptide conformations. CRC Crit Rev Biochem. 1980;9(1):1–44. doi: 10.3109/10409238009105471. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]


