Abstract
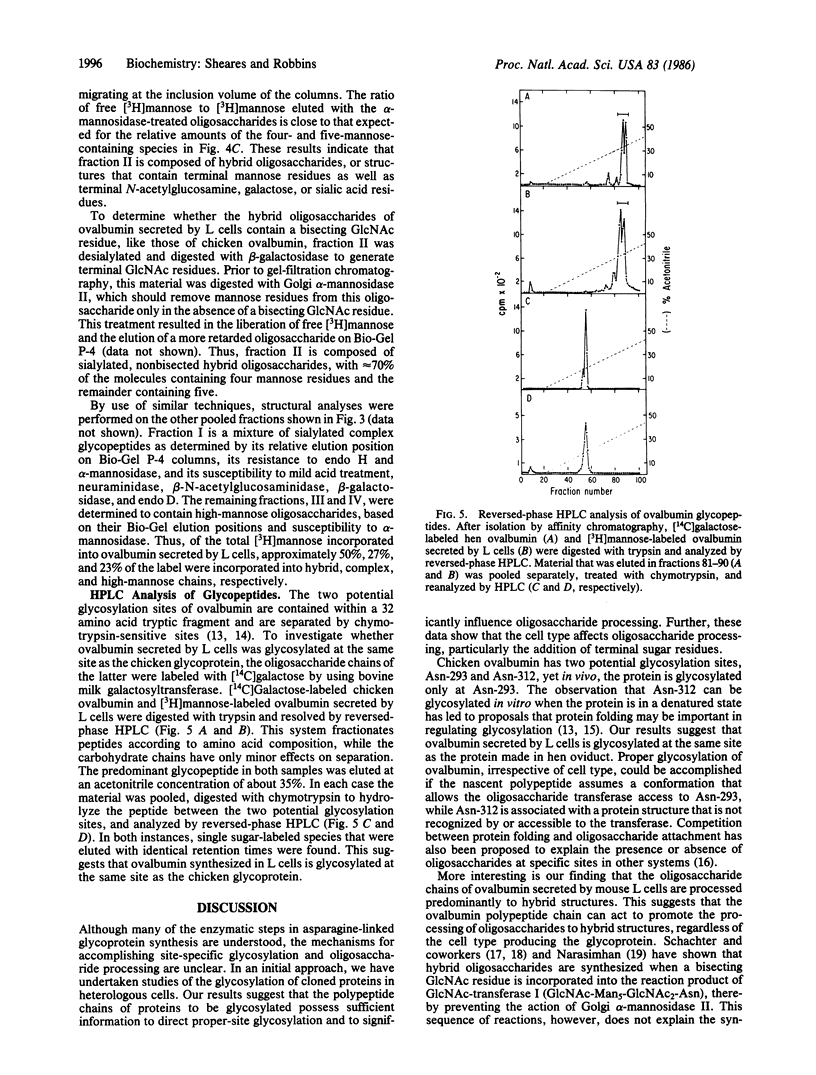
In an effort to understand the factors that influence protein glycosylation, we are studying the expression of the chicken ovalbumin gene in a heterologous cell. Ovalbumin synthesized in mouse L-353 cells is glycosylated, as judged by incorporation of [3H]mannose and susceptibility to endo-beta-N-acetylglucosaminidases. Sequential digestion of ovalbumin synthesized in L cells with trypsin and chymotrypsin yields material migrating as one peak on HPLC coincident with similarly treated material from chicken ovalbumin, suggesting that the protein synthesized in L cells is glycosylated at the correct site. As in the case of chicken ovalbumin, the protein synthesized in L cells contains large amounts of hybrid oligosaccharides. Approximately 50% of the [3H]mannose incorporated into ovalbumin secreted by L cells is found in such hybrid structures. These results suggest that it is the polypeptide chain of ovalbumin that is responsible for proper glycosylation and subsequent processing of a substantial fraction of the oligosaccharide chains to hybrid structures. However, differences do exist between ovalbumin synthesized in L cells and the chicken glycoprotein. The hybrid oligosaccharides of ovalbumin secreted by L cells are completely sialylated and do not contain a bisecting GlcNAc residue, distinguishing them from hybrid chains in chicken ovalbumin. In addition to high-mannose and hybrid oligosaccharide chains, ovalbumin synthesized in L cells contains oligosaccharides of the complex type. To date, this type of sugar chain has not been observed in chicken ovalbumin. These differences in fine structure, between the oligosaccharides derived from ovalbumin secreted by L cells and those known to be present in the chicken egg glycoprotein, suggest that the cell type also plays a role in oligosaccharide processing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S., Hubbard S. C., Strominger J. L. HLA-DR antigens of autologous melanoma and B lymphoblastoid cell lines: differences in glycosylation but not protein structure. J Immunol. 1984 Jul;133(1):315–320. [PubMed] [Google Scholar]
- Allen S. D., Tsai D., Schachter H. Control of glycoprotein synthesis. The in vitro synthesis by hen oviduct membrane preparations of hybrid asparagine-linked oligosaccharides containing 5 mannose residues. J Biol Chem. 1984 Jun 10;259(11):6984–6990. [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Glabe C. G., Hanover J. A., Lennarz W. J. Glycosylation of ovalbumin nascent chains. The spatial relationship between translation and glycosylation. J Biol Chem. 1980 Oct 10;255(19):9236–9242. [PubMed] [Google Scholar]
- Harpaz N., Schachter H. Control of glycoprotein synthesis. Processing of asparagine-linked oligosaccharides by one or more rat liver Golgi alpha-D-mannosidases dependent on the prior action of UDP-N-acetylglucosamine: alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. J Biol Chem. 1980 May 25;255(10):4894–4902. [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Host-dependent variation of asparagine-linked oligosaccharides at individual glycosylation sites of Sindbis virus glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2548–2554. [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Selective cleavage by endo-beta-N-acetylglucosaminidase H at individual glycosylation sites of Sindbis virion envelope glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2555–2561. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Narasimhan S. Control of glycoprotein synthesis. UDP-GlcNAc:glycopeptide beta 4-N-acetylglucosaminyltransferase III, an enzyme in hen oviduct which adds GlcNAc in beta 1-4 linkage to the beta-linked mannose of the trimannosyl core of N-glycosyl oligosaccharides. J Biol Chem. 1982 Sep 10;257(17):10235–10242. [PubMed] [Google Scholar]
- Pless D. D., Lennarz W. J. Enzymatic conversion of proteins to glycoproteins. Proc Natl Acad Sci U S A. 1977 Jan;74(1):134–138. doi: 10.1073/pnas.74.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Norrild B., Simpson T., Roizman B. Chicken ovalbumin gene fused to a herpes simplex virus alpha promoter and linked to a thymidine kinase gene is regulated like a viral gene. Mol Cell Biol. 1982 Mar;2(3):233–240. doi: 10.1128/mcb.2.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvidou G., Klein M., Grey A. A., Dorrington K. J., Carver J. P. Possible role for peptide-oligosaccharide interactions in differential oligosaccharide processing at asparagine-107 of the light chain and asparagine-297 of the heavy chain in a monoclonal IgG1 kappa. Biochemistry. 1984 Jul 31;23(16):3736–3740. doi: 10.1021/bi00311a026. [DOI] [PubMed] [Google Scholar]
- Savvidou G., Klein M., Horne C., Hofmann T., Dorrington K. J. A monoclonal immunoglobulin G1 in which some molecules possess glycosylated light chains--I. Site of glycosylation. Mol Immunol. 1981 Sep;18(9):793–805. doi: 10.1016/0161-5890(81)90001-8. [DOI] [PubMed] [Google Scholar]
- Schachter H., Narasimhan S., Gleeson P., Vella G. Control of branching during the biosynthesis of asparagine-linked oligosaccharides. Can J Biochem Cell Biol. 1983 Sep;61(9):1049–1066. doi: 10.1139/o83-134. [DOI] [PubMed] [Google Scholar]
- Swiedler S. J., Freed J. H., Tarentino A. L., Plummer T. H., Jr, Hart G. W. Oligosaccharide microheterogeneity of the murine major histocompatibility antigens. Reproducible site-specific patterns of sialylation and branching in asparagine-linked oligosaccharides. J Biol Chem. 1985 Apr 10;260(7):4046–4054. [PubMed] [Google Scholar]
- Swiedler S. J., Hart G. W., Tarentino A. L., Plummer T. H., Jr, Freed J. H. Stable oligosaccharide microheterogeneity at individual glycosylation sites of a murine major histocompatibility antigen derived from a B-cell lymphoma. J Biol Chem. 1983 Oct 10;258(19):11515–11523. [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ito S., Kobata A. Structures of the carbohydrate moiety of ovalbumin glycopeptide III and the difference in specificity of endo-beta-N-acetylglucosaminidases CII and H. J Biol Chem. 1977 Oct 10;252(19):6687–6694. [PubMed] [Google Scholar]
- Tai T., Yamashita K., Kobata A. The substrate specificities of endo-beta-N-acetylglucosaminidases CII and H. Biochem Biophys Res Commun. 1977 Sep 9;78(1):434–441. doi: 10.1016/0006-291x(77)91273-6. [DOI] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Maley F. endo-beta-N-Acetylglucosaminidase from Streptomyces plicatus. Methods Enzymol. 1978;50:574–580. doi: 10.1016/0076-6879(78)50065-7. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. Swainsonine causes the production of hybrid glycoproteins by human skin fibroblasts and rat liver Golgi preparations. J Biol Chem. 1983 Jun 25;258(12):7578–7585. [PubMed] [Google Scholar]
- Williams D. B., Lennarz W. J. Control of asparagine-linked oligosaccharide chain processing: studies on bovine pancreatic ribonuclease B. An in vitro system for the processing of exogenous glycoproteins. J Biol Chem. 1984 Apr 25;259(8):5105–5114. [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Hitoi A., Kobata A. Sialic acid-containing sugar chains of hen ovalbumin and ovomucoid. Carbohydr Res. 1984 Jul 15;130:271–288. doi: 10.1016/0008-6215(84)85285-4. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Kobata A. The structures of the galactose-containing sugar chains of ovalbumin. J Biol Chem. 1978 Jun 10;253(11):3862–3869. [PubMed] [Google Scholar]
- Yamashita K., Ueda I., Kobata A. Sulfated asparagine-linked sugar chains of hen egg albumin. J Biol Chem. 1983 Dec 10;258(23):14144–14147. [PubMed] [Google Scholar]