Abstract
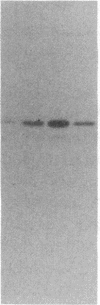
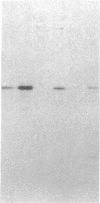
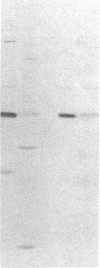
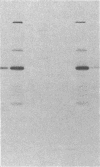
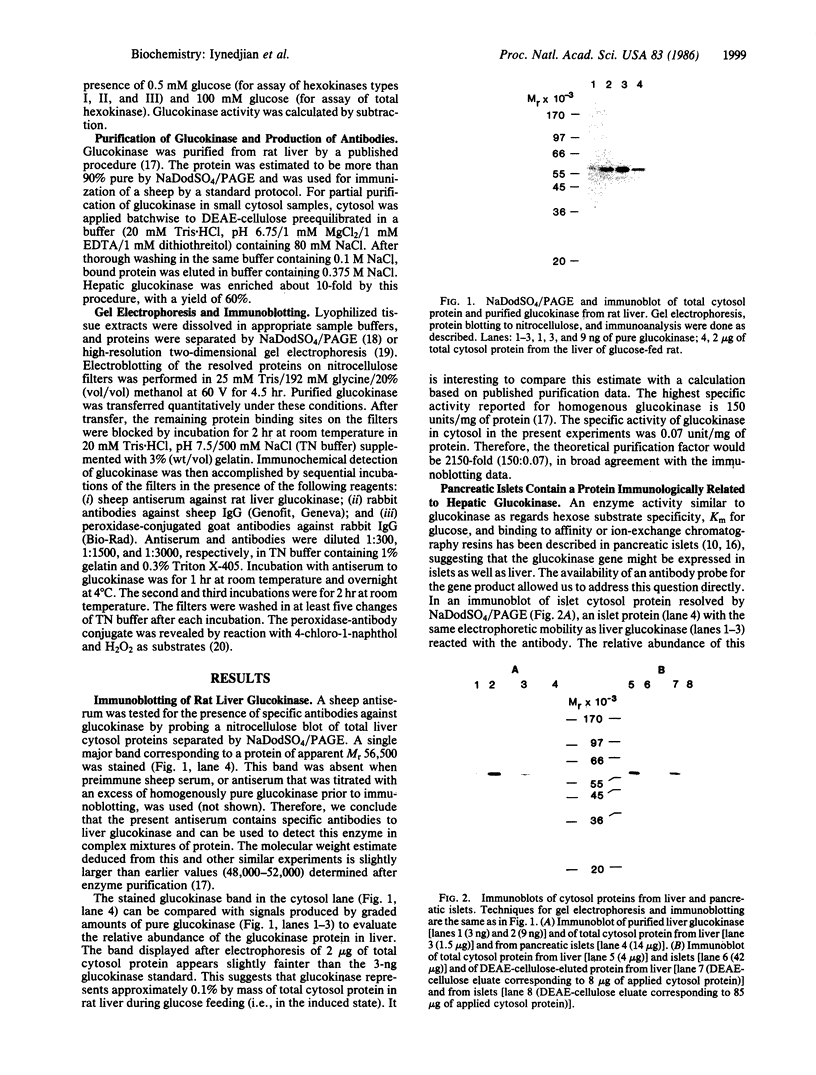
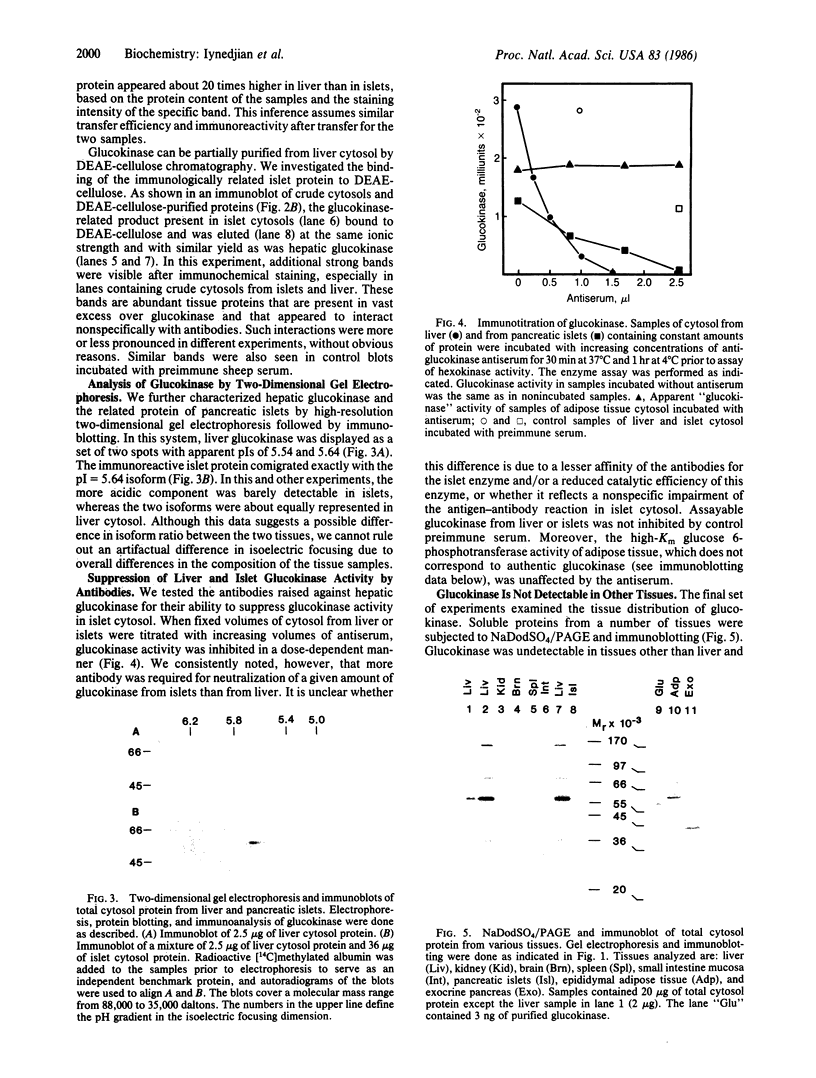
The tissue distribution of glucokinase (ATP:D-hexose 6-phosphotransferase, EC 2.7.1.1) was examined by protein blotting analysis. Antibodies raised against rat liver glucokinase recognized a single protein subunit with an apparent Mr of 56,500 on nitrocellulose blots of cytosol protein from liver, separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. A protein of identical electrophoretic mobility was detected by immunoblotting of cytosol protein from pancreatic islets. Hepatic glucokinase and the immunoreactive islet product bound to and were eluted from DEAE-cellulose at the same ionic strength. Glucokinase was displayed as a set of two spots with apparent pI values of 5.54 and 5.64 by immunoblotting after two-dimensional gel electrophoresis. The two isoforms appeared equally abundant in liver extract, whereas the component with a pI of 5.64 was predominant in islets. By quantitative immunoblotting, glucokinase was estimated to represent 0.1% of total cytosol protein in liver and 1/20th as much in islets. The glucokinase activity of both liver and islet cytosols was suppressed by the antibodies to hepatic glucokinase. Immunoblotting of cytosol protein from intestinal mucosa, exocrine pancreas, epididymal adipose tissue, kidney, brain, and spleen failed to reveal the glucokinase protein. Thus, significant expression of the glucokinase gene appears restricted to the liver and pancreatic islets.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Brockelbank J. L., Walker D. G. Apparent 'glucokinase' activity in non-hepatic tissues due to N-acetyl-D-glucosamine kinase. Biochim Biophys Acta. 1980 Aug 7;614(2):357–366. doi: 10.1016/0005-2744(80)90225-9. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., Hickman B. J. Analytical techniques for cell fractions. XXIV. Isoelectric point stadnards for two-dimensional electrophoresis. Anal Biochem. 1979 Mar;93(2):312–320. doi: 10.1016/s0003-2697(79)80157-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIPIETRO D. L., SHARMA C., WEINHOUSE S. Studies on glucose phosphorylation in rat liver. Biochemistry. 1962 May 25;1:455–462. doi: 10.1021/bi00909a014. [DOI] [PubMed] [Google Scholar]
- Davagnino J., Ureta T. The identification of extrahepatic "glucokinase" as N-acetylglucosamine kinase. J Biol Chem. 1980 Apr 10;255(7):2633–2636. [PubMed] [Google Scholar]
- Hansen R., Pilkis S. J., Krahl M. E. Properties of adaptive hexokinase isozymes of the rat. Endocrinology. 1967 Dec;81(6):1397–1404. doi: 10.1210/endo-81-6-1397. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hers H. G., Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- Holroyde M. J., Allen M. B., Storer A. C., Warsy A. S., Chesher J. M., Trayer I. P., Cornish-Bowden A., Walker D. G. The purification in high yield and characterization of rat hepatic glucokinase. Biochem J. 1976 Feb 1;153(2):363–373. doi: 10.1042/bj1530363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Burch P. T., Berner D. K., Najafi H., Vogin A. P., Matschinsky F. M. Chromatographic resolution and kinetic characterization of glucokinase from islets of Langerhans. Proc Natl Acad Sci U S A. 1983 Jan;80(1):85–89. doi: 10.1073/pnas.80.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meglasson M. D., Burch P. T., Hoenig M., Chick W. L., Matschinsky F. M. Identification and significance of glucokinase in transplantable insulinomas. J Biol Chem. 1983 Feb 25;258(4):2094–2097. [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. New perspectives on pancreatic islet glucokinase. Am J Physiol. 1984 Jan;246(1 Pt 1):E1–13. doi: 10.1152/ajpendo.1984.246.1.E1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H., O'Farrell P. Z. Two-dimensional polyacrylamide gel electrophoretic fractionation. Methods Cell Biol. 1977;16:407–420. doi: 10.1016/s0091-679x(08)60116-8. [DOI] [PubMed] [Google Scholar]
- SCHARFF R., WOOL I. G. CONCENTRATION OF AMINO ACIDS IN RAT MUSCLE AND PLASMA. Nature. 1964 May 9;202:603–604. doi: 10.1038/202603a0. [DOI] [PubMed] [Google Scholar]
- Shatton J. B., Morris H. P., Weinhouse S. Kinetic, electrophoretic, and chromatographic studies on glucose-ATP phosphotransferases in rat hepatomas. Cancer Res. 1969 Jun;29(6):1161–1172. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus M. D., Zawalich W. S., Burch P. T., Berner D. K., Weill V. A., Matschinsky F. M. Regulation of glucose metabolism in pancreatic islets. Diabetes. 1981 Nov;30(11):911–922. doi: 10.2337/diab.30.11.911. [DOI] [PubMed] [Google Scholar]
- Walker D. G., Holland G. The development of hepatic glucokinase in the neonatal rat. Biochem J. 1965 Dec;97(3):845–854. doi: 10.1042/bj0970845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse S. Regulation of glucokinase in liver. Curr Top Cell Regul. 1976;11:1–50. [PubMed] [Google Scholar]