Introduction
Colorectal cancer is considered one of the most preventable cancers. It is the third most prevalent cancer and the second leading cause of cancer death in the United States, with projected estimates of more than 146,970 new cases and about 49,920 deaths for the year 2009 (1). Disparities exist in both incidence and mortality by race/ethnicity and age. Additionally, approximately 90% of colorectal cancer is diagnosed in people aged 50 years or older, and about 75% of colorectal cancer is diagnosed in persons without any well-established predisposing risk factors such as personal history of polyps or colorectal cancer, a family history of colorectal or polyps, or bowel diseases (2). It is generally accepted that most, if not all, colorectal cancers arise from precancerous lesions: the adenomatous polyp (2). Early-stage diagnosis of colorectal cancer through regular screening is associated with survival rates of more than 90% for colon cancer and 80% for rectal cancer, indicating the need for broad-based interventions promoting increased screening among individuals over age 50 years. (3) The diffusion of colorectal cancer screening into clinical and public health practice has contributed to the trend of small decreases in colorectal cancer-related mortality over the past several decades (3); screening, however, remains suboptimal for minorities and older individuals (4).
Experts in the U.S. recommend regular colorectal cancer screening starting at age 50 for those at average risk. Screening options include annual fecal occult blood testing (stool blood test); flexible sigmoidoscopy (sigmoidoscopy) every 5 years; sigmoidoscopy every 5 years with annual stool blood test; and colonoscopy every 10 years (2, 5). Screening guidelines in other countries differ slightly from the U.S. and many offer national population-based screening programs. For example, in Australia, stool blood test is strongly recommended at least once every two years; and screening sigmoidoscopy is advised every five years from age 50 (6). In the United Kingdom, England and Wales have chosen to implement biennial stool blood test for all those aged between 60 and 69 years by 2010. Scotland is currently screening those aged between 50 and 74 years. National population-based programs also exist in Finland, France, Italy, and Poland, and regional based screening in advance of a national program is underway in Portugal, Slovenia, Sweden, and Spain. In Germany, annual stool blood testing is offered for those between 50 and 54 years and colonoscopy for those between 55 and 65 years (7).
Published reports of behavioral interventions to promote colorectal cancer screening target specific populations (8–12) and investigate different types of screening procedures such as stool blood testing (13), and combinations of stool blood testing, sigmoidoscopy, and colonoscopy (14–16). Despite available guidelines and strong evidence demonstrating mortality reductions from screening for this potentially preventable disease, an alarming proportion (39%) of screening-eligible adults are not up to date with screening recommendations (17). Low participation in screening for the disease results in lost opportunities to reduce morbidity and mortality through early detection and treatment (2, 18).
With a variety of screening promotion interventions reported, evidence indicates that tailored communication is among the most promising approaches to increase screening participation (19–22). Moreover, while there are no published trials testing motivational interview interventions promoting colorectal cancer screening, it has shown promise in health promotion (23–24) and was developed into a viable intervention to be used in our clinical trial (25).
Recently published randomized clinical trials testing tailored education/counseling strategies have shown significant increases in screening adoption. Significant intervention effects were reported with : 1) tailored telephone counseling (19); 2) non-tailored brochure plus telephone support calls from a prevention care manager (26); targeted video with non-tailored print information combined with a provider-directed education, performance feedback, and a quality improvement intervention (27); an ethnically-matched patient navigator (28); a brief educational message with multiple mailings of tailored print materials (20); tailored and non-tailored print materials mailed with fecal immunochemical test kit and 30-day reminders (29); an interactive web-based computer program designed to establish user preferences for colorectal cancer screening (21); and an annual mailed screening invitation with tailored education booklet plus follow-up phone call (22). While the majority of studies were guided by a theoretical framework, samples tended to be primarily Caucasian or included too few minority participants to make meaningful comparisons. Lack of standardized reporting of effects, reported as percent increases to odds ratios, also made making comparisons of the effectiveness of interventions across studies difficult. In general however, significant odds ratios for tailored interventions range from 1.08 (30) to 4.4 (19) and 7.7 (31).
Personalized behavioral interventions using computerized tailoring (28), motivational interviewing (24), or both (32) have shown promise over targeted (not individually personalized) interventions in improving health promotion behavior, including cancer screening. Few studies, however, have examined remote personalized interventions designed to promote colorectal cancer screening in diverse primary care patient populations (33), and to date there has been no direct comparison of a tailored intervention to a motivational interviewing intervention designed to promote colorectal cancer screening.
We report results of the first randomized clinical trial comparing the efficacy of two personalized telephone-based interventions—tailored counseling and motivational interviewing—to usual care in increasing colorectal cancer screening. The primary hypothesis guiding this 3-arm, randomized behavioral intervention trial was that colorectal cancer screening rates would be higher among individuals who received either tailored counseling or motivational interviewing than among those who were randomized to the usual-care (control) group.
Methods
Eligibility criteria included being 50 years or older; having no personal or family history of colorectal cancer; and being non-adherent with stool blood test, sigmoidoscopy, and colonoscopy. The last criterion was verified by checking medical records. Based on the policies of the Institutional Review Boards of the two Midwestern research sites, we could not verify any eligibility criteria until the individual had consented to participate in the study. Participants were enrolled in primary care clinics where a trained research assistant used self-reported information to determine eligibility and to obtain informed consent. Those who consented were then assigned by block randomization to one of three groups: control, tailored counseling, or motivational interview. Random allocation was done by pre-assigned identification and group numbers. Trained data collectors assigned participants to an identification number and corresponding group. Consenting and baseline data collection was completed by trained data collectors. After medical records were checked for these consenting individuals, either they were informed by phone of ineligibility (i.e., they had a recorded colorectal cancer screening test per recommendations in their medical record) or a baseline phone interview was conducted on knowledge of and beliefs about colorectal cancer and screening and past screening practices. Within 2 weeks of the baseline interview, those in the intervention groups received a counseling call and then follow-up interviews at one month and 6 months post-intervention. Those in the usual-care group had similar calls after the baseline interview. Participants in the usual-care group may have received a referral for a colorectal cancer screening test or not at the provider’s discretion. No special education or academic detailing was present in any of the sites during the study period.
Intervention Calls
Interventionists for both tailored counseling and motivational interviewing were trained in separate groups using training manuals that allowed us to standardize training for new staff (34–35). Additionally, 10% of both tailored counseling and motivational interview calls were randomly selected for audio-recording and review by study investigators (UM and SW). Process evaluations of the recorded calls were then discussed with each interventionist, and strategies for improvement were discussed if needed. Sample intervention protocols for both study conditions (tailored counseling and motivational interviewing) are available as electronic supplementary material.
Theoretical Frameworks for the interventions
Two behavior change theories were integrated to guide the intervention content. The Health Belief Model, one of the most commonly used theoretical frameworks in cancer screening research posits that an individual may change behavior around the action of interest (in this context, colorectal cancer screening) if knowledge about screening, perceived risk of developing the disease, perceived benefits (positive outcomes associated with colorectal cancer screening), and self-efficacy (confidence in one’s ability to complete a colorectal cancer screening test) are high, and barriers (obstacles to colorectal cancer screening) are low (36). Both interventions tested in the study addressed beliefs such as benefits, self-efficacy, and barriers. Behavior change is often seen as a dichotomous event. For example, not screened for cancer versus screened for cancer. In the Transtheoretical Model of Change, behavior change is seen as occurring on a continuum of stages from pre-contemplation (not thinking about having colorectal cancer screening) to contemplation (thinking about colorectal cancer screening) to action (completing a screening test) rather than as a dichotomous event (37). Beliefs may differ at different stages; as such targeted messages can be delivered on perceived risk, benefits, barriers, and self-efficacy based on the stage of readiness to complete colorectal cancer screening (38).
Tailored counseling
The tailored counseling was enabled by an expert computer system TIMS© (35). All participants answered a standardized survey over phone administered by trained collectors. Participants’ baseline demographics and beliefs were imported into TIMS©, and a script, tailored to baseline stage of readiness (pre-contemplation, contemplation), demographics (age, gender, race/ethnicity), and perceptions (most important benefit and barrier items, perceived risk and perceived self-efficacy), was printed. TIMS© is a computer program that contains tailored messages on all the variables identified above. Messages are connected to participants’ responses with algorithms. TIMS© is described in detail elsewhere (35). One of the trained interventionist then contacted the participants by phone, read the script to them, and answered questions. The script was written in a conversational style and was previously tested via focus groups for ease of understanding. The average call time was 13.7 minutes.
Motivational Interview
The motivational interview intervention consisted of a single, telephone-based motivational interview session. A detailed discussion of the design, delivery, and training of the motivational interview interventionists has been published elsewhere (25). Interventionists did not refer to the responses given by participants in the baseline survey. Rather, interventionists were trained to use motivational interviews to help people explore and resolve their ambivalence regarding colorectal cancer screening, as well as to explore and enhance their motivation(s) to get screened. Key intervention components and techniques included establishing rapport; asking permission to discuss colorectal cancer and screening; eliciting what the participant already knew about colorectal cancer and screening; providing additional education and information when necessary; assessing motivation, confidence and readiness to get screened; exploring ambivalence; eliciting change talk; rolling with resistance; and (if appropriate) supporting self-efficacy and commitment to get screened. We created a sample road map to provide interventionists (particularly those new to motivational interviewing) with some prompts that reflected the range of motivational interview skills and techniques available to them throughout the call. Intervention calls averaged 21.2 minutes.
Research Sites
This study was conducted at three U.S. sites: two large Midwestern medical centers (a Veteran’s Administration Medical Center and an academic health center) and one Southeastern medical center. Recruitment for this study took place in the primary care clinic or internal medicine clinics’ waiting areas, where trained research assistants distributed IRB-approved flyers to those interested in participating in the study and obtained consent. Recruitment occurred between 2005–2008; follow-up was up to six months post baseline assessment. Participants were mailed a $15 gift card to a department store of their choice for each data collection call but not the intervention session.
Statistical Analysis
Sample Size Justification
All power calculations were estimated using SAS Proc Power. We calculated a priori, a sample size of 420 (140 per study group) based on a detecting a 20% difference between the treatment groups with 80% power and an alpha level of 0.05 (using a Bonferroni adjustment to account for multiple comparisons, alpha = 0.025). Screening achieved was 18.1% for any colorectal cancer test. Descriptive statistics were used to examine the distribution of socio-demographic indicators and pre- and post-intervention colorectal cancer screening among study participants. Contingency table analyses were employed to examine the bivariate relationships between treatment group assignment and covariates of interest mentioned above.
We conducted a per-protocol analysis to assess the intervention effect. All cases were analyzed according to original assignment group (Table 1). Logistic regression models were fit to the data to estimate adjusted odds ratios (AORs) and 95% confidence intervals (CIs), indicating the relationships among completion of post-intervention colorectal cancer screening test and treatment group and socio-demographic indicators. The probability of receiving any colorectal cancer screening test was modeled. Crude odds ratios were estimated for each covariate with respect to the colorectal cancer screening outcome (Table 2, column 1). The adjusted odds ratios (AOR) reported in Table 2 account for all covariates simultaneously (study group, race, gender, age, employment, marital status, income, site, education, prior screening test, physician recommendation) with respect to colorectal cancer screening outcome. Adjusted odds ratios and confidence intervals are included for variables remaining significant at the p=0.05 level.
Table 1.
Sample characteristics
| Characteristic | All (n=515) %(n) | Usual Care (n=169) %(n) | TC (n=168) %(n) | MI (n=178) %(n) | X2 Statistic |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 69.7(n=359) | 68.0(n=115) | 72.6(n=122) | 68.5(n=122) | X2 =1.01 (df=2) |
| Female | 29.3(n=156) | 32.0(n=54) | 27.4(n=46) | 31.5(n=56) | |
| Race | |||||
| Black | 72.4(n=373) | 74.6(n=126) | 78.0(n=131) | 65.2(n=116) | X2 =10.76* (df=4) |
| White | 17.7(n=91) | 13.6(n=23) | 16.0(n=27) | 23.0(n=41) | |
| Other | 9.9(n=51) | 11.8(n=20) | 6.0(n=10) | 11.8(n=21) | |
| Age (mean[sd]) | 58.1 (7.9) | 56.9 (7.2) | 58.5 (8.3) | 58.8 (8.2) | F =2.76 (df=2) |
| Employment Status | |||||
| Working | 21.0(n=108) | 23.2(n=39) | 19.1(n=32) | 20.8(n=37) | X2 =0.89 (df=2) |
| Marital Status | |||||
| Single/divorce/separated/widowed | 71.8(n=375) | 72.8(n=117) | 72.6(n=126) | 70.2(n=132) | X2 =1.66 (df=2) |
| Married/partnered | 28.2(n=140) | 27.2(n=52) | 27.4(n=42) | 29.8(n=46) | |
| Income | |||||
| < 15k | 48.5(n=233) | 45.2(n=70) | 50.0(n=79) | 50.3(n=84) | X2 =3.32 (df=24) |
| 15k–30k | 28.3(n=136) | 32.2(n=5) | 24.0(n=38) | 28.7(n=48) | |
| <30k | 23.2(n=111) | 22.6(n=39) | 26.0(n=41) | 21.0(n=35) | |
| Site | |||||
| Midwest 1 | 29.0(n=149) | 29.6(n=50) | 26.4(n=44) | 30.5(n=55) | X2 =2.25 (df=4) |
| Midwest 2 | 65.0(n=335) | 62.(n=106) | 68.3(n=114) | 64.4(n=114) | |
| Southeast | 6.0(n=41) | 7.7(n=13) | 5.3(n=9) | 5.1(n=9) | |
| Education | |||||
| < HS, HS or trade school | 44.7(n=230) | 43.2(n=73) | 42.9(n=72) | 47.7(n=85) | X2 =1.06 (df=2) |
| BS or greater | 55.3(n=285) | 56.8(n=96) | 57.1(n=96) | 52.3(n=93) | |
| Prior screening test | |||||
| % Yes | 48.5(n=250) | 51.5(n=87) | 44.1(n=74) | 50.0(n=89) | X2 =2.1 (df=2) |
| Physician recommended any test | |||||
| % Yes | 65.4(n=337) | 60.4(n=102) | 66.7(n=113) | 69.1(n=122) | X2 =2.37 (df=2) |
| Had any test post-treatment | |||||
| %Yes | 18.1(n=93) | 11.83(n=20) | 23.8(n=40) | 18.5(n=33) | X2 =7.80* (df=4) |
TC=Tailored Counseling; MI=Motivational Interviewing;
p<.05
Table 2.
Unadjusted and adjusted odds ratios and 95% confidence intervals of colorectal cancer screening
| Characteristic | Unadjusted OR(CI) Any screening test | Adjusted OR(CI)-AOR Any screening test |
|---|---|---|
| Study Group | ||
| TC versus control | 2.3 (1.3,4,1)* | 2.2 (1.2, 4.0)* |
| MI versus control | 1.6 (0.9,3.0) | 1.6 (0.9, 2.9) |
| Gender | ||
| Male versus female | 1.7 (1.1,2.9)* | 1.5(0.7,3.5) |
| Race | ||
| White | Reference | |
| Black | 0.9 (0.5,1.6) | 0.9(0.5,1.7) |
| Other | 1.3 (0.6,3.0) | 1.9(0.8,4.7) |
| Age | 1.0 (0.9,1.0) | 1.0(.9,1.0) |
| Employment Status | ||
| Working versus not | 1.2 (0.7,2.1) | 1.2(0.6,2.4) |
| Working | ||
| Marital Status | ||
| Married/partner | Reference | |
| Single/divorced/separated/ | 0.7 (0.4,1.2) | 0.8 (0.4,1.4) |
| Widowed | ||
| Income | ||
| < 15k | Reference | |
| 15k–30k | 1.0 (0.5,1.6) | 1.1 (0.6,1.9) |
| >30k | 1.2 (0.7,2.2) | 1.4 (0.7,2.8) |
| Site | ||
| Midwest 1 | Reference | |
| Midwest 2 | 0.9 (0.3,2.4) | 1.5(0.6,3.4) |
| Southeast | 1.3 (0.5,3.5) | 1.9(0.6,5.5) |
| Education | ||
| < HS, HS, or trade | Reference | |
| BS or greater | 0.9 (0.6,1.4) | 0.7 (0.4,1.2) |
| Had prior test | 1.1 (0.7,1.8) | 1.3 (0.8,2.2) |
| Physician-recommended colorectal cancer test | 2.4 (1.4, 4.0)** | 2.3 (1.3, 3.8)** |
p < .05
p < .01
Adjsted Odds Ratio in Column 2 accounted for all covariates in the table TC=Tailored Counseling; MI=Motivational Interview
Measurement
Outcome measure
The outcome of interest was the completion of any screening test stool blood test, sigmoidoscopy, or colonoscopy within 12 months of the intervention. We assessed the three most recommended tests for colorectal cancer screening (2, 18). Other recommended tests such as double-contrast barium enema and virtual colonoscopy were not included because the former may have involved patients with multiple comorbidities and the latter had not had proven efficacy at the beginning of this trial. Completion of the three tests was confirmed through medical record review for each participant, and the verified status was used for analysis in the current report. Concordance between self-report and medical record data will be assessed in ongoing analysis.
Main Effect
The main effect of interest was treatment group: Usual care, tailored counseling, or motivational interview.
Other Covariates
Sociodemographic variables were race (African American, Caucasian, other), sex, age, employment status (currently working or not), marital status (married/partnered or not), and household income (<15k, 15–30k, >30k). Marital status is measured as partnered/or not, since colorectal cancer screening participation has been found to be related to having a cohabitating partner who can assist with test procedures or not (39). Educational status is presented as two grouping of less than high school, high school or trade school, and bachelor’s degree or greater. Having more than a high-school education (versus less than high school) has been found to be associated with participation in colorectal cancer screening (40).
Screening History
Whether or not the participant ever had a stool blood test, sigmoidoscopy, or colonoscopy.
Provider Recommendation
Self-reported information on whether or not a provider recommended that the participant have a stool blood test, sigmoidoscopy, or colonoscopy post-study enrollment.
Results
Sample
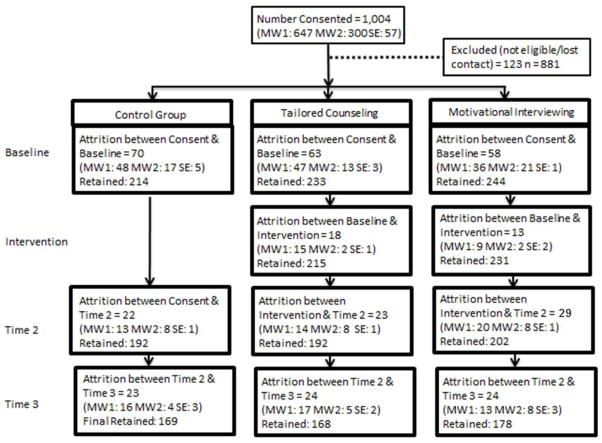
The average age of participants was approximately 58 years (SD = 8.0). The majority of our sample was male (69.7%), African American (72.4%), and currently not working (79%). While more than half of the participants had a bachelor’s degree or higher (55.3%), almost half had an income of less than $15,000 per year (48.5 %). Less than a third of participants were married or had a partner (28.2%). The majority of participants were recruited from the VA sites (65%). Table 1 displays the descriptive results for all covariates of interest and the bivariate relationships between covariates and intervention group. The response rate, defined as the number of people who signed a consent form and participated in the baseline interview, across sites was 70%, and attrition across sites was 24%. A detailed breakdown of participant flow by research site is presented in Figure 1. Attrition was not significantly different across sites or study groups.
Figure 1.
Study Participant Flow Diagramabc
MW1 = Midwest site 1 MW2 = Midwest site 2 SE = Southeast site
Reasons for attrition: refused, unable to contact; deceased
Final Total Study n = 515
Over two-thirds of the participants reported having had colorectal cancer screening recommended by a physician (65.4%) and had had a screening test (48.5%) in the past. Approximately 18% of the participants had a post-intervention colorectal cancer screening test. A total of 76 participants had a colonoscopy, 6 had a sigmoidoscopy, and 23 had a stool blood test for a total of 105 screening tests completed. This number includes 12 individuals who had more than one screening test. However, for analysis of intervention effect each individual was only counted once for a total of 93 completed screening tests at post-intervention (Table 1). By study group, the proportion who completed a colorectal cancer screening test (measured by medical record data) post-intervention was 11.8% (usual care), 23.8% (tailored counseling), and 18.5% (motivational interview) (X2 [df = 4] = 7.80, p< .05).
Odds ratios representing the relationship between patient and study characteristics and completion of post-intervention colorectal cancer screening are presented in Table 2. Crude odds ratios (table 2, column 1) indicates that men had increased odds of obtaining to obtain colorectal cancer screening compared to females (OR=1.7, 95% CI 1.1, 2.9). Additionally, the tailored counseling group had increased odds of colorectal cancer screening compared to the control group(OR=2.3, 95% CI 1.3, 4.1) and participants who reported receiving physician recommendation had higher odds of completing colorectal cancer screening compared to participants who did not receive a recommendation (OR=2.4, 95% CI 1.4, 4.0).
After controlling for socio-demographic characteristics (Table 1, Column 2), there were only two significant predictors of post-intervention colorectal cancer screening: intervention group and physician recommendation. Participants in the tailored counseling group had 2.2 times the odds of completing post-intervention colorectal cancer screening than did the participants in the usual-care group (AOR = 2.2, 95% CI 1.2, 4.0). Motivational interviewing was not significantly associated with greater probability of screening post-intervention compared with usual care (Table 2). Participants who reported having a physician recommend a screening test had just over two times greater odds of completing post-intervention screening than those who reported no physician recommendation (AOR = 2.3, 95% CI 1.3, 3.8).
Discussion and Conclusions
Overall we detected a moderate intervention effect in this study, with the tailored counseling calls being significantly more effective in increasing any colorectal cancer screening than usual care. Both tailored counseling and motivational interviewing produced small increases in screening, but the tailored counseling was not significantly more effective than motivational interviewing (analyses not shown). Our findings do compare favorably with previous studies reporting tailored interventions to increase stool blood testing and endoscopic screening (19–22, 35, 27–29, 41). Demographic characteristics such as sex, race/ethnicity, age, and education, shown to be significant predictors in past research, were, surprisingly, not significant in our study. It must be noted, however, that the current study sample had a greater proportion of male participants in comparison with other studies. There were not, however, any significant differences by biological sex. Our sample was also 72% African Americans, which was higher than most mixed-ethnicity study samples. Race was significantly different across groups, perhaps because the African American patient population was significantly higher at two sites: 50% and 55%. We controlled for the effect of race/ethnicity and site in all logistic regression analyses. Furthermore, we examined potential race/ethnicity by site interaction, which was not statistically significant.
The study response rate was good, at 70% overall, with the lowest response rate for the Southeastern site. Attrition rates were within anticipated range: 24% overall, with the Southeastern site being the highest at 38%. This site was added last to the study and had few overall study participants (under 10% of the sample). We believe that lower recruitment resulted from study personnel not being in the clinics long enough to establish a rapport with patients. At the other two Midwestern sites, study personnel were on site for at least 4 years.
Our retention strategies included 10 follow-up contacts on different days and at different times for unreachable participants, followed by a letter explaining we were trying to reach them by phone. Medical records were checked for address and phone number updates for those who remained lost to contact. Additionally, a series of mailings were sent to all those marked “unable to contact” before and after the third wave of data collection. All strategies yielded modest numbers who responded; overall, however, this added to a lower attrition rate for the study.
The overall numbers of those who completed screening were lower than anticipated: 18% of the sample had had any colorectal cancer screening test at 6 months post-intervention; of these, the significant majority (23.8%) was in the tailored counseling group. Recent meta-analyses of tailored interventions to increase mammography cancer screening show modest effects: 10–20% (42), which is similar to our findings (23.8% increase in screening in the tailored counseling intervention group).
The motivational interview calls were longer than the tailored calls, which we attribute to the nature of the intervention itself. Counselors in the tailored counseling group were given scripts to read based on participants’ responses at baseline, whereas the motivational interview counselors elicited this information (e.g., benefits, barriers) during the call. Although acceptability of both interventions was high, the systematic focus on exploring ambivalence for colorectal cancer screening in the motivational interview intervention may have been counterproductive (25). In some of their more recent conceptualizations of motivational interviewing, Miller and Rollnick (43) reflect that exploring ambivalence may in fact be “contraindicated” in some instances; this reflection was not published at the time we designed the intervention reported on in this study. According to Miller and Rollnick (43), “There is nothing fundamental, essential, or definitive about the decisional balance technique in the practice of motivational interviews. In fact, it could be contraindicated within our original conception of motivational interviews, wherein the focus is on eliciting the client’s own change talk and taking care not to reinforce counter-change talk” (p. 133). The motivational interviewing intervention was designed according to the Miller & Rollnick (44) definition and conceptualization of motivational interviewing that centered on ambivalence as a focal point of motivational interviewing practice. It is quite possible that the combined use of a “road map”(25) that included a systematic exploration of ambivalence regardless of a person’s motivation, confidence or readiness to get screened may have reinforced reasons not to get screened rather than facilitated change talk which is thought to be facilitative of actual change (45). Perhaps, for some study participants, talking about why they did not want to get screened, or not being ready to get screened actually enhanced and strengthened their lack of motivation, readiness or confidence to get screened - their “sustain talk” regarding maintaining status quo was enhanced and strengthened as a result.
Another possible reason for the lack of efficacy in the motivational interviewing intervention may be attributed to treatment fidelity. As discussed in detail elsewhere (25), while interventionists’ motivational interviewing proficiency increased over the course of the study, across some areas (namely global ratings for empathy, understanding and spirit), not all interventionists were motivational interviewing proficient from the beginning of the study, and some interventionists were more skilled than others.
In this study, the tailored messages focused on the primary benefit and barrier identified by the participant, his or her overall self-efficacy, and stage of readiness (pre-contemplation or contemplation) to complete screening. Future studies could pare down these concepts to the most important ones effective for screening completion, making computer-based tailored colorectal cancer screening education a viable practice in primary care. While results for tailored counseling are promising, we believe this intervention will need to be further tested before it can be applied in clinical practice. It is likely that compared to a generic referral for a test, any form of a personalized or tailored inquiry into patients’ reasons for not completing a colorectal cancer screening test could lead to increased adherence. However, to implement a tailored intervention in clinical practice, further analysis to understand the underlying mechanisms through which the tailoring worked is needed. For example, does tailoring to barriers produce higher rates of screening as opposed to tailoring to other beliefs such as self-efficacy or benefits, or some combination of beliefs?
Provider recommendation has been reported to predict screening post-intervention (e.g., 38, 46–47), which is similar to our findings, underscoring the need to find a way for providers to consistently endorse cancer screening in primary care visits. For example, studies of academic detailing and provider training have also reported only moderate effect. Interventions directed at providers or that are practice-based included developing academic detailing systems for tracking overdue patients (41, 48), expanding office staff responsibilities for screening (48), offering educational workshops (40), giving performance feedback, undertaking other types of quality improvement initiatives, or combining these strategies (27, 41, 48). Only one study reported a significant increase in screening (an academic detailing intervention combined with letter from provider, an educational brochure, and stool blood test kit with instructions) (41). Such results clearly underscore the need to examine more closely the mechanisms (e.g., mediating and moderating variables) by which such interventions work as well as combined patient and provider-directed interventions.
Our study had several limitations, including the significant difference by race/ethnicity across study groups at baseline for which we controlled in analyses. Although we enrolled participants during their primary care visit, the intervention was not scheduled until after the visit (at least 3–4 weeks later). Patients may not have been scheduled for another primary care visit post-intervention, thereby decreasing our rates of colorectal cancer screening completion. At all three sites, no screening test could be obtained without a referral or script from a provider, which meant that unless the participant had another primary care visit, they may not have had the opportunity to discuss screening with their providers. Therefore, provider recommendation as reported by participants may have occurred before the intervention, or the time line for recommendation may have been affected by recall bias. Future research should consider a more immediate intervention in primary care, ideally, just before the patient sees the provider. Additional strategies may be the inclusion of simultaneous provider reminders (at the visit when the patient is counseled or educated), especially given the significance of provider recommendation in increasing screening. Results from this study may be generalizable to primary care clinic settings where colorectal cancer screening referrals are made.
In summary, tailored interventions continue to show promise as an educational strategy to increase colorectal cancer screening. Development of strategies to optimize implementation of tailored interventions in practice settings is needed. Given the promise of early detection to reduce colorectal cancer morbidity and mortality, the timing and method of delivery of tailored interventions aimed at increasing cancer screening rates warrants continued research. Implementation and dissemination would be premature without a better understanding of the mechanism through which these interventions work or the minimal interventions needed for behavior change related to colorectal cancer screening.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institute of Nursing Research: R01NR8425. The data collection for this study was conducted while the first author was employed at the University of Illinois-Chicago, College of Nursing. We gratefully acknowledge the patients who consented to participate in the study. This study was approved by the Institutional Review Boards of the Jesse Brown Veterans Administration Medical Center, the University of Illinois-Chicago, Vanderbilt University, Meharry Medical College, and Arizona State University. This material is the result of work supported with resources and the use of facilities at the Jesse Brown Veterans Administration Medical Center, 820 S. Damen, Chicago, IL. Clinical trial registration: NCT01099826 (www.ClinicalTrails.gov). The authors thank Kelly Martin and Amy Woof for assistance with preparation of this manuscript.
Footnotes
Authors have no conflicts of interest to disclose.
Contributor Information
Usha Menon, Email: Usha.Menon@asu.edu, College of Nursing & Health Innovation, Arizona State University.
Rhonda Belue, Email: rzb10@psu.edu, Pennsylvania State University.
Stéphanie Wahab, Email: wahabs@pdx.edu, Portland State University.
Kathryn Rugen, Email: Kathryn.rugen@va.gov, Jesse Brown Veterans Administration Medical Center.
Anita Y. Kinney, Email: Anita.Kinney@hci.utah.edu, Department of Internal Medicine and Huntsman Cancer Institute, University of Utah
Peter Maramaldi, Email: peter.maramaldi@simmons.edu, Simmons College School of Social Work, Harvard Medical School.
Debra Wujcik, Email: debbie.wujcik@vanderbilt.edu, Vanderbilt University School of Nursing.
Laura A. Szalacha, Email: Laura.Szalacha@asu.edu, College of Nursing & Health Innovation, Arizona State University
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US multi-society task force on colorectal cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Cancer Statistics Working Group. [Accessibility verified January 10, 2010];United States cancer statistics: 1999–2005 incidence and mortality web-based report. Available at http:www.cdc.gov/uscs.
- 4.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Preventive Services Task Force. 2009 clinical guidelines: screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Prev Med. 2009;149:627–637. [Google Scholar]
- 6.Australian Cancer Network Colorectal Cancer Guidelines Revision Committee. [Accessibility verified May 1, 2010];Guidelines for the Prevention, Early Detection and Management of Colorectal Cancer. 2005 http://www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/cp106/cp106.pdf.
- 7.National Cancer Screening Service. [Accessibility verified May 1, 2010];Recommendations for a colorectal cancer screening programmer in Ireland. 2008 http://www.cancerscreening.ie/publications/Colorectal-Recommendations.pdf.
- 8.Bao Y, Fox SA, Escarce JJ. Socioeconomic and racial/ethnic differences in the discussion of cancer screening: “between-“ versus “within-“ physician differences. Health Serv Res. 2007;42(3 Pt 1):950–970. doi: 10.1111/j.1475-6773.2006.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastani R, Glenn BA, Maxwell AE, Ganz PA, Mojica CM, Chang LC. Validation of self-reported colorectal cancer (CRC) screening in a study of ethnically diverse first-degree relatives of CRC cases. Cancer Epidemiol Biomarkers Prev. 2008;17(4):791–798. doi: 10.1158/1055-9965.EPI-07-2625. [DOI] [PubMed] [Google Scholar]
- 10.Larkey LK, Lopez AM, Minnal A, Gonzalez J. Storytelling for promoting colorectal cancer screening among underserved latina women: A randomized pilot study. Cancer Control. 2009;16(1):79–87. doi: 10.1177/107327480901600112. [DOI] [PubMed] [Google Scholar]
- 11.Pollack CE, Mallya G, Polsky D. The impact of consumer-directed health plans and patient socioeconomic status on physician recommendations for colorectal cancer screening. J Gen Intern Med. 2008;23(10):1595–1601. doi: 10.1007/s11606-008-0714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu SP, Taylor V, Yasui Y, et al. Promoting culturally appropriate colorectal cancer screening through a health educator: A randomized controlled trial. Cancer. 2006;107(5):959–966. doi: 10.1002/cncr.22091. [DOI] [PubMed] [Google Scholar]
- 13.Jimbo M, Myers RE, Meyer B, et al. Reasons patients with a positive fecal occult blood test result do not undergo complete diagnostic evaluation. Ann Fam Med. 2009;7(1):11–16. doi: 10.1370/afm.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Federici A, Valle S, Giorgi Rossi P, Grassi A, Borgia P, Guasticchi G. Colorectal cancer screening: recommendations and guideline adherence by physicians from digestive endoscopy centers in the Lazio region, Italy. Prev Med. 2006;43(3):183–186. doi: 10.1016/j.ypmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Griffith JM, Lewis CL, Brenner AR, Pignone MP. The effect of offering different numbers of colorectal cancer screening test options in a decision aid: a pilot randomized trial. BMC Med Inform Decis Mak. 2008;8:4. doi: 10.1186/1472-6947-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169(4):364–371. doi: 10.1001/archinternmed.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control (CDC) Use of colorectal cancer tests United States, 2002, 2004, and 2006. MMWR Morb Mortal Wkly Rep. 2008;57(10):253–238. [PubMed] [Google Scholar]
- 18.American Cancer Society. Colorectal cancer facts & figures 2008–2010. Atlanta, GA: American Cancer Society; 2008. [Google Scholar]
- 19.Basch CE, Wolf RL, Brouse CH, et al. Telephone outreach to increase colorectal cancer screening in an urban minority population. Am J Public Health. 2006;96(12):2246–2253. doi: 10.2105/AJPH.2005.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcus AC, Mason M, Wolfe P, et al. The efficacy of tailored print materials in promoting colorectal cancer screening: results from a randomized trial involving callers to the National Cancer Institute’s Cancer Information Service. J Health Commun. 2005;10(Suppl 1):83–104. doi: 10.1080/10810730500257754. [DOI] [PubMed] [Google Scholar]
- 21.Ruffin MT, Fetters MD, Jimbo M. Preference-based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial. Prev Med. 2007;45(4):267–273. doi: 10.1016/j.ypmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Tilley BC, Vernon SW, Myers R, et al. The next step trial: impact of a worksite colorectal cancer screening promotion program. Prev Med. 1999;28(3):276–283. doi: 10.1006/pmed.1998.0427. [DOI] [PubMed] [Google Scholar]
- 23.Apodaca TR, Longabaugh R. Mechanisms of change in motivational interviewing: a review and preliminary evaluation of the evidence. Addiction. 2009;104(5):705–715. doi: 10.1111/j.1360-0443.2009.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clin Psychol Rev. 2009;29(4):283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Wahab S, Menon U, Szalacha L. Motivational interviewing and colorectal cancer screening: a peek from the inside out. Patient Educ Couns. 2008;72(2):210–217. doi: 10.1016/j.pec.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietrich AJ, Tobin JN, Cassells A, Robinson CM, Greene MA, Sox CH, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Ann IntMed. 2006 Apr 18;144(8):563–713. doi: 10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira MR, Dolan NC, Fitzgibbon ML, Davis TC, Gorby N, Ladewski L, et al. Health care provider-directed intervention to increase colorectal cancer screening among veterans: results of a randomized controlled trial. J Clin Oncol. 2005 Mar 1;23(7):1548–54. doi: 10.1200/JCO.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 28.Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005 Jun;82(2):216–24. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. CA. 2007 Nov 1;110(9):2083–91. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 30.Ling BS, Schoen RE, Trauth JM, et al. Physicians encouraging colorectal screening: a randomized controlled trial of enhanced office and patient management on compliance with colorectal cancer screening. Arch Intern Med. 2009;169(1):47–55. doi: 10.1001/archinternmed.2008.519. [DOI] [PubMed] [Google Scholar]
- 31.Rawl SM, Champion VL, Scott LL, et al. A randomized trial of two print interventions to increase colon cancer screening among first-degree relatives. Patient Educ Couns. 2008;71(2):215–227. doi: 10.1016/j.pec.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valanis BG, Glasgow RE, Mullooly J, et al. Screening HMO women overdue for both mammograms and pap tests. Prev Med. 2002;34(1):40–50. doi: 10.1006/pmed.2001.0949. [DOI] [PubMed] [Google Scholar]
- 33.Myers RE. Patient preferences for colorectal cancer screening. Am J Manag Care. 2007;13(11):633–4. author reply 634, 636. [PubMed] [Google Scholar]
- 34.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol. 2004;23(5):443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 35.Menon U, Szalacha LA, Belue R, Rugen K, Martin KR, Kinney AY. Interactive, culturally sensitive education on colorectal cancer screening. Med Care. 2008;46(9 Suppl 1):S44–50. doi: 10.1097/MLR.0b013e31818105a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Champion VL, Skinner CS. The Health Belief Model. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior & Health Education: Theory, Research & Practice. Jossey-Bass, CA: 2008. pp. 45–62. [Google Scholar]
- 37.Prochaska JO, Redding CA, Evers KE. The Transtheoretical Model and Stages of Change. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior & Health Education: Theory, Research & Practice. Jossey-Bass, CA: 2008. pp. 97–117. [Google Scholar]
- 38.Menon U, Belue R, Sugg Skinner C, Rothwell BE, Champion V. Perceptions of colon cancer screening by stage of screening test adoption. Cancer Nurs. 2007;30(3):178–185. doi: 10.1097/01.NCC.0000270706.80037.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Jaarsveld CH, Miles A, Edwards R, Wardle J. Marriage and cancer prevention: does marital status and inviting both spouses together influence colorectal cancer screening participation? J Med Screen. 2006;13(4):172–6. doi: 10.1177/096914130601300403. [DOI] [PubMed] [Google Scholar]
- 40.Doubeni CA, Laiyemo AO, Young AC, Klabunde CN, Reed G, Field TS, Fletcher RH. Primary care, economic barriers to health care, and use of colorectal cancer screening tests among medicare enrollees over time. Ann Fam Med. 2010 Jul-Aug;8(4):299–307. doi: 10.1370/afm.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh JM, Posner SF, Perez-Stable EJ. Colon cancer screening in the ambulatory setting. Prev Med. 2002;35(3):209–218. doi: 10.1006/pmed.2002.1059. [DOI] [PubMed] [Google Scholar]
- 42.Sohl SJ, Moyer A. Tailored interventions to promote mammography screening: a meta-analytic review. Prev Med. 2007;45(4):252–261. doi: 10.1016/j.ypmed.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychother. 2009;37(2):129–140. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 44.Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. New York: The Guildford Press; 2002. [Google Scholar]
- 45.Miller WR, Rose SG. Toward a theory of motivational interviewing. Am Psyc. 2009;64:527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitzgibbon ML, Ferreira MR, Dolan NC, et al. Process evaluation in an intervention designed to improve rates of colorectal cancer screening in a VA medical center. Health Promot Pract. 2007;8(3):273–281. doi: 10.1177/1524839907302210. [DOI] [PubMed] [Google Scholar]
- 47.Menon U, Champion VL, Larkin GN, Zollinger TW, Gerde PM, Vernon SW. Beliefs associated with fecal occult blood test and colonoscopy use at a worksite colon cancer screening program. J Occup Environ Med. 2003;45(8):891–898. doi: 10.1097/01.jom.0000083038.56116.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruffin MT, Gorenflo DW. Interventions fail to increase cancer screening rates in community-based primary care practices. Prev Med. 2004;39(3):435–440. doi: 10.1016/j.ypmed.2004.04.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.