Abstract
We identified the molecular target by histone deacetylase (HDAC) inhibitors for exploring their potential prostate cancer (PCa) therapy. Upon HDAC inhibitors-treatment, LNCaP cell growth was suppressed, correlating with increased cellular prostatic acid phosphatase (cPAcP) expression, an authentic protein tyrosine phosphatase. In those cells, ErbB-2 was dephosphorylated, histone H3/H4 acetylation and methylation increased and cyclin proteins decreased. In PAcP shRNA-transfected C-81 cells, valproic acid (VPA) efficacy of growth suppression was diminished. Further, VPA pre-treatment enhanced androgen sensitivity of C-81, C4-2 and MDA PCa2b-AI cells. Thus, cPAcP expression is involved in growth suppression by HDAC inhibitors in PCa cells, and VPA pre-treatments increase androgen sensitivity.
Keywords: Prostate cancer, HDAC inhibitors, cPAcP, ErbB-2, androgen sensitivity
1. Introduction
Prostate cancer (PCa) is the most commonly diagnosed solid tumor and the second leading cause of cancer death in men in United States [1]. Despite the fact that androgen ablation therapy provides the first line of treatment for metastatic PCa, effective therapy for relapsed HR or CR PCa is limited. Further modalities are required for treating this patient population.
The epigenetic modifications including DNA methylation and histone modifications play an important role in the patho-physiology of cancer [2,3]. Methylation of cytosine in CpG islands leads to gene silencing and is closely associated with cancer development. However, about 40% of human gene promoters do not contain the CpG island; other epigenetic factors such as chromatin modification and nucleosome remodeling are involved in establishing this mode of regulation [3,4]. Histone modifications predominantly acetylation and deacetylation by histone acetyl transferases and HDACs are the major events for epigenetic regulation [4]. HDAC activity is enhanced in various cancers and histone deacetylation is one of common mechanisms used by cancer cells to down-regulate tumor suppressor genes and/or to up-regulate tumor promoting genes [3,4]. Several lines of evidence show that HDACs are abundantly expressed and up-regulated in PCa cells and cancerous tissues [2,5,6]. Therefore HDAC inhibitors, for example, VPA, are in clinical trials for PCa therapy [7]. However, the functional targets regulated by HDAC inhibitors remain further identification.
Human PAcP is the major phosphatase in normal well-differentiated prostate epithelial cells. In those cells, there are two forms of PAcP: the cellular form (cPAcP) and the secretory form (sPAcP), differing in several biochemical properties [8-10]. The serum activity of sPAcP is frequently elevated in PCa patients and correlated with tumor progression [11,12]. As a consequence, serum sPAcP was used as a surrogate marker for monitoring PCa until the availability of PSA [12]. Several lines of evidence show that cPAcP level negatively correlates with prostate carcinogenesis, i.e. its cellular level decreases in PCa cells, lower than in adjacent non-cancerous cells, despite the fact that sPAcP is elevated in the circulation [13-17]. Further, cPAcP activity inversely correlates with the growth rate of prostate epithelia and PCa cell lines [13-15,18-20]. It is thus proposed that cPAcP functions as a negative growth regulator of prostate epithelia [10,18,19,21]. This cPAcP function is at least in part due to the fact that this enzyme possesses the intrinsic protein tyrosine phosphatase activity [10,15,22,23]. In prostate epithelia, cPAcP dephosphorylates HER-2/ErbB-2/Neu at its phosphotyrosine residues and down-regulates its kinase specific activity, which is associated with diminished growth rates and reduced tumorigenicity of xenograft tumors [15,18,24-26]. Conversely, knockdown cPAcP expression by shRNA associates with hyper-tyrosyl phosphorylation of HER-2 and activation of its downstream signaling, which results in CR growth in culture and tumor development in female xenograft animals [10,27,28]. Further, in PAcP-knockout mice, prostates develop adenocarcinomas spontaneously [29]. These observations provide insights into one of the molecular mechanisms involved in CR PCa progression. Despite the importance of cPAcP in prostate carcinogensis, the molecular mechanism by which the expression of cPAcP is decreased or silenced in PCa cells remains an enigma.
In this communication, we explored the functional molecules targeted by HDAC inhibitors and concurrently, investigated the regulation of cPAcP expression by epigenetic events including histone acetylation and methylation in AR-positive, androgen-independent PCa cells, representing the majority of advanced PCa population in clinic [20,30]. We used LNCaP C-81 cells as the model system because these cells express functional AR, secrete PSA under androgen-deprived conditions and exhibit intracrine ability as CR PCa in clinic [20,30,31]. Our data provide strong evidence for the role of cPAcP in growth suppression by HDAC inhibitors and the regulation of cPAcP expression by epigenetic control in PCa. Importantly, VPA-treated C-81 cells obtained the enhanced androgen sensitivity of cell growth and PSA expression. Similar effects by VPA-pretreatment on androgen sensitivity were also observed in androgen-independent LNCaP C4-2 and MDA PCa2b-AI cells. The results thus have important clinical impacts on developing novel therapy toward advanced CR PCa.
2. Materials and methods
2.1. Materials
RPMI 1640 medium, gentamicin, FBS and trypsin/EDTA reagents were purchased from Invitrogen Corporation (Carlsbad, CA). Charcoal/dextran-treated, certified FBS was from Atlanta Biologicals (Lawrenceville, GA, USA). Acrylamide, protein molecular weight standard markers and Protein Estimation Kit were obtained from Bio-Rad (Hercules, CA). The ECL reagent kit was purchased from Pierce Biotechnology Inc. (Rockford, IL). FBS, histone deacetylase inhibitors including NaB, VPA, Trichostatin A and anti-β-Actin Ab (AC-15) were from Sigma (St Louis, MO, USA). Anti-phospho-HER-2/ErbB-2 (Tyr1221/1222) Ab was from Cell Signaling Technology (Beverly, MA). The Ab against histone H3-acetylation (Lys-9/14) and histone H4-acetylation (Lys-16) were from Santa Cruz Biotechnology (Santa Cruz, CA). The Ab against pY1248-ErbB-2, H3-methylation (trimethyl-Lys27) and H4-methylation (trimethyl-Lys20) were from Upstate Biotechnology (Lake Placid, NY, USA). Other Abs were procured from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-PAcP antiserum (ATM-3) was described previously [24].
2.2. Cell Culture
Human prostate carcinoma cell line LNCaP and MDA PCa2b was originally purchased from the American Type Culture Collection (Rockville, MD). LNCaP C4-2 cells were purchased from DIANON Company (Oklahoma City, OK). LNCaP cells were routinely maintained in the regular medium, i.e., phenol red-positive RPMI 1640 medium supplemented with 5% FBS, 2 mM glutamine and 50 μg/ml gentamicin. MDA PCa2b cells were cultured in BRFF-HPC1 medium containing 20 % FBS, 2 mM glutamine and 50 μg/ml gentamicin [27,32]. LNCaP C4-2 cells were grown in DMEM/F12 medium containing 10 % FBS, 2mM glutamine, 50 μg/ml gentamicin, 1 mM sodium pyruvate, 2× vitamin C and 1× MEM non-essential amino acid [33]. Cells were split once a week by trypsinization, which was defined as one passage. The LNCaP PCa cell progression model was described originally by Lin et al. [20] and further characterized by Igawa et al. [30] with passage number less than 33 defined as C-33, passage numbers between 80 and 120 as C-81. In these experiments, cells with passage numbers between 100 and 115 were used. LNCaP C-81 cells exhibit many biochemical properties similar to advanced CR PCa, including AR expression and PSA secretion with rapid growth in the steroid-deprived condition [20,30] and obtaining intracrine regulation [31]. Similarly, LNCaP C4-2 cells and the high passage MDA PCa2b cells exhibit androgen-independent proliferation [27,32,33]. Since these high passage MDA PCa2b cells obtain the androgen-independent phenotype, we named these cells as MDA PCa2b-AI cells. In this set of experiments, the passage numbers of MDA PCa2b-AI cells were between about 110 and 125.
2.3. Northern blot hybridization
Total RNA was prepared from LNCaP cells by the guanidine isothiocynate method [18,20]. Ten μg each of total RNA sample were electrophoresed on 1.2% agarose gels containing formaldehyde as a denaturing agent. After electrophoresis, the gel was stained with ethidium bromide (EtBr) and visualized to ensure approximately equal amounts of RNA per lane, then blotted to nitrocellulose membranes by standard techniques [18]. Filters were hybridized and washed under stringent conditions as described previously [18,20]. cDNA probes for PAcP were labeled with (α-32P)-dCTP using random oligonucleotide-primed synthesis with a commercial system. GAPDH cDNA probes were used to detect GAPDH mRNA as an internal control.
2.4. Cell growth analysis
LNCaP C-33 and C-81 cells were plated with 1×104 cells/well in 12-well plates in regular medium and treated with different concentrations of reagents as specified in each experiment for 3 days. Cells were counted using a Cellometer Auto T4 Image-based cell counter (Nexcelom Bioscience) [27]. Results shown are an average of three sets of independent experiments performed in triplicates.
2.5. CAT reporter gene assay and cPAcP shRNA plasmid transfection
For CAT reporter gene assay, LNCaP C-81 cells were plated at a density of 1×104 cells/well in 12-well plates and transfected with 0.1 μg of PAcP promoter-reporter gene including p779 and p1258, using Lipofectamine and Plus reagents in serum-free medium [34]. Four hours after transfection, cells were fed with RPMI media containing 5% FBS for 24 hr. The cells were then treated with different concentrations of NaB for 48 hr. Cells were then harvested, lysed and the CAT activity in cell lysates was determined. For shRNA plasmid transfections, C-81 cells were plated at a density of 1×105 cells/well in 6-well plates for 72 hr and then transfected with PAcP shRNA-126 plasmids [27]. Control cells were transfected with the vector containing the scramble DNA. Five hours after transfection, the cells were fed with RPMI media containing 10% FBS for 24 hr. The cells were then treated with 1mM VPA for 2 or 3 days. Total cell lysate proteins from 3 days treatment were analyzed for cPAcP protein expression.
2.6. Effect of HDAC inhibitor on the androgen sensitivity of cell growth
For analyzing the androgen sensitivity of HDAC inhibitor-treated cells, LNCaP C-81, C4-2 and MDA PCa2b cells were plated at a density of 3×104, 3×104 and 1×105 cells/well, respectively, in 6-well plates for 3 days and then treated with 1 mM VPA in regular medium for 2 days. Subsequently, cells were maintained in a steroid-reduced medium plus or minus 10 nM 5α-dihydrotestosterone (DHT) for 2 or 3 days. Cells were then harvested, counted and lysed for analyzing cPAcP, AR and PSA protein expression.
2.7. Immunoblotting
For detecting cellular proteins, subconfluent cells were harvested by scraping. The cell pellet was rinsed with ice-cold 20 mM HEPES-buffered saline (pH 7.0) and then lysed in ice-cold hypotonic cell lysis buffer containing protease and phosphatase inhibitors and the total lysate protein was prepared accordingly [27]. An aliquot of total cellular lysate having 50-120 μg protein was subjected to electrophoresis on SDS-polyacrylamide gels (7.5-12% acrylamide) for western blot analyses [27]. The proteins of interest were visualized by an ECL detection system. For rehybridization, the membranes were stripped with a stripping buffer [27], blocked and re-probed with specific Abs.
2.8. Flow cytometry analysis
LNCaP C-81 cells were treated with 1 mM NaB, 1 mM VPA or 300 nM TSA for 48 hr. The cells were then washed with ice-cold 20 mM HEPES-buffered saline (pH 7.0) and harvested by trypsinization. A minimum of 1×106 cells was fixed with chilled 75% ethanol for at least 1 hr at 4°C. Cells were then incubated with ice cold Telford DNA staining reagent (50 μg/ml RNase A and 50 μg/ml propidium iodide in HEPES) for overnight at 4°C. Data acquisition and analyses were carried out using a flow cytometry system [35].
2.9. Statistical analysis
Each experiment was performed in triplicates, repeated 2-3 times and the mean and standard error values were calculated. The significance of difference (p-value) was calculated using independent Student t-test and the p-value less than 0.05 was considered as significant [20,35].
3. Results
3.1. Effect of NaB on cell growth and PAcP expression in AR-positive PCa cells
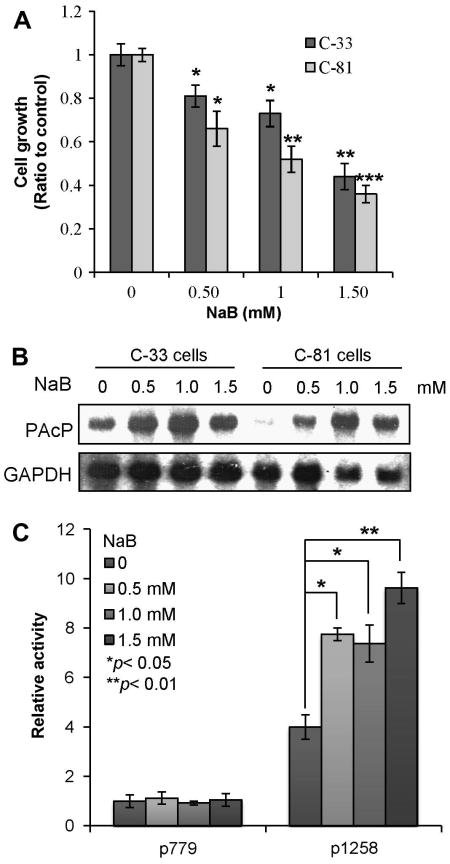
To explore the functional molecules targeted by HDAC inhibitors, we initially treated LNCaP C-33 and C-81 cells with NaB, a classical HDAC inhibitor. Both cells express functional AR; while C-33 cells are androgen-sensitive, C-81 cells obtain many biochemical properties of advanced CR PCa, including rapid proliferation in a steroid-reduced condition and intracrine regulation [15,20,30,31]. Cell growth analyses showed that NaB treatment is associated with decreased cell proliferation, following a dose-dependent manner in both C-33 and C-81 cells (Fig. 1A). Since cPAcP expression is associated with growth suppression [26,27], we investigated NaB effect on cPAcP expression. Northern blot analyses showed that in the absence of NaB, C-33 cells expressed a higher basal level of PAcP mRNA than C-81 cells in which PAcP mRNA was only marginally detected (Fig. 1B). PAcP mRNA level was greatly elevated in both NaB-treated C-33 and C-81 cells, following a bell-shape dose-responsive fashion. We further examined if NaB up-regulated PAcP mRNA at the transcriptional level by analyzing its promoter (p779 and p1258) activity utilizing the CAT reporter gene assay in C-81 cells because these cells express a very low level of PAcP in control cells (Fig. 1B). The p779 promoter exhibited a low basal promoter activity, as shown previously [34,36], and NaB had no effect on its reporter gene activity (Fig. 1C). The p1258 promoter exhibited a high basal activity and was further activated by NaB (Fig. 1C), in the face of growth suppression (Fig. 1A). Thus, NaB inhibits LNCaP cell growth and concurrently induces the expression of PAcP, an authentic growth suppressor [10,27], at the transcriptional level in these cells.
Fig. 1.
Effect of NaB on cell growth and PAcP expression. LNCaP C-33 and C-81 cells were treated with different concentrations of NaB for 3 days. Cells were harvested for (A) cell growth analysis and (B) total RNA preparation. The expression of PAcP mRNA was analyzed by northern blot analyses. The same membrane was hybridized with a GAPDH probe as a loading control. (C) C-81 cells were transfected with the CAT reporter gene drived by PAcP promoters in the presence of different concentrations of NaB. Cells were harvested and the CAT activity was determined. The values represent the means ± SD of three independent experiments (n=3×3). *p < 0.05; **p < 0.01; ***p < 0.001.
3.2. Effect of HDAC inhibitors on cPAcP protein levels in dosage and kinetic manner
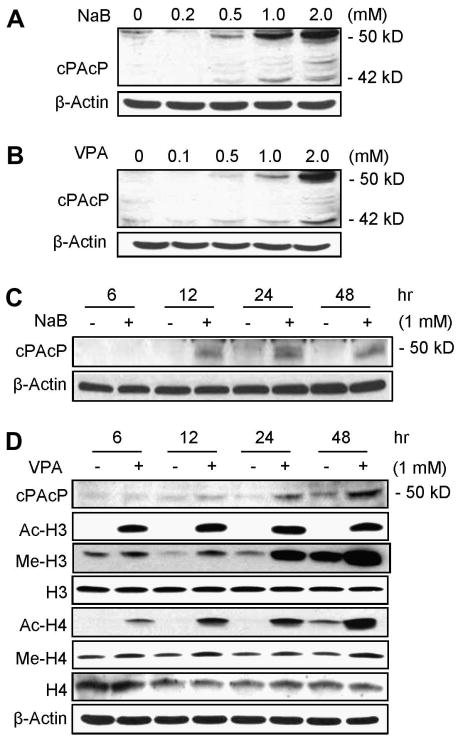
We focused our efforts on examining NaB effect on PAcP expression at protein level, the functional molecule, in C-81 cells since these cells exhibit the CR phenotype with a low level of PAcP expression. In NaB-treated C-81 cells, cPAcP protein level elevated, following a dose-dependent fashion (Fig. 2A). The 50 kDa protein is the mature glycosylated form of PAcP protein, and the 42 kDa protein is its intermediate form protein (Fig. 2A; [10,27]). Since NaB exhibits diverse activities other than HDAC inhibition, we examined the effect of another HDAC inhibitor TSA on cPAcP protein expression in C-81 cells. Western blot analyses showed that cPAcP protein level is also elevated in TSA-treated C-81 cells, following a dose-dependent manner (data not shown). We further examined VPA effect on cPAcP protein expression since VPA exhibits an activity as a HDAC inhibitor and is currently in clinical usages [4,7]. In VPA-treated C-81 cells, cPAcP protein level elevated (Fig. 2B) and concurrently, cell growth decreased (data not shown), as seen by NaB (Fig. 1A). Therefore, HDAC inhibitors effectively suppress PCa cell growth and concurrently up-regulate cPAcP expression in a dose-dependent fashion.
Fig.2.
Dosage and kinetic effects of HDAC inhibitors on cPAcP protein expression in LNCaP C-81 cells. LNCaP C-81 cells that were seeded at a density of 5×105 cells/T25 for 2 days in regular medium were treated with different concentrations of (A) NaB or (B) VPA for 48 hr, or treated with (C) 1 mM NaB or (D) 1 mM VPA for different time periods. The total protein was harvested and subjected to western blot analyses of cPAcP protein expression. (D) The acetylation and the methylation levels of H3 and H4 proteins were also analyzed in VPA-treated C-81 cells for different time periods. β-Actin was analyzed and used as a loading control in each experiment. Similar results were obtained from five sets of independent experiments.
We investigated the kinetic effect of HDAC inhibitors on cPAcP protein expression in LNCaP C-81 cells. As shown in Fig. 2C & 2D, NaB and VPA up-regulated the 50 kDa mature form of cPAcP protein level in C-81 cells initially seen at 12 hr treatment, which was greatly elevated at 24 hr and 48 hr time points (Fig. 2C & 2D). Thus, in HDAC inhibitor-treated cells, increased cPAcP expression followed a time-dependent manner.
HDAC inhibitors have effects on histone acetylation and also methylation [37-39]. Due to the clinical applicability of VPA, we focused on analyzing VPA effects on histone H3 and H4 acetylation levels at the residue Lysine-9/14 and Lysine-16, respectively, for representation. In VPA-treated C-81 cells, histone H3/H4 acetylation levels were greatly up-regulated in a time-dependent fashion between 0-12 hr and the up-regulated acetylation levels were remained essentially the same from 12 hr to 48 hr. A low level of acetylation in control cells was seen after a prolonged period of exposure (data not shown). The methylation levels of histone H3 (Lys-27) and H4 (Lys-20) were only moderately up-regulated seen at the 12 hr time point and through 48 hr (Fig. 2D). The data revealed that in C-81 cells, the low level of PAcP gene expression is apparently in part down-regulated by epigenetic mechanism including histone deacetylation and possibly demethylation.
3.3. Role of cPAcP expression in PCa cell growth suppression by HDAC inhibitors
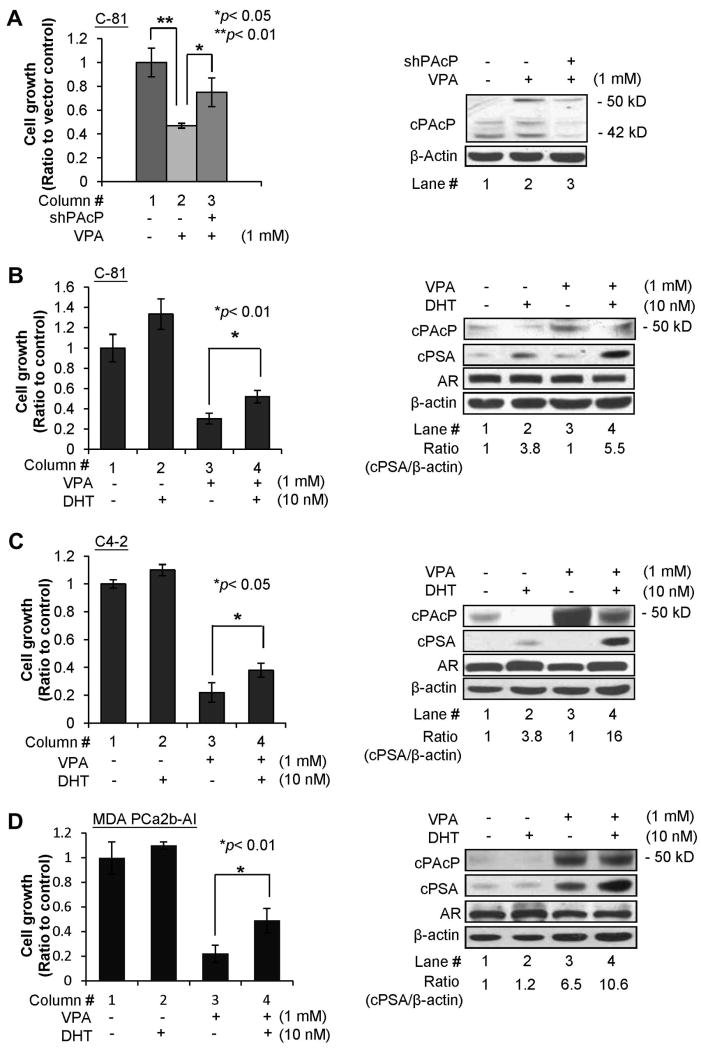
To clarify the role of cPAcP expression in growth suppression by HDAC inhibitors, C-81 cells were transfected with shRNA plasmid to cPAcP and followed by VPA treatment. Control cells were transfected with the vector containing scramble oligonucleotides. As shown in Fig 3A, VPA suppressed the growth of vector alone transfected C-81 cells by about 53% (column #1 vs. 2). Interestingly, the degree of VPA-growth suppression on shPAcP-transfected C-81 cells was decreased by about 50%, i.e., from 53% growth inhibition (Fig. 3A, column #2 vs. #1) reduced to 25% suppression (Fig. 3A, column #3 vs. #1). Similar results were obtained from both 48 hr (Fig. 3A, left panel) and 72 hr (data not shown) VPA treatments. Furthermore, western blot results validated that cPAcP protein level was decreased by about 60% in shPAcP-transfected C-81 cells, lower than that in control vector-transfected cells after 72 hr VPA-treatment (Fig. 3A, lane #3 vs. #2, right panel). Thus, the data collectively support the notion that VPA efficacy on growth suppression is in part through cPAcP expression.
Fig. 3.
Effect of cPAcP expression on VPA growth suppression and VPA effect on androgen sensitivity of PCa cell lines. (A) LNCaP C-81 cells were plated at a density of 1×105 cells/well in 6-well plates for 72 hr and then transfected with PAcP shRNA-126 plasmids. Control cells were transfected with the vector containing the scramble DNA. Five hours after transfection, the cells were fed with RPMI medium containing 10% FBS for 24 hr. The cells were then treated with 1mM VPA for 48 hr. The cell numbers were counted. The ratio of cell growth was calculated by normalizing the cell number to that of the control cells transfected with the control vector and treated with the solvent alone. The result shown is the average from two sets of independent experiments in triplicates (left panel, n= 3×2). Total cell lysate proteins from 3-day VPA treatments were analyzed for cPAcP protein expression. β-Actin was used as a loading control (right panel). (B) LNCaP C-81; (C) C4-2; (D) MDA PCa2b-AI cells were plated at a density of 3×104, 3×104 and 1×105 cells/well, respectively, in 6-well plates for 72 hr and then treated with 1 mM VPA. Control cells were treated with solvent alone. After 48 hr VPA treatment, cells were maintained in a steroid-reduced medium in the absence or presence of 10 nM DHT for 48 hr. The cell numbers were counted. The ratio of cell growth was calculated by normalizing the cell number to that of the control cells without any treatment (column #1). The result shown is the average from three sets of independent experiments in triplicates (n= 3×3). Total cell lysate proteins from 3-day DHT treatment were analyzed for cPAcP, PSA, AR protein expression. The AR protein in Fig. 3B was obtained after 3-hr hybridization with anti-AR Ab followed by an exposure time period of less than 1 min; while the AR proteins in Fig. 3C & 3D were obtained after overnight hybridization with anti-AR Ab and with an exposure time period of 1 hr. β-Actin was used as a loading control and it was obtained with the same reaction time periods.
3.4. Effect of HDAC inhibitor on the androgen sensitivity of PCa cells
cPAcP has been shown for its role in regulating androgen sensitivity of PCa cells which has been reported in several publications [20,27,28]. We thus investigated the effect of HDAC inhibitor on androgen sensitivity. Androgen-independent C-81 cells were treated with 1 mM VPA for 48 hr followed by 10 nM DHT in a steroid-reduced condition. The usage of 10 nM DHT is to determine if VPA pre-treatment has an effect on androgen sensitivity of these cells. In the presence of DHT for 48 hr, the growth of VPA-pretreated C-81 cells increased by about 70% (column #4 vs. #3, Fig. 3B, left panel, p<0.01), compared with only about 35% stimulation of control cells without VPA pre-treatment (column #2 vs. #1, Fig. 3B, left panel). Western blot results validated that the cPAcP 50 kDa mature form protein was elevated by VPA treatment (lane #3 vs. #1, Fig. 3B, right panel), which was decreased by subsequent DHT-treatment (lane #4 vs. #3, Fig. 3B, right panel), inversely correlating with cell growth stimulation [20,27,28]. In parallel, cellular PSA level was greatly elevated by DHT in VPA-pretreated cells, about 1.5-fold of that in control cells without VPA-pretreatment (lane #4 vs. #2, Fig. 3B, right panel). In those same cells, AR expression level was not significantly changed after a total of 5 days treatments including 2-day by VPA and 3-day by DHT.
Due to the clinical importance of androgen sensitivity of PCa cells, we investigated whether VPA treatment could similarly increase the degree of androgen stimulation in other androgen-independent PCa cells, including LNCaP C4-2 and MDA PCa2b-AI cells. As shown in Fig. 3C for LNCaP C4-2 cells and Fig. 3D for MDA PCa2b-AI cells, DHT alone could increase the basal cell growth by approximately 10% (column #1 vs. #2). Interestingly, DHT could greatly increase the growth of VPA-pretreated cells by about 70% and 120%, respectively, (column #4 vs. #3, Fig. 3C & 3D, left panels). We subsequently validated DHT effect by semi-quantifying PSA levels in those treated cells. Interestingly, PSA basal level was elevated in VPA alone-treated MDA PCa2b-AI cells in the absence of DHT (lane #3 vs. lane #1, Fig. 3D, right panel). Importantly, cellular PSA level was greatly elevated by DHT in VPA-pretreated cells, approximately 4- and 9-fold of that in control cells without VPA-pretreatment, respectively (lane #4 vs. #2, Fig. 3C & 3D, right panels). It should also be noted that due to the low expression level of AR protein in these two PCa cell lines, prolonged hybridization with primary Ab to AR with longer exposure time periods were required. In summary, VPA pre-treatment enhances DHT effect on the increments of cell growth and PSA expression, which indicates that HDAC inhibitors can enhance androgen sensitivity of PCa cells.
3.5. Effect of HDAC inhibitors on ErbB-2 tyrosyl phosphorylation level
We elucidated the molecular mechanism by which cPAcP contributes to HDAC inhibitor-induced PCa cell growth suppression in C-81 cells as our model system. Cell density has a significant effect on the expression of functional genes involving in growth regulation [40-42]. In prostate epithelia, including LNCaP C-33 cells, cPAcP protein level elevates when cell density increases in which cell growth is decreased [10,41]. Therefore, we investigated the effect of 2-day VPA treatment on cPAcP protein expression in different cell densities of C-81 cells. Western blot analyses showed that in the absence of VPA, the basal level of cPAcP 50 kDa protein in higher density cells was slightly higher than that of lower density cells (lane #3 & #5 vs #1, Fig. 4). In the presence of VPA, cPAcP protein level was further elevated in the face of growth suppression by VPA. Nevertheless, neither the cell density nor VPA had an effect on β-actin protein level. Thus, VPA activates the silenced cPAcP gene expression in C-81 cells under growth suppression, independent of the cell density. Similar results were observed in NaB-treated cells (data not shown).
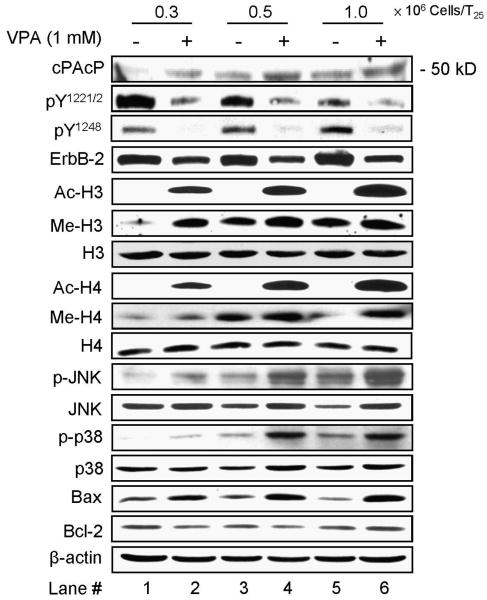
Fig. 4.
Effects of VPA on cPAcP protein expression and ErbB-2 tyrosyl phosphorylation. LNCaP C-81 cells were plated in three cell dinsities (0.3, 0.5 and 1×106 cells/T25) in regular medium for 2 days and then treated with 1 mM VPA for 48 hr. The cells were harvested and the total protein was subjected to western blot analyses of functional proteins expression. VPA effects on cPAcP protein expression, ErbB-2 phosphorylation at Tyr1221/2 and Tyr1248, histone H3 and H4 acetylation and methylation, JNK and p38 phosphorylation, Bcl-2 and Bax protein levels. β-Actin was detected as a loading control. The data shown is a representative from three sets of independent experiments.
Since cPAcP can regulate ErbB-2 tyrosyl phosphorylation in PCa cells [15,24-27], we analyzed ErbB-2 tyrosyl phosphorylation in 2-day VPA-treated cells. As shown in Fig. 4, the basal phosphorylation level of Tyr1221/2 of ErbB-2 decreased in the high-density, slow-growing cells in which cPAcP proteins level is elevated, concurring with the notion that Tyr1221/2 are the primary sites of dephosphorylation by cPAcP [27]. Unexpectedly, the basal phosphorylation level of Tyr1248 at ErbB-2 was increased in high-density cells (Fig. 4). In VPA-treated cells, independent of cell density, cPAcP protein level elevated and ErbB-2 phosphorylation decreased at both Tyr1221/2 and Tyr1248 (Fig. 4), indicating cPAcP can dephosphorylate both phosphorylation sites. Similar results of the inverse correlation between cPAcP elevation and ErbB-2 dephosphorylation were observed in NaB-treated cells (data not shown). ErbB-2 protein level was also diminished in HDAC inhibitors-treated cells although to a lesser degree than that of phosphorylation level.
We analyzed acetylation and methylation levels of H3 and H4 in VPA-treated cells of different densities. Western blot analyses showed that the acetylation levels of histone H3 and H4 were greatly up-regulated in 2-day VPA-treated LNCaP C-81 cells, following a cell density-dependent fashion (Fig. 4); while there was a very low level of acetylation in the absence of VPA which was seen after a long term exposure (data not shown). Interestingly, in the absence of VPA, the basal levels of H3 Lys-27 and H4 Lys-20 methylation increased, following the density of cells and correlating with basal cPAcP expression. VPA up-regulated these methylation levels primarily in low density cells (Fig. 4). Nevertheless, neither the cell density nor HDAC inhibitors had an effect on histone H3 and H4 protein levels. Thus, in these HDAC inhibitors-treated cells, cPAcP protein increases and ErbB-2 Tyr1221/2 and Tyr1248 phosphorylation decreases, which apparently contribute to growth suppression.
3.6. Effect of HDAC inhibitors on PCa cell proliferation and apoptotic proteins
We analyzed stress-induced signal pathway in 2-day VPA-treated C-81 cells. As shown in Fig. 4, in the absence of VPA, increased phosphorylation levels of JNK/MAPK and p38/MAPK were following a cell density-dependent manner. In VPA-treated cells, the levels of JNK and p38 MAPK phosphorylation and Bax pro-apoptotic protein were greatly elevated. However, there was no significant change in bcl-2 anti-apoptotic protein level. Thus, HDAC inhibitor-induced growth suppression is apparently in part via JNK and p38 MAPK activation of Bax.
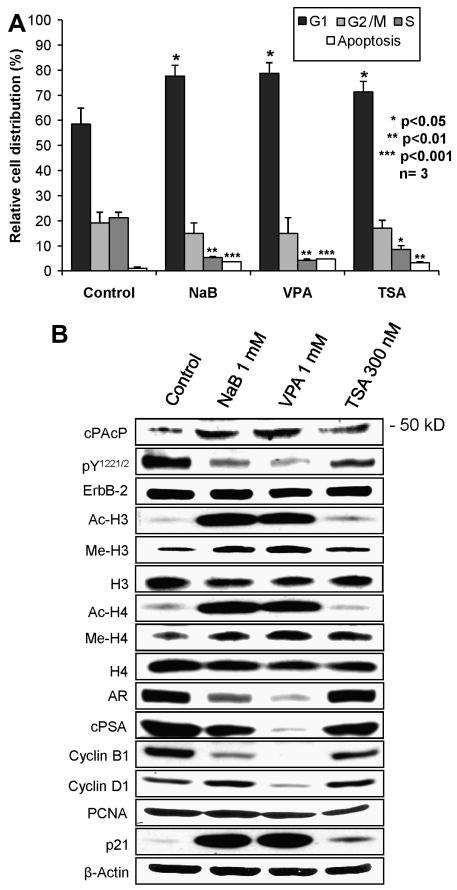
We examined cell cycle distribution and apoptosis in HDAC inhibitors-treated LNCaP C-81 cells. Flow cytometry analyses showed that approximately 60% and 20% control cells were at the G1- and S-phase, respectively, of the cell cycle (Fig. 5A). HDAC inhibitors caused the accumulation of cells (70-75%) in the G1-phase with only 3-6% cells at S-phase, indicating diminished cell growth as observed in Fig. 1A. Further, HDAC inhibitors induced a significant increase of apoptosis in C-81 cells, compared with control cells (Fig. 5A, p<0.01). Interestingly, among HDAC inhibitors examined, TSA exhibited the least efficacy on cell cycle arrest and apoptosis induction in C-81 cells.
Fig. 5.
Effects of HDAC inhibitors on cell cycle distribution, apoptosis and cell cycle protein expression in LNCaP C-81 cells. LNCaP C-81 cells were plated in regular medium for 48 hr and treated with 1 mM NaB, 1 mM VPA or 300 nM TSA for 48 hr. All cells were harvested and subjected to cell cycle analyses using flow cytometry and western blot analyses of indicated proteins. (A) Cell cycle analyses. The data shown is the mean of three sets of independent experiments. (B) Western blot analyses. The total cellular lysate proteins were analyzed for cPAcP protein, ErbB-2 phosphorylation, histone H3/H4 acetylation and methylation, AR, cPSA, cyclin B1 and D1, PCNA and p21 protein levels. β-Actin was analyzed as a loading control in each experiment. The data shown is a representative of three sets of independent experiments.
We further investigated the effects of HDAC inhibitors on cell cycle proteins. Western blot results (Fig. 5B) showed that the expression levels of cyclin B1 and D1 were greatly abolished by VPA and to a lesser degree by NaB and TSA after 2-day treatment. On the contrary, the expression level of p21, a cyclin-dependent kinase inhibitor protein, was greatly increased by NaB and VPA, and to a much lesser degree by TSA (Fig. 5B). Nevertheless, β-actin was not changed in HDAC inhibitor-treated cells. Unexpectedly, HDAC inhibitors did not have an effect on the expression level of PCNA, a cell proliferation marker, despite the observed growth inhibition. Further analyses showed the down-regulation of AR and intracellular PSA (cPSA) in VPA-treated C-81 cells and to a lesser degree in NaB-treated cells; while there was no significant change in TSA-treated cells (Fig. 5B). In these HDAC inhibitors-treated cells (Fig. 5B), cPAcP protein level was inversely correlated with ErbB-2 tyrosyl phosphorylation and was positively correlated with H3/H4 acetylation and methylation. The data collectively indicate that HDAC inhibitors suppress androgen-independent, AR-positive PCa cells in part via cPAcP induction for ErbB-2 dephosphorylation, leading to cell cycle arrest and apoptosis.
4. Discussion
Epigenetic regulation including histone modifications plays critical roles in tumor initiation and progression. In PCa, HDAC activity is increased in their pre-malignant and malignant stages [2,38,43]. Therefore, HDAC inhibitors are emerging as an exciting class of potential anticancer agents [5,6,38]. HDAC inhibitors including NaB and VPA have been examined for antitumor activity in phase I and II clinical trials, respectively [2,7,44,45]. Among them, VPA exhibits an efficacy on PCa cell growth [46,47] and xenograft tumor suppression [46]. However, the molecular mechanism and the functional targets of these HDAC inhibitors in PCa remain further illustration for exploring their potential uses in PCa therapy.
The restoration of cPAcP expression in PCa cells may provide a new avenue for treating advanced CR PCa in which the expression of PAcP is decreased or silenced. The notion is supported by the observation that treatment of androgen-independent LNCaP C-81 cells with HDAC inhibitors leads to their growth suppression, which correlates with elevated cPAcP expression. Conversely, knockdown cPAcP expression by pre-transfecting with shRNA reduces growth suppression by VPA (Fig. 3A, column #2 vs. #3). The data collectively support the notion that cPAcP expression contributes to growth suppression efficacy by VPA. Importantly, the pre-treatment of HDAC inhibitor enhances the androgen-sensitivity of LNCaP C-81 and C4-2 and MDA PCa2b-AI cells (Fig. 3B, 3C & 3D). The data imply that intermit treatments with HDAC inhibitors may prolong the efficacy of androgen ablation therapy. Nevertheless, it should be pointed out that in VPA-treated C-81 cells, different AR and PSA protein levels were observed in Fig. 3B vs. Fig. 5B; while the PSA level correlates with AR level in respective experiments. Because AR level in PCa cells is regulated by a dynamic process, and also because Fig. 3B was generated from cells with a total of 5-day treatment including 2-day by VPA and 3-day by DHT while Fig. 5B was from cells of 2-day VPA treatment, we thus proposed it is in part caused by different protocols with different time periods of treatments. Further exploration of this avenue is apparently timely important in developing novel strategies for treating CR PCa.
Another finding in this study is that HDAC inhibitors up-regulate the expression of PAcP gene. Despite the fact that cPAcP can function as a tumor suppressor [26,27,29], and its decreased expression correlates with the CR phenotype [27], the molecular mechanism of its loss of expression in PCa cells remains an enigma. Southern blot analyses reveal that PAcP gene is apparently retained intact in PAcP-null cells [48]. Interestingly, the promoter of PAcP gene does not contain the classical CpG island (Klinkebiel D, Christman JK and Lin MF; unpublished observations). The involvement of DNA methylation in silencing PAcP gene requires further investigation. In this study, the expression of cPAcP is elevated in HDAC inhibitors-treated AR-positive PCa cells and is associated with decreased cell growth. In NaB-treated C-81 cells, the PAcP promoter activity increases as evidenced by the reporter gene activity and the PAcP mRNA level is elevated (Fig. 1). Further, increased expression of cPAcP is correlated with both increased acetylation and methylation of histone H3/H4. Thus, the silencing of PAcP gene in LNCaP C-81 cells is at least in part down-regulated by the epigenetic mechanism [48]. In those cells, histone deacetylation and/or demethylation act cooperatively to silence the PAcP gene, leading to CR PCa progression. Similarly effects by VPA on cPAcP expression are also observed in androgen-independent C4-2 and MDA PCa2b-AI cells (Fig. 3C & 3D). Due to the potential importance of PAcP expression in tumor suppression, the regulatory mechanism of PAcP expression requires further exploration.
Our data also demonstrate that in the absence of VPA, the expression level of cPAcP protein increased when the cell density increases and this increased expression of cPAcP correlates with decreased ErbB-2 phosphorylation at Tyr1221/2 residues, but not Tyr1248 (Fig. 4). The increased expression of cPAcP associates with slow growth of high density PCa cells may imply that cPAcP is a functional molecule in down-regulating cell growth. The data also indicate that Tyr1221/2 is the primary site of mediating cell proliferation signaling in these PCa cells, concurring with biochemical observations [27]. Thus, inhibiting Tyr1221/2 phosphorylation at ErbB-2 may serve as a novel approach for CR PCa therapy.
Decreased EGFR and ErbB-2 proteins and their phosphorylation levels can be involved in apoptosis via p38 MAPK-dependent activation of Bax in various cancer cells [49,50]. Our results show that in HDAC inhibitors-treated PCa cells, increased cPAcP expression is associated with decreased ErbB-2 protein and its tyrosyl phosphorylation; while stress signal pathways JNK and p38/MAPK are activated and Bax is elevated in addition to the increased apoptotic protein p21 in those cells. These data reveal that HDAC inhibitors-induced suppression of HR PCa cells is in part via the inhibition of ErbB-2 downstream cell survival signaling pathway where MAPK-dependent activation of Bax is involved. Additionally, HDAC inhibitors can induce the expression of various tumor suppressor genes including p21Waf/Cip1, p27Kip1 and p53, leading to cell cycle arrest at the G1- or G2/M phase [51,52]. In the present study, in HDAC inhibitors-treated androgen-independent, AR-positive PCa cells, elevated cPAcP protein correlates with decreased cell-cycle up-regulating proteins cyclin B1 and cyclin D1, and increased cyclin-dependent kinase inhibitor p21 protein. Together, cell growth is arrested.
5. Conclusion
Our study shows that in HDAC inhibitors-treated androgen-independent, AR-positive PCa cells, growth suppression is associated with cPAcP induction, ErbB-2 dephosphorylation and importantly, increased androgen sensitivity. The enhanced androgen sensitivity by HDAC inhibitors pretreatment may indicate that intermit HDAC inhibitor treatments can prolong the efficacy of androgen ablation therapy, therefore, further investigation is necessary. Further, the effect of HDAC inhibitors on PCa cell growth suppression is at least in part via up-regulating cPAcP expression. Therefore, the status of inactivation of PAcP gene could potentially serve as a surrogate marker for identifying the lethal form of PCa. Our results also reveal a novel mechanism of HDAC inhibitors on PCa growth suppression, which can lead to the development of a new class of agents for treating CR PCa.
Acknowledgments
This study is supported in part by NIH Grant (2R01CA88184), DOD (PC050769 and PC074289) and Nebraska Research Initiative. We thank Dr. Stanislav Zelivianski for his support in the early phase of the reporter gene assay and Drs. David Klinkebiel and Judith Christman for analyzing the methylation status of PAcP promoter.
The abbreviations used are
- Ab
antibody
- Ac-H3/H4
acetylation of histone-3 and -4
- AI
androgen-independent
- AR
androgen receptor
- cPAcP
cellular prostatic acid phosphatase
- CR
castration-resistant
- ECL
enhanced chemiluminescence
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- HDAC
histone deacetylases
- HR
hormone-refractory
- MAPK
mitogen activated protein kinase
- Me-H3/H4
methylation of histone-3 and -4
- NaB
sodium butyrate
- PCa
prostate cancer
- sPAcP
secretory prostatic acid phosphatase
- PSA
prostate-specific antigen
- TSA
trichostatin A
- VPA
valproic acid
Footnotes
Conflict of interest: The authors declare that there are no financial and personal relationships with other people or organizations that could inappropriately influence (bias) our work.
References
- 1.Jemal A, Siegel R, Ward E, Hao YP, Xu JQ, Murray T, Thun MJ. Cancer Statistics, 2008, CA. Cancer. J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Abbas A, Gupta S. The role of histone deacetylases in prostate cancer. Epigenetics. 2008;3:300–309. doi: 10.4161/epi.3.6.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 4.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 5.Waltregny D, North B, Van Mellaert F, de Leval J, Verdin E, Castronovo V. Screening of histone deacetylases (HDAC) expression in human prostate cancer reveals distinct class I HDAC profiles between epithelial and stromal cells. Eur J Histochem. 2004;48:273–290. [PubMed] [Google Scholar]
- 6.Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, Kristiansen G. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98:604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez R. Sidney kimmel Comprehensive Cancer Center at Johns Hopkins, Valproic acid in treating patients with progressive, non-metastatic prostate cancer. National cancer institute NCT00670046. 2009 [Google Scholar]
- 8.Vihko P. Human prostatic acid phosphatases: purification of a minor enzyme and comparisons of the enzymes. Invest Urol. 1979;16:349–352. [PubMed] [Google Scholar]
- 9.Lad PM, Cooper JF, Learn DB, Olson CV. Identification of structural and secretory lectin-binding glycoproteins of normal and cancerous human prostate. Biochim Biophys Acta. 1984;791:186–197. doi: 10.1016/0167-4838(84)90008-6. [DOI] [PubMed] [Google Scholar]
- 10.Veeramani S, Yuan TC, Chen SJ, Lin FF, Petersen JE, Shaheduzzaman S, Srivastava S, MacDonald RG, Lin MF. Cellular prostatic acid phosphatase: a protein tyrosine phosphatase involved in androgen-independent proliferation of prostate cancer. Endocr Relat Cancer. 2005;12:805–822. doi: 10.1677/erc.1.00950. [DOI] [PubMed] [Google Scholar]
- 11.Huggins C, Hodges CV. Studies on prostatic cancer: the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- 12.Chu TM, Lin MF. PSA and acid phosphatase in the diagnosis of prostate cancer. J Clin Ligand Assay. 1998;21:24–34. [Google Scholar]
- 13.Reif AE, Schlesinger RM, Fish CA, Robinson CM. Acid phosphatase isozymes in cancer of the prostate. Cancer. 1973;31:689–699. doi: 10.1002/1097-0142(197303)31:3<689::aid-cncr2820310331>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Hakalahti L, Vihko P, Henttu P, Autio-Harmainen H, Soini Y, Viko R. Evaluation of PAP and PSA gene expression in prostatic hyperplasia and prostatic carcinoma using northern-blot analyses, in situ hybridization and immunohistochemical stainings with monoclonal and bispecific antibodies. Int J Cancer. 1993;55:590–597. doi: 10.1002/ijc.2910550413. [DOI] [PubMed] [Google Scholar]
- 15.Lin MF, Lee MS, Zhou XW, Andressen JC, Meng TC, Johansson SL, West WW, Taylor RJ, Anderson JR, Lin FF. Decreased expression of cellular prostatic acid phosphatase increases tumorigenicity of human prostate cancer cells. J Urol. 2001;166:1943–1950. [PubMed] [Google Scholar]
- 16.Sakai H, Yogi Y, Minami Y, Yushita Y, Kanetake H, Saito Y. Prostate specific antigen and prostatic acid phosphatase immunoreactivity as prognostic indicators of advanced prostatic carcinoma. J Urol. 1993;149:1020–1023. doi: 10.1016/s0022-5347(17)36285-7. [DOI] [PubMed] [Google Scholar]
- 17.Sinha AA, Gleason DF, Wilson MJ, Wick MR, Reddy PK, Blackar CE. Relationship of prostatic acid phosphatase localization in human prostate by a monoclonal antibody with the Gleason Grading System. Prostate. 1988;13:1–15. doi: 10.1002/pros.2990130102. [DOI] [PubMed] [Google Scholar]
- 18.Lin MF, DaVolio J, Garcia-Arenas R. Expression of human prostatic acid phosphatase activity and the growth of prostate carcinoma cells. Cancer Res. 1992;52:4600–4607. [PubMed] [Google Scholar]
- 19.Lin MF, Garcia-Arenas R, Xia XZ, Biela B, Lin FF. The cellular level of prostatic acid phosphatase and the growth of human prostate carcinoma cells. Differentiation. 1994;57:143–149. doi: 10.1046/j.1432-0436.1994.5720143.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin MF, Meng TC, Rao PS, Chang C, Schonthal AH, Lin FF. Expression of human prostatic acid phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines. J Biol Chem. 1998;273:5939–5947. doi: 10.1074/jbc.273.10.5939. [DOI] [PubMed] [Google Scholar]
- 21.Lin MF, Clinton GM. Human prostatic acid phosphatase has phosphotyrosyl protein phosphatase activity. Biochem J. 1986;235:351–357. doi: 10.1042/bj2350351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li HC, Chernoff J, Chen LB, Kirschonbaum A. A phosphotyrosyl-protein phosphatase activity associated with acid phosphatase from human prostate gland. Eur J Biochem. 1984;138:45–51. doi: 10.1111/j.1432-1033.1984.tb07879.x. [DOI] [PubMed] [Google Scholar]
- 23.Lin MF, Clinton GM. The epidermal growth factor receptor from prostate cells is dephosphorylated by a prostate-specific phosphotyrosyl phosphatase. Mol Cell Biol. 1988;8:5477–5485. doi: 10.1128/mcb.8.12.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng TC, Lin MF. Tyrosine Phosphorylation of c-ErbB-2 Is Regulated by the Cellular Form of Prostatic Acid Phosphatase in Human Prostate Cancer Cells. J Biol Chem. 1998;273:22096–22104. doi: 10.1074/jbc.273.34.22096. [DOI] [PubMed] [Google Scholar]
- 25.Zhang XQ, Lee MS, Zelivianski S, Lin MF. Characterization of a prostate-specific tyrosine phosphatase by mutagenesis and expression in human prostate cancer cells. J Biol Chem. 2001;276:2544–2550. doi: 10.1074/jbc.M006661200. [DOI] [PubMed] [Google Scholar]
- 26.Igawa T, Lin FF, Rao P, Lin MF. Suppression of LNCaP prostate cancer xenograft tumors by a prostate-specific protein tyrosine phosphatase, prostatic acid phosphatase. Prostate. 2003;55:247–258. doi: 10.1002/pros.10240. [DOI] [PubMed] [Google Scholar]
- 27.Chuang TD, Chen SJ, Lin FF, Veeramani S, Kumar S, Batra SK, Tu Y, Lin MF. Human prostatic acid phosphatase, an authentic tyrosine phosphatase, dephosphorylates ErbB-2 and regulates prostate cancer cell growth. J Biol Chem. 2010;285:23598–23606. doi: 10.1074/jbc.M109.098301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng TC, Lee MS, Lin MF. Interaction between protein tyrosine phosphatase and protein tyrosine kinase is involved in androgen-promoted growth of human prostate cancer cells. Oncogene. 2000;19:2664–2677. doi: 10.1038/sj.onc.1203576. [DOI] [PubMed] [Google Scholar]
- 29.Zylka MJ, Sowa NA, Taylor-Blake B, Twomey MA, Herrala A, Voikar V, Vihko P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008;60:111–122. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Igawa T, Lin FF, Lee MS, Karan D, Batra SK, Lin MF. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 2002;50:222–235. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- 31.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen SJ, Karan D, Johansson SL, Lin FF, Zeckser J, Singh AP, Batra SK, Lin MF. Prostate-derived factor as a paracrine and autocrine factor for the proliferation of androgen receptor-positive human prostate cancer cells. Prostate. 2007;67:557–571. doi: 10.1002/pros.20551. [DOI] [PubMed] [Google Scholar]
- 33.Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, Pathak S, Chung LWK. LNCaP progression model of human prostate cancer: Androgen-independence and osseous metastasis. Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 34.Zelivianski S, Igawa T, Lim S, Taylor R, Lin MF. Identification and characterization of regulatory elements of the human prostatic acid phosphatase promoter. Oncogene. 2002;21:3696–3705. doi: 10.1038/sj.onc.1205471. [DOI] [PubMed] [Google Scholar]
- 35.Veeramani S, Igawa T, Yuan TC, Lin FF, Lee MS, Lin JS, Johansson SL, Lin MF. Expression of p66(Shc) protein correlates with proliferation of human prostate cancer cells. Oncogene. 2005;24:7203–7212. doi: 10.1038/sj.onc.1208852. [DOI] [PubMed] [Google Scholar]
- 36.Zelivianski S, Larson C, Seberger J, Taylor R, Lin MF. Expression of human prostatic acid phosphatase gene is regulated by upstream negative and positive elements. Biochim Biophys Acta. 2000;1491:123–132. doi: 10.1016/s0167-4781(00)00037-3. [DOI] [PubMed] [Google Scholar]
- 37.Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, Yamochi T, Urano T, Furukawa K, Kwabi-Addo B, Gold DL, Sekido Y, Huang TH, Issa JP. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet. 2008;40:741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- 38.Wedel SA, Sparatore A, Soldato PD, Al-Batran SE, Atmaca A, Juengel E, Hudak L, Jonas D, Blaheta RA. New histone deacetylase inhibitors as potential therapeutic tools for advanced prostate carcinoma. J Cell Mol Med. 2008;12:2457–2466. doi: 10.1111/j.1582-4934.2008.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu LP, Wang X, Li L, Zhao Y, Lu S, Yu Y, Zhou W, Liu X, Yang J, Zheng Z, Zhang H, Feng J, Yang Y, Wang H, Zhu WG. Histone Deacetylase Inhibitor Depsipeptide Activates Silenced Genes through Decreasing both CpG and H3K9 Methylation on the Promoter. Mol Cell Biol. 2008;28:3219–3235. doi: 10.1128/MCB.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter T, Cooper JA. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981;24:741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- 41.Lin MF, Garcia-Arenas R. Effect of cell density on androgen regulation of the mRNA level of human prostatic acid phosphatase. Mol Cell Endocrinol. 1994;99:R21–24. doi: 10.1016/0303-7207(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 42.Pfeiffer D, Kimmig R, Herrmann J, Ruge M, Fisseler-Eckhoff A, Scheidel P, Jensen A, Schatz H, Pfeiffer A. Epidermal-growth-factor receptor correlates negatively with cell density in cervical squamous epithelium and is down-regulated in cancers of the human uterus. Int J Cancer. 1998;79:49–55. doi: 10.1002/(sici)1097-0215(19980220)79:1<49::aid-ijc10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 43.Li LC, Carroll PR, Dahiya R. Epigenetic changes in prostate cancer: implication for diagnosis and treatment. J Natl Cancer Inst. 2005;97:103–115. doi: 10.1093/jnci/dji010. [DOI] [PubMed] [Google Scholar]
- 44.Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 46.Xia Q, Sung J, Chowdhury W, Chen CL, Hoti N, Shabbeer S, Carducci M, Rodriguez R. Chronic administration of valproic acid inhibits prostate cancer cell growth in vitro and in vivo. Cancer Res. 2006;66:7237–7244. doi: 10.1158/0008-5472.CAN-05-0487. [DOI] [PubMed] [Google Scholar]
- 47.Iacopino F, Urbano R, Graziani G, Muzi A, Navarra P, Sica G. Valproic acid activity in androgen-sensitive and -insensitive human prostate cancer cells. Int J Oncol. 2008;32:1293–1303. doi: 10.3892/ijo_32_6_1293. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Arenas R, Lin FF, Lin D, Jin LP, Shih CC, Chang C, Lin MF. The expression of prostatic acid phosphatase is transcriptionally regulated in human prostate carcinoma cells. Mol Cell Endocrinol. 1995;111:29–37. doi: 10.1016/0303-7207(95)03544-h. [DOI] [PubMed] [Google Scholar]
- 49.Campiglio M, Locatelli A, Olgiati C, Normanno N, Somenzi G, Viganò L, Fumagalli M, Ménard S, Gianni L. Inhibition of proliferation and induction of apoptosis in breast cancer cells by the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor ZD1839 (‘Iressa’) is independent of EGFR expression level. J Cell Physiol. 2004;198:259–268. doi: 10.1002/jcp.10411. [DOI] [PubMed] [Google Scholar]
- 50.Sheng G, Guo J, Warner BW. Epidermal growth factor receptor signaling modulates apoptosis via p38alpha MAPK-dependent activation of Bax in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:599–606. doi: 10.1152/ajpgi.00182.2007. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch CL, Bonham K. Histone deacetylase inhibitors regulate p21WAF1 gene expression at the post-transcriptional level in HepG2 cells. FEBS Lett. 2004;570:37–40. doi: 10.1016/j.febslet.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y, Lu S, Wu L, Chai G, Wang H, Chen Y, Sun J, Yu Y, Zhou W, Zheng Q, Wu M, Otterson GA, Zhu WG. Acetylation of p53 at Lysine 373/382 by the Histone Deacetylase Inhibitor Depsipeptide Induces Expression of p21Waf1/Cip1. Mol Cell Biol. 2006;26:2782–2790. doi: 10.1128/MCB.26.7.2782-2790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]