Abstract
This study aimed to identify the sources and routes of transmission of Campylobacter in intensively reared poultry farms in the Republic of Ireland. Breeder flocks and their corresponding broilers housed in three growing facilities were screened for the presence of Campylobacter species from November 2006 through September 2007. All breeder flocks tested positive for Campylobacter species (with C. jejuni and C. coli being identified). Similarly, all broiler flocks also tested positive for Campylobacter by the end of the rearing period. Faecal and environmental samples were analyzed at regular intervals throughout the rearing period of each broiler flock. Campylobacter was not detected in the disinfected house, or in one-day old broiler chicks. Campylobacter jejuni was isolated from environmental samples including air, water puddles, adjacent broiler flocks and soil. A representative subset of isolates from each farm was selected for further characterization using flaA-SVR sub-typing and multi-locus sequence typing (MLST) to determine if same-species isolates from different sources were indistinguishable or not. Results obtained suggest that no evidence of vertical transmission existed and that adequate cleaning/disinfection of broiler houses contributed to the prevention of carryover and cross-contamination. Nonetheless, the environment appears to be a potential source of Campylobacter. The population structure of Campylobacter isolates from broiler farms in Southern Ireland was diverse and weakly clonal.
Introduction
Campylobacter continues to be the most commonly reported cause of bacterial gastroenteritis in the European Union (EU). In total there were 198,252 confirmed cases of campylobacteriosis in 2009, giving an overall crude incidence rate (CIR) of 45.6 per 100,000 population [1].
While a range of risk factors for infection with Campylobacter have been identified, the most common is the handling and/or consumption of undercooked poultry, in particular chicken. According to a recent opinion of the European Food Safety Authority's (EFSA) Biological Hazards (BIOHAZ) Panel, 50 to 80% of human cases of campylobacteriosis may be attributed to the chicken reservoir [2]. This report also outlined the considerable underreporting of cases of campylobacteriosis and suggested that no less than 2 million and up to 20 million cases of clinical campylobacteriosis occur per year in the EU.
In 2008, a baseline survey on Campylobacter in broiler batches and carcasses in the EU was undertaken [3]. The prevalence at community level of Campylobacter-colonized broiler batches was reported to be 71.2% and the prevalence of Campylobacter-contaminated broiler carcasses was found to be 75.8%. The colonization of broiler flocks with Campylobacter is therefore a significant food safety issue and a reduction in the number of contaminated poultry products entering the food chain would reduce the negative impact on public health.
It is thought that the reduction of Campylobacter-contaminated poultry meat can be achieved most effectively by implementing on-farm control measures [4].
Extensive research into the most important source of Campylobacter in poultry production units has been carried out. Vertical transmission has previously been implicated [5], [6], [7], [8]. Carryover to subsequent flocks as a result of inadequate disinfection of broiler houses has also been identified as a risk factor [9]. Horizontal transmission from the surrounding environment to the broiler house either via the farm workers or other vectors such as wild birds, vermin and house flies, is considered to be the most likely source of contamination [10], [11]. Risk factors for infection may vary from country to country because of differing farming practices and associated climatic conditions. Therefore, efforts to understand the relative importance of each potential source and transmission route of Campylobacter infection on-farm, continues to have an important role in extending our understanding of the epidemiology of this important pathogen.
In 2009, there were 1,808 confirmed cases of campylobacteriosis in Ireland [12]. The prevalence of Campylobacter-colonized broiler batches in Ireland in 2008 was 83.1% and of that Campylobacter-contaminated broiler carcasses accounted for 98.3% [3]. In order to implement effective control measures and reduce the prevalence of Campylobacter in Irish poultry products, the most significant transmission routes must be identified. The aim of this study was to investigate the occurrence of Campylobacter in a subset of intensively reared Irish poultry flocks and to identify sources of Campylobacter in each farm environment. Molecular sub-typing methods were used to identify the genotypes present in Irish broiler farms and to shed further light on possible transmission routes of Campylobacter in poultry farms.
Materials and Methods
Description of Farms in this Study
Three housed broiler flocks (denoted as broiler flocks 1, 2 and 3), located on three different farms (denoted as broiler farms 1, 2 and 3) in different geographical locations of Ireland, were studied throughout their 6–7 week life span. The flocks were screened between November 2006 and September 2007. Farm 1 consisted of three poultry house units sampled from November 2006 to January 2007. Farm 2 consisted of two poultry house units that were sampled between April 2007 and May 2007. Farm 3 consisted of one poultry house unit and was sampled between July 2007 and September 2007. Broiler flock sizes ranged from approximately 18,000 to 34,000 birds per house. Three breeder flocks, located on breeder farm 1 (denoted as breeder farm 1, flocks 1–3), supplied chicks for broiler farm 1. Broiler farm 2 was supplied by breeder farms 2 and 3. Broiler farm 3 was supplied by two flocks from breeder farm 1 (denoted as breeder farm 1, flocks 4 and 5), and one flock from both breeder farms 4 and 5. Each breeder flock comprised approximately 5,000 birds per house.
Sample Collection
Samples were collected approximately every 14 days from (i) the cleaned and disinfected broiler houses prior to chick placement, (ii) the chickens and (iii) the environments inside and outside the broiler houses. The breeder flocks supplying each broiler house were also tested. Samples were transported to the laboratory at 4°C in a cool box and processed on the same day.
Breeder flocks
Sixty fresh faecal samples (5 pooled faecal samples each containing 12 fresh faecal droppings) were collected from the floor of the house of the corresponding breeder farms supplying broiler hatching eggs for each flock in this study.
Broiler flocks
One-day old broiler chicks were tested by enriching the faeces-soiled paper that lined the crates used to transport the birds from the hatchery to the broiler house (100 birds per crate). Faecal samples were then taken at regular intervals throughout the rearing period of each flock (at days 14, 28 and 42 approximately). Sixty individual fresh faecal samples were collected from the broiler house floor and combined (as outlined previously).
Environmental samples
Samples from walls, floors, structural support columns, feed and water dispensers along with concrete aprons (the concreted area outside the front of the house) were taken using sterile swabs pre-moistened with 10 ml Maximum Recovery Diluent (MRD, Lab M Ltd., Bury, UK). An area of 0.1 m2 of the object's surface was chosen for sampling, and swabbing continued outside this area until either the entire surface was sampled or the swab was dry. Two litres of water supplying the broiler house drinkers were also taken. Aspiration of air samples onto one Campylobacter blood-free selective agar base (CCDA, CM0739, Oxoid, Cambridge, UK), supplemented with cefoperazone and amphotericin B (CCDA selective supplement) (Oxoid), and one Tryptone Soya Agar (TSA, Oxoid Ltd., Cambridge, UK) plate was performed using a Sampl'air MK2 double agar plate sampler (AES Laboratoire Groupe, Combourg, France). Two air samples (500 l×2) were taken from inside and one directly outside each broiler house on each sampling occasion (at days -1, 14, 28 and 42). Occasionally, samples were taken opportunistically inside and outside the broiler house environment (Table 1).
Table 1. Campylobacter sources during the sampling period across farms.
| Flock | Sample | Number of positive samples/number of samples tested | ||||
| Day -1* | Day 1 | Day 14* | Day 28* | Day 42* | ||
| 1 | Broilers | - | 0/48c | 3/5 c | 5/5 c | 5/5 c |
| Adjacent Broiler 1 | - | - | 0/2c | 2/2 c | 2/2 c | |
| Adjacent Broiler 2 | - | - | 2/2 c | 2/2 c | - | |
| Air | 0/6 | - | 1/6 | 0/6 | 0/6 | |
| Puddle | 0/1 | - | 1/2 | 0/1 | 0/2 | |
| Soil | 0/2 | - | 0/2 | 0/2 | 1/2 | |
| Other | 0/20ap, cl co, d, f, fe, fl, h, w, wa | - | 0/7ap, h, i, wa | 0/9ap, h, i, wa | 0/11ap, h, i, wa | |
| 2 | Broilers | - | 0/48c | 0/5c | 0/5c | 5/5 c |
| Adjacent Broiler | - | - | 0/2c | 0/2c | - | |
| Soil | 0/2 | - | 0/2 | 1/2 | 0/2 | |
| Other | 0/22a, ap, co, d, fe, fl, p, w, wa | - | 0/10a, ap, wa | 0/13a, ap, i, wa | 0/12a, ap, p, r, wa | |
| 3 | Broilers | - | 0/48c | 0/5c | 5/5 c | 5/5 c |
| Soil | 0/2 | - | 0/2 | 0/2 | 1/2 | |
| Other | 0/24a, ap, cl co, d, fe, fl, p, w, wa | - | 0/12a, ap, p, wa | 0/12a, ap, p, wa | 0/10a, ap, wa | |
Bold type indicates positive result; -, not tested; *, approximate day of sampling; a, air; ap, apron; cl, clothing; co, support columns; d, drinkers; f; fan, fe; feeder, fl; floor, h; horse faeces; i, insects; p, puddles; r, rodent faeces; w, walls; wa, water; c, composite samples.
Isolation of Campylobacter
To determine the presence/absence of Campylobacter, samples were examined by direct plating and/or enrichment culture methodologies based on the Horizontal Method for Detection and Enumeration of Campylobacter spp. (ISO 10272-1:2006) and the Detection and Semi-quantitative Enumeration of Thermotolerant Campylobacter spp. (ISO 17995:2005). Isolation of emerging Campylobacter species, based on the method previously described [13], was also performed for faecal samples. Following incubation, five suspect colonies were randomly selected from plates and subcultured to obtain pure colonies.
For the lined transport crates, 60 papers from each flock were collected at random and divided into six piles consisting of ten papers on top of one another. Each pile of ten papers was then aseptically cut into 8 strips. Each strip was enriched in 200 ml Campylobacter Enrichment Broth (CEB, Lab M Ltd., Bury, UK) supplemented with 5% (v/v) lysed horse blood (TCS Biosciences, Buckingham, UK) and cefoperazone, vancomycin, trimethoprim and cyclohexamide (CVTC supplement, Lab M Ltd., Bury, UK). Forty-eight composite samples, each consisting of ten strips, from each flock were tested in this way. Swabs were enriched in 100 ml of CEB supplemented with 5% (v/v) lysed horse blood and CVTC. Water samples were filtered using 0.45 µm filters (Millipore, Billerica, MA., USA), which were then enriched in sterile 30 ml containers containing 20 ml of CEB supplemented with 5% (v/v) lysed horse blood and CVTC. For the air samples, the agar from each TSA plate was aseptically removed and enriched in 100 ml CEB supplemented with 5% (v/v) lysed horse blood and CVTC, while each CCDA plate was incubated directly. Faecal samples from horses, crushed flies and beetles were enriched with CEB supplemented with 5% (v/v) lysed horse blood and CVTC using a 1∶10 ratio of sample to broth. Water from puddles was enriched with an equivalent volume of double-strength CEB supplemented with 5% (v/v) lysed horse blood and CVTC.
Biochemical confirmation
Presumptive Campylobacter isolates were confirmed using standard biochemical procedures including a Gram lysis test (3% [w/v] KOH, Sparks Lab Supplies, Dublin, Ireland), oxidase test (Oxoid, Cambridge, UK), catalase test (3% H2O2, Sigma Aldrich, St. Louis, MO, USA) and l-alanine aminopeptidase test (Aminopeptidase Test, Fluka Biochemika, Buchs, Switzerland). A latex agglutination test was also used (DrySpot Campylobacter Test Kit, Oxoid, Cambridge, UK). Following biochemical confirmation, isolates were stored at −20 and −80°C on Protect cryobeads (Technical Service Consultants Ltd., Heywood, Lancashire, UK) containing 80% [v/v] glycerol, for further analysis.
Genotyping
Multiplex Polymerase Chain Reaction (mPCR)
DNA purification was carried out using a commercial kit (Wizard Genomic DNA Purification Kit, Promega, Madison, WI, USA). Isolates were identified to species level as described previously [14]. Amplicon sizes were determined by comparison with a molecular weight marker (50 bp ladder, Promega) following migration on a 1.5% [w/v] agarose gel.
flaA-SVR sequencing
Sequencing of an internal 321-bp fragment of the flagellin A short variable region (SVR), was performed using the primer pair Fla242FU [5′-CTA TGG ATG AGC AAT T (AT) A AAAT-3′] and Fla625RU [5′-CAA G (AT) C CTG TTC C (AT)A CTG AAG-3′], as previously described by Meinersmann et al. [15]. All flaA-derived amplicons were purified using a QIAquick PCR Purification kit (Qiagen GmbH, Hilden, Germany) and sequenced commercially by MWG Biotech (Ebersberg, Germany). The nucleotide sequences were deposited in the internet accessible flaA-SVR sequence database (http://hercules.medawar.ox.ac.uk/flaA/) and SVR allele numbers were assigned by sequence comparisons against the existing flaA-SVR sequences. The DNA trace files were submitted for confirmation of novel flaA-SVR alleles.
Multi Locus Sequence Typing (MLST)
Internal fragments of seven gene targets were amplified by PCR and their nucleotide sequences determined with primers and reaction conditions in accordance with the published MLST scheme for C. jejuni and C. coli. Allele identification followed by sequence type (ST) and clonal complex (CC) assignments were done by interrogation of the Campylobacter MLST database for each isolate (http://pubmlst.org/campylobacter).
Data Analysis
Trimmed flaA-SVR sequences (including STs) were imported into BioNumerics, Version 6.1 (Applied Maths, Sint-Martens-Latem, Belgium). A dendrogram was created using the unweighted-pair group method with arithmetic mean (UPGMA).
Results
Presence of Campylobacter on poultry farms
Broiler breeders
All broiler breeder flocks supplying each rearing farm were found to be colonized with both C. jejuni and C. coli (Table 2). A total of 27 C. jejuni and 19 C. coli were isolated from breeder farm 1 (containing flocks 1, 2 and 3). Two C. jejuni and 42 C. coli were isolated from breeder farm 2. One C. coli isolate and 22 C. jejuni isolates were recovered from breeder farm 3. There were 6 C. jejuni and 14 C. coli isolates recovered from breeder farm 1, flock 4, along with 7 C. jejuni and 12 C. coli from breeder farm 1, flock 5. A total of 13 C. jejuni and 7 C. coli were isolated from breeder farm 4, while 26 C. jejuni and 25 C. coli were recovered from breeder farm 5.
Table 2. Campylobacter species recovered from all farms tested.
| Farm | Day | Source | Sample | Species (no.) |
| 1 | - | Breeder 1 Flock 1 | Faeces | C. jejuni (6), C. coli (2) |
| - | Breeder 1 Flock 2 | Faeces | C. jejuni (6), C. coli (9) | |
| - | Breeder 1 Flock 3 | Faeces | C. jejuni (15), C. coli (8) | |
| 13 | Broiler | Faeces | C. jejuni (10) | |
| 13 | Adjacent Broiler 2 | Faeces | C. jejuni (3) | |
| 13 | Environment Inside | Air | C. jejuni (2) | |
| 13 | Environment Outside | Puddle | C. jejuni (1) | |
| 32 | Broiler | Faeces | C. jejuni (9) | |
| 32 | Adjacent Broiler 1 | Faeces | C. jejuni (1) | |
| 32 | Adjacent Broiler 2 | Faeces | C. jejuni (8) | |
| 42 | Broiler | Faeces | C. jejuni (26) | |
| 42 | Adjacent Broiler 1 | Faeces | C. jejuni (5) | |
| 42 | Environment Outside | Soil | C. jejuni (1) | |
| Total | C. jejuni (93) C. coli (19) | |||
| 2 | - | Breeder 2 | Faeces | C. jejuni (2), C. coli (42) |
| - | Breeder 3 | Faeces | C. jejuni (22), C. coli (1) | |
| 27 | Environment Outside | Soil | C. jejuni (5) | |
| 41 | Broiler | Faeces | C. jejuni (24) | |
| Total | C. jejuni (53) C. coli (43) | |||
| 3 | - | Breeder 4 | Faeces | C. jejuni (13), C. coli (7) |
| - | Breeder 5 | Faeces | C. jejuni (26), C. coli (25) | |
| - | Breeder 1 Flock 4 | Faeces | C. jejuni (6), C. coli (14) | |
| - | Breeder 1 Flock 5 | Faeces | C. jejuni (7), C. coli (12) | |
| 27 | Broiler | Faeces | C. jejuni (23) | |
| 41 | Broiler | Faeces | C. jejuni (26) | |
| 41 | Environment Outside | Soil | C. jejuni (1) | |
| Total | C. jejuni (102) C. coli (58) | |||
-, not applicable.
Broiler chickens
Campylobacter could not be cultured from the transport crate paper liners on the day the chicks arrived at the rearing house. All three broiler flocks were found to be contaminated with Campylobacter by the end of the rearing period. Campylobacter jejuni was the only species isolated from all three poultry flocks under investigation (Table 2). Faecal samples from broiler flock 1 were contaminated with Campylobacter on days 13, 32 and 42. Faecal samples from broiler flock 2 were negative until the final sampling day (day 41). Broiler flock 3 was found to be contaminated with Campylobacter on days 27 and 41.
Presence of Campylobacter in the poultry farm environment
On all three farms, Campylobacter could not be detected in the empty poultry house structure/environment after the cleaning and disinfection procedure had been carried out. All samples taken from the external environment of the cleaned house, including soil, air, the concrete apron and horse faeces were negative for Campylobacter (Table 1).
During the rearing period of each flock, the environment was found to be contaminated with Campylobacter (Table 1). In the case of broiler farm 1 (day 13), an air sample taken inside the rearing house, an adjacent broiler flock and a puddle outside the house were sampled and found to be contaminated with Campylobacter. On day 32, two adjacent broiler flocks tested positive for C. jejuni. On day 42, one adjacent broiler house had been depopulated. Campylobacter jejuni was isolated from the remaining adjacent flock and was also recovered from an environmental soil sample. In the case of broiler farm 2, C. jejuni was isolated from a soil sample taken on day 27. On broiler farm 3, C. jejuni was recovered from a soil sample taken on day 41.
Molecular sub-typing and spatio-temporal tracking of Campylobacter isolates
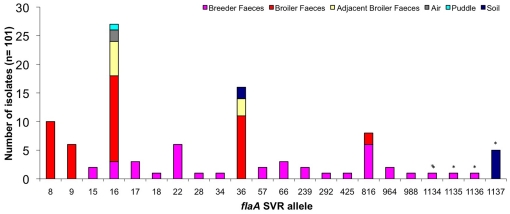
A representative subset of Campylobacter isolates was chosen from each farm and characterized by DNA sequence analysis of the SVR containing region of the flaA gene (flaA-SVR), to determine if same-species isolates from different sources could be distinguished. Figure 1 shows the distribution of allele types discovered in relation to the source of each isolate. A total of 22 flaA-SVR alleles were identified in the 101 isolates investigated.
Figure 1. Distribution of flaA-SVR alleles detected according to source, (asterisks signify new flaA-SVR alleles).
Figures 2, 3 and 4 show the spatio-temporal distribution of flaA-SVR alleles identified on each sampling day across each of the three broiler farms. This sub-typing analysis revealed a diverse and weakly clonal population structure of C. jejuni, with multiple subtypes present throughout the lifecycle of each flock. Campylobacter isolates originating from each set of breeders and the faeces from their respective progeny presented with non-identical flaA-SVR DNA sequences.
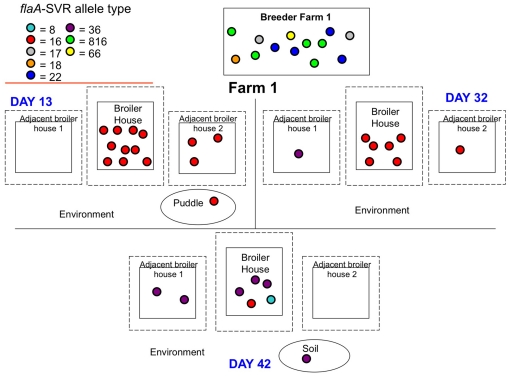
Figure 2. Schematic diagram of the flaA-SVR alleles detected on farm 1.
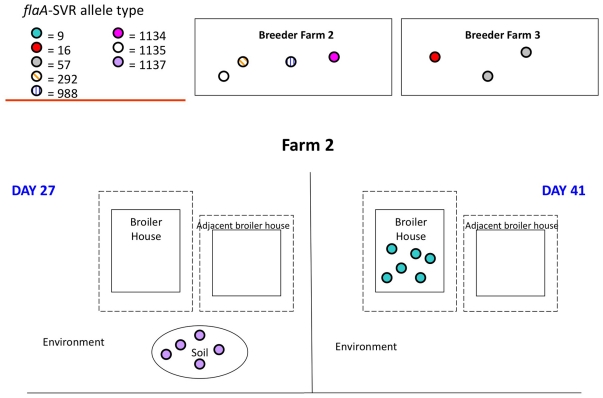
Figure 3. Schematic diagram of the flaA-SVR alleles detected on farm 2.
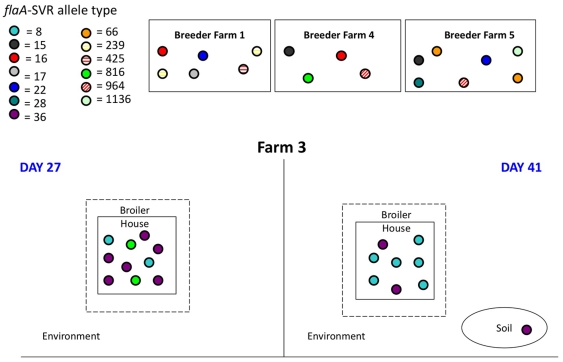
Figure 4. Schematic diagram of the flaA-SVR alleles detected on farm 3.
In the case of farm 1, flaA-SVR allele number 16 was detected on day 13 in faeces from the broiler flock, faeces from adjacent broiler flock 2, and in a puddle located outside the house (Figure 2). On day 32, allele number 16 continued to be identified in the broiler and adjacent broiler flock 2 faeces. On the same day, allele number 36 was detected in faeces from adjacent broiler flock 1. On day 42, allele number 36 could be detected in faeces from adjacent broiler flock 1, and was also now identified in broiler flock 1 and in an environmental soil sample. Allele numbers 8 and 16 were also detected in the broiler faeces.
In the case of farm 2, allele number 1137 was identified in an environmental soil sample taken on day 27 (Figure 3). Campylobacter was not isolated from broiler flock 2 faeces until the final sampling day, when allele number 9 was detected.
In the case of farm 3, allele numbers 8, 36 and 816 were detected in the broiler faeces on day 27 (Figure 4). On day 41, alleles 8 and 36 were again identified. Allele 26 was also found to be present in a soil sample outside the house on this day.
Diversity of MLST Sequence Types (STs) and Clonal Complexes (CCs)
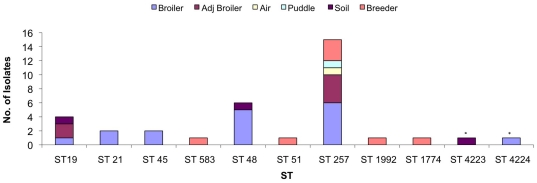
In order to substantiate flaA-SVR findings, a subset of isolates from each farm was chosen for MLST analysis.Eleven STs were identified among the 35 isolates (34 C. jejuni, 1 C. coli) chosen. Five STs were assigned to more than one isolate while six STs were assigned to single isolates. Figure 5 shows that ST 257 was the most frequently detected sub-type (in 15/35 isolates), followed by ST 48 (in 6/35 isolates) and ST19 (in 4/35 isolates). ST 21 and ST 45 were assigned to two isolates each. ST 583, ST 51, ST 1922, ST 1744, ST 4223 and ST 4224 were assigned to single isolates. Two novel sequence types (ST 4223 and ST 4224) were identified and submitted to the C. jejuni MLST database (http://pubmlst.org/campylobacter/).
Figure 5. Distribution of STs detected according to source (asterisks signify new STs).
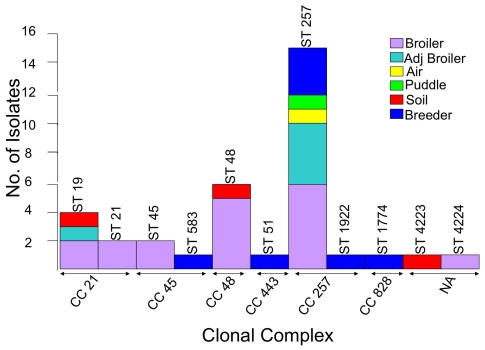
All 11 STs were grouped into 6 CCs. The two novel sequences could not be grouped into a defined CC. The largest CC was found to be CC 257 (consisting of 16 isolates), followed by CC 21 and CC 48 (6 isolates each). In total, 80% (28/35) of isolates analyzed by MLST were grouped into one of these three CCs. Isolates grouped in CC 257 were found to have originated from a variety of sources (Figure 6).
Figure 6. Distribution of CCs and STs according to source.
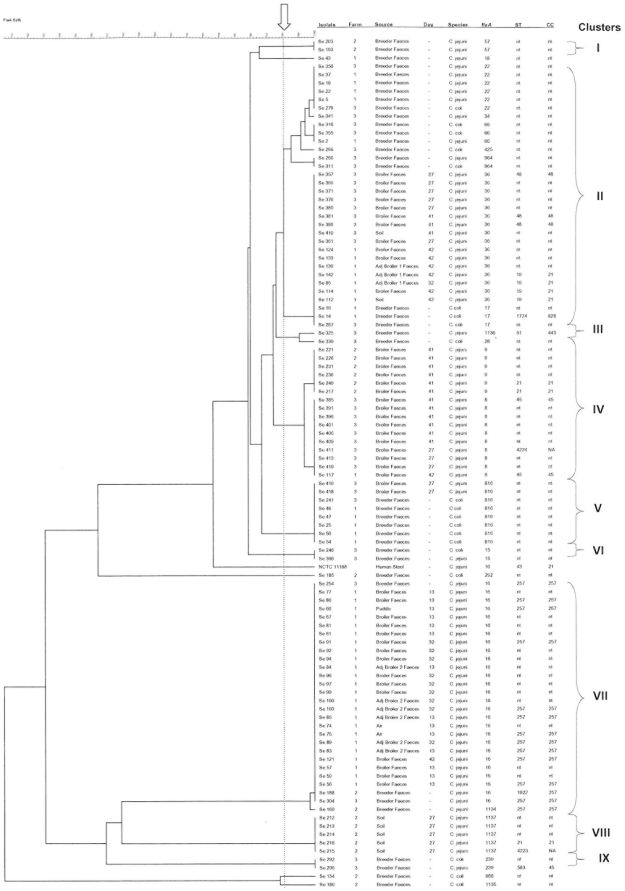
Figure 7 depicts the phylogenetic relationship between the 22 flaA-SVR types and the 11 STs identified during the study. Nine cluster genotypes were observed using a threshold genetic similarity of 98% as a cut-off coefficient value.
Figure 7. Comparison of flaA-SVR sequences of Campylobacter isolates from breeder and broiler farms and farm environments.
A total of 101 isolates were included. Strain Campylobacter jejuni NCTC11168 was included as a control strain in the pairwise analysis. The arrow indicates the 98% similarity cut-off point. flaA, flaA-SVR allele number; ST, sequence type; CC, clonal complex; Nt, not tested; NA, not assigned; -, not applicable.
Discussion
Despite extensive research, the definitive sources of infection and routes of transmission of C. jejuni in the poultry reservoir remain to be fully elucidated.
The purpose of this study was to identify sources of Campylobacter in intensively reared broiler flocks in Ireland.
Molecular sub-typing techniques have previously shown the population structure of C. jejuni to be highly diverse and weakly clonal [16], [17]. This study confirmed that genetic diversity also exists among Irish C. jejuni strains isolated from broiler flocks, adjacent flocks and the farm environment.
The occurrence of vertical transmission of Campylobacter in poultry has been a controversial issue. The isolation of Campylobacter species from the reproductive tract of broiler breeders has been reported [6], [7] and transmission from breeder hens to broiler chickens has been suggested [18]. Conflicting reports have also been published suggesting that vertical transmission is unlikely or of little importance [19], [20]. In this study, while breeder flocks were found to be colonized with Campylobacter, molecular characterization confirmed the isolates to be of different flaA-SVR and ST types, suggesting that vertical transmission was not involved in the colonization of these broiler flocks with Campylobacter. This work is in agreement with a related study conducted by Patriarchi et al., where none of the genotypes identified in breeder flocks were subsequently identified on any of the broiler farms [21]. Colonization of chickens with Campylobacter usually occurs between 3 and 5 weeks of age, and once infected, prevalence in a flock can often be close to 100% [22]. Interestingly, Campylobacter was not detected in any of the three broiler flocks in this study before day 13 of the rearing cycle. A possible factor contributing to the delay in colonization of chickens with Campylobacter is the presence of protective maternal antibodies in young chicks [23], [24].
In this study, Campylobacter was not isolated from broiler flock 2 until after the process of partial depopulation or thinning had been carried out. The practice of thinning has previously been reported as an important risk factor for Campylobacter colonization of residual birds [25], [26], [27]. It has been shown in vitro that the presence of the neurotransmitter noradrenaline stimulates the growth and motility of C. jejuni [28]. As a result of triggering the release of noradrenaline, the stressful thinning process could be expected to contribute to rapid growth of the bacterium in the avian gastrointestinal tract leading to increased shedding of Campylobacter by birds, and the subsequent rapid spread of the bacteria.
Challenges in maintaining biosecurity during the thinning process can result in cross-contamination from environmental sources. Allen et al. reported the isolation of Campylobacter from transport vehicles, equipment, personnel and the farm driveways prior to the thinning process [25]. Using flaA-SVR and MLST subtyping methods, Patriarchi et al. also identified the practice of partial depopulation as a potential source and route of flock contamination on Irish broiler farms. Molecular evidence of the role of transport crates in introducing Campylobacter spp. into the broiler house was also reported [21]. In the case of farm 1 in this study, a C. jejuni isolate identified as flaA-SVR allele type 36 was isolated from an adjacent broiler flock prior to thinning. On the final sampling day, this flaA-SVR allele type was again isolated in the adjacent broiler flock, and was also identified in broiler flock 1 and in an environmental soil sample. Improved biosecurity measures in relation to the depopulation process may contribute to the prevention or delayed colonization of chickens with Campylobacter.
Previous studies have reported that poultry strains are frequently found to be genetically distinct from environmental isolates [29], [30]. In the case of broiler farm 2, C. jejuni flaA-SVR allele types isolated from broilers and environmental soil samples were confirmed as non-identical. However, identical C. jejuni strains were identified from air, soil, water puddles and chickens on broiler farms 1 and 3, suggesting that transfer of campylobacters between these environments may be occurring. However, it is not possible based on the epidemiological data presented here to establish the direction of a given exchange. Bi-directional movement of Campylobacter between sources cannot be ruled out and has been implicated previously [31]. In the case of two broiler flocks studied here, contamination of the farm environment was not detected until after the chickens had become infected, highlighting the broilers as a possible source of environmental contamination. MLST data from flock 1 further demonstrate that genetically identical strains can be isolated from broiler faeces and environmental samples. On day 13, ST 257 was found to be present in 2 broiler houses and in a water puddle outside the house. This ST was identified in the broiler flock on all sampling days. On day 42, ST 19 and ST 45 were also detected. These results reflect the findings of previous spatio-temporal studies on broiler farms, where different STs were identified in chicken faeces as the rearing period progressed [11].
During 2007, New Zealand experienced a 50% decline in the rate of campylobacteriosis notifications and hospitalisations [32]. This decline followed the introduction of voluntary and regulatory interventions to reduce contamination of poultry with Campylobacter species and was sustained in 2008 and 2009. A number of other countries have reported a reduced incidence of campylobacteriosis infections following the implementation of poultry-focussed control strategies [33], [34], [35], [36]. Various interventions were employed in each country however all strategies included strengthening on-farm biosecurity and monitoring the prevalence of Campylobacter-positive flocks. The implementation of similar measures in poultry farms in Ireland could contribute to a reduction in human campylobacteriosis infection rates and lead to improved public health protection.
The population structure of Campylobacter isolates from broiler farms in Southern Ireland was determined, (based on these data), to be weakly clonal. Such genetic diversity complicates the challenge of managing Campylobacter species population dynamics within the poultry farm environment. Nevertheless, our data point to the importance of applying more than one sub-typing method as part of our epidemiological studies to carefully describe this dynamic process.
It is reasonable to conclude that there are multiple sources from which Campylobacter can be transmitted on broiler farms. Following their introduction into broiler flocks, the spread of Campylobacter is influenced by various host and environmental factors, such as biosecurity measures in place, farming practices, the immune status of the chickens, and the presence of other animals on the farm. A combined protocol individually targeting each potential source and route of transmission is required as a logical approach to effectively reduce the colonization of broiler chickens with Campylobacter in a step-wise fashion.
Acknowledgments
The authors wish to acknowledge the cooperation of the poultry farmers who participated in the study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by Safefood (04-RESR-04). www.safefood.eu. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.EFSA. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2009. EFSA Journal 2011. 2011;9(3):2090. [378pp.] doi:10.2903/j.efsa.2011.2090. Available online: www.efsa.europa.eu/efsajournal [last accessed 02/07/2011] [Google Scholar]
- 2.EFSA. EFSA Panel on Biological Hazards (BIOHAZ); Scientific Opinion on Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA Journal 2010. 2010;8(1):1437. [89 pp.]. doi:10.2903/j.efsa.2010.1437. Available online: www.efsa.europa.eu [last accessed 02/07/2011] [Google Scholar]
- 3.EFSA. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008 Part A: Campylobacter and Salmonella prevalence estimates. EFSA Journal 2010. 2010;8(03):1503. [99 pp.]. doi:10.2903/j.efsa.2010.1503. Available online: www.efsa.europa.eu [last accessed 02/07/2011] [Google Scholar]
- 4.ACMSF. The Advisory Committee on the Microbiological Safety of Food. 2005. Second report on Campylobacter. [PubMed]
- 5.Buhr RJ, Musgrove MT, Richardson LJ, Cox NA, Wilson JL, et al. Recovery of Campylobacter jejuni in feces and semen of caged broiler breeder roosters following three routes of inoculation. Avian Dis. 2005;49:577–581. doi: 10.1637/7337-020205R.1. [DOI] [PubMed] [Google Scholar]
- 6.Buhr RJ, Cox NA, Stern NJ, Musgrove MT, Wilson JL, et al. Recovery of Campylobacter from segments of the reproductive tract of broiler breeder hens. Avian Dis. 2002;46:919–924. doi: 10.1637/0005-2086(2002)046[0919:ROCFSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Cox NA, Stern NJ, Wilson JL, Musgrove MT, Buhr RJ, et al. Isolation of Campylobacter spp. from semen samples of commercial broiler breeder roosters. Avian Dis. 2002;46:717–720. doi: 10.1637/0005-2086(2002)046[0717:IOCSFS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Pearson AD, Greenwood MH, Feltham RK, Healing TD, Donaldson J, et al. Microbial ecology of Campylobacter jejuni in a United Kingdom chicken supply chain: intermittent common source, vertical transmission, and amplification by flock propagation. Appl Environ Microbiol. 1996;62:4614–4620. doi: 10.1128/aem.62.12.4614-4620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen L, Wedderkopp A. Evidence that certain clones of Campylobacter jejuni persist during successive broiler flock rotations. Appl Environ Microbiol. 2001;67:2739–2745. doi: 10.1128/AEM.67.6.2739-2745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newell DG, Fearnley C. Sources of Campylobacter colonization in broiler chickens. Appl Environ Microbiol. 2003;69:4343–4351. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bull SA, Allen VM, Domingue G, Jorgensen F, Frost JA, et al. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl Environ Microbiol. 2006;72:645–652. doi: 10.1128/AEM.72.1.645-652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HPSC. Health Protection Surveillance Centre. 2010. Infectious Disease Notifications in Ireland, 2004–2009. http://www.hpsc.ie/hpsc/NotifiableDiseases/AnnualIDStatistics/ [last accessed 02/07/2010]
- 13.Lynch OA, Cagney C, McDowell DA, Duffy G. A method for the growth and recovery of 17 species of Campylobacter and its subsequent application to inoculated beef. J Microbiol Methods. 2010 doi: 10.1016/j.mimet.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Clark CG, Taylor TM, Pucknell C, Barton C, et al. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J Clin Microbiol. 2002;40:4744–4747. doi: 10.1128/JCM.40.12.4744-4747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meinersmann RJ, Helsel LO, Fields PI, Hiett KL. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. J Clin Microbiol. 1997;35:2810–2814. doi: 10.1128/jcm.35.11.2810-2814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dingle KE, Colles FM, Wareing DRA, Ure R, Fox AJ, et al. Multilocus sequence typing system for Campylobacter jejuni. J Clin Microbiol. 2001;39:14–23. doi: 10.1128/JCM.39.1.14-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meinersmann RJ, Phillips RW, Hiett KL, Fedorka-Cray P. Differentiation of Campylobacter populations as demonstrated by flagellin short variable region sequences. Appl Environ Microbiol. 2005;71:6368–6374. doi: 10.1128/AEM.71.10.6368-6374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox NA, Stern NJ, Hiett KL, Berrang ME. Identification of a new source of Campylobacter contamination in poultry: transmission from breeder hens to broiler chickens. Avian Dis. 2002;46:535–541. doi: 10.1637/0005-2086(2002)046[0535:IOANSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs-Reitsma WF. Campylobacter bacteria in breeder flocks. Avian Dis. 1995;39:355–359. [PubMed] [Google Scholar]
- 20.Sahin O, Kobalka P, Zhang Q. Detection and survival of Campylobacter in chicken eggs. J Appl Microbiol. 2003;95:1070–1079. doi: 10.1046/j.1365-2672.2003.02083.x. [DOI] [PubMed] [Google Scholar]
- 21.Patriarchi A, Fox A, Maunsell B, Fanning S, Bolton D. Molecular characterization and environmental mapping of Campylobacter isolates in a subset of intensive poultry flocks in Ireland. Foodborne Pathog Dis. 2011;8:99–108. doi: 10.1089/fpd.2010.0637. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs-Reitsma WF. Aspects of epidemiology of Campylobacter in poultry. Vet Q. 1997;19:113–117. doi: 10.1080/01652176.1997.9694753. [DOI] [PubMed] [Google Scholar]
- 23.Sahin O, Luo N, Huang S, Zhang Q. Effect of Campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl Environ Microbiol. 2003;69:5372–5379. doi: 10.1128/AEM.69.9.5372-5379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cawthraw SA, Newell DG. Investigation of the presence and protective effects of maternal antibodies against Campylobacter jejuni in chickens. Avian Dis. 2010;54:86–93. doi: 10.1637/9004-072709-Reg.1. [DOI] [PubMed] [Google Scholar]
- 25.Allen VM, Weaver H, Ridley AM, Harris JA, Sharma M, et al. Sources and spread of thermophilic Campylobacter spp. during partial depopulation of broiler chicken flocks. J Food Prot. 2008;71:264–270. doi: 10.4315/0362-028x-71.2.264. [DOI] [PubMed] [Google Scholar]
- 26.Hue O, Le Bouquin S, Laisney MJ, Allain V, Lalande F, et al. Prevalence of and risk factors for Campylobacter spp. contamination of broiler chicken carcasses at the slaughterhouse. Food Microbiol. 2010;27:992–999. doi: 10.1016/j.fm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Hald B, Rattenborg E, Madsen M. Role of batch depletion of broiler houses on the occurrence of Campylobacter spp. in chicken flocks. Letters in Applied Microbiology. 2001;32:253–256. doi: 10.1046/j.1472-765x.2001.00896.x. [DOI] [PubMed] [Google Scholar]
- 28.Cogan TA, Thomas AO, Rees LE, Taylor AH, Jepson MA, et al. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut. 2006 doi: 10.1136/gut.2006.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nesbit EG, Gibbs P, Dreesen DW, Lee MD. Epidemiologic features of Campylobacter jejuni isolated from poultry broiler houses and surrounding environments as determined by use of molecular strain typing. American Journal of Veterinary Research. 2001;62:190–194. doi: 10.2460/ajvr.2001.62.190. [DOI] [PubMed] [Google Scholar]
- 30.Petersen L, Nielsen EM, Engberg J, On SL, Dietz HH. Comparison of genotypes and serotypes of Campylobacter jejuni isolated from Danish wild mammals and birds and from broiler flocks and humans. Appl Environ Microbiol. 2001;67:3115–3121. doi: 10.1128/AEM.67.7.3115-3121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern NJ, Fedorka-Cray P, Bailey JS, Cox NA, Craven SE, et al. Distribution of Campylobacter spp. in selected U.S. poultry production and processing operations. J Food Prot. 2001;64:1705–1710. doi: 10.4315/0362-028x-64.11.1705. [DOI] [PubMed] [Google Scholar]
- 32.Sears A, Baker MG, Wilson N, Marshall J, Muellner P, et al. Marked campylobacteriosis decline after interventions aimed at poultry, New Zealand. Emerg Infect Dis Jun. 2011 doi: 10.3201/eid1706.101272. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofshagen M, Kruse H. Reduction in flock prevalence of Campylobacter spp. in broilers in Norway after implementation of an action plan. J Food Prot. 2005;68:2220–2223. doi: 10.4315/0362-028x-68.10.2220. [DOI] [PubMed] [Google Scholar]
- 34.Stern NJ, Hiett KL, Alfredsson GA, Kristinsson KG, Reiersen J, et al. Campylobacter spp. in Icelandic poultry operations and human disease. Epidemiol Infect. 2003;130:23–32. doi: 10.1017/s0950268802007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansson I, Forshell LP, Gustafsson P, Boqvist S, Lindblad J, et al. Summary of the Swedish Campylobacter program in broilers, 2001 through 2005. J Food Prot. 2007;70:2008–2014. doi: 10.4315/0362-028x-70.9.2008. [DOI] [PubMed] [Google Scholar]
- 36.Rosenquist H, Boysen L, Galliano C, Nordentoft S, Ethelberg S, et al. Danish strategies to control Campylobacter in broilers and broiler meat: facts and effects. Epidemiol Infect. 2009:1–9. doi: 10.1017/S0950268809002684. [DOI] [PubMed] [Google Scholar]