Abstract
Bacillus cereus causes a uniquely rapid and blinding intraocular infection, endophthalmitis. B. cereus replicates in the eye, synthesizes numerous toxins, and incites explosive intraocular inflammation. The mechanisms involved in the rapid and explosive intraocular immune response have not been addressed. Because Toll-like receptors (TLRs) are integral to the initial recognition of organisms during infection, we hypothesized that the uniquely explosive immune response observed during B. cereus endophthalmitis is directly influenced by the presence of TLR2, a known Gram-positive pathogen recognition receptor. To address this hypothesis, we compared the courses of experimental B. cereus endophthalmitis in wild type C57BL/6J mice to that of age-matched homozygous TLR2-/- mice. Output parameters included analysis of bacterial growth, inflammatory cell (PMN) infiltration, cytokine/chemokine kinetics, retinal function testing, and histology, with N≥4 eyes/assay/time point/mouse strain. B. cereus grew at similar rates to108 CFU/eye by 12 h, regardless of the mouse strain. Retinal function was preserved to a greater degree in infected TLR2-/- eyes compared to that of infected wild type eyes, but infected eyes of both mouse strains lost significant function. Retinal architecture was preserved in infected TLR2-/- eyes, with limited retinal and vitreal cellular infiltration compared to that of infected wild type eyes. Ocular myeloperoxidase activities corroborated these results. In general, TNFα, IFNγ, IL6, and KC were detected in greater concentrations in infected wild type eyes than in infected TLR2-/- eyes. The absence of TLR2 resulted in decreased intraocular proinflammatory cytokine/chemokine levels and altered recruitment of inflammatory cells into the eye, resulting in less intraocular inflammation and preservation of retinal architecture, and a slightly greater degree of retinal function. These results demonstrate TLR2 is an important component of the initial ocular response to B. cereus endophthalmitis.
Introduction
Bacillus cereus is a Gram-positive sporulating rod found throughout the environment. B. cereus is most commonly known as a food contaminant, causing self-limiting gastrointestinal symptoms such as vomiting or diarrhea. However, B. cereus can also cause severe infections, such as meningitis, food poisoning, bacteremia, and pneumonia. As one of the most feared ocular pathogens, B. cereus causes a uniquely rapid form of intraocular infection (endophthalmitis) that typically results in explosive intraocular inflammation, significant vision loss, and sometimes loss of the eye, within hours [1]. Its involvement in endophthalmitis typically occurs after open globe injuries. B. cereus has been isolated in as many as 46% of reported cases of post-traumatic endophthalmitis [2]. Post-traumatic endophthalmitis cases caused by B. cereus resulted in less than 30% of patients retaining useful vision, while only 9% of infected patients retained 20/70 vision or better. Nearly 50% of B. cereus endophthalmitis cases require enucleation of the eye [2].
During B. cereus endophthalmitis, loss of vision or the eye itself can occur despite proper and aggressive therapeutic intervention that may otherwise cure infection caused by other ocular pathogens such as Staphylococcus aureus or Streptococcus pneumoniae [1]. B. cereus endophthalmitis typically results in involvement of both the anterior and posterior segments, leading to inflammation of the vitreous (vitritis), aqueous humor, and cornea, with a hallmark corneal ring abscess. Rapid vision loss and severe ocular pain occurs, with systemic symptoms including fever and an elevated leukocyte count [1]. During the early stages of B. cereus endophthalmitis, the eye mounts an aggressive inflammatory response in an effort to eradicate intraocular organisms. Previous studies have demonstrated that metabolically inactive B. cereus triggers the explosive intraocular inflammatory response [3], suggesting that cell wall components play a role in inciting inflammation. B. cereus also synthesizes multiple toxins in the eye during infection [4], which are likely responsible for the rapid loss of vision that is a trademark of this disease [5]–[8]. The mechanisms underlying the inflammatory response and vision loss during B. cereus endophthalmitis remain an open question.
Initial recognition of bacteria during the acute stage of infection is critical in mounting an effective immune response. Bacteria are recognized by pattern recognition receptors known as Toll-like receptors (TLRs), which signal through the NF-κB pathway and upregulate the synthesis of cytokines and chemokines responsible for recruiting immune cells to the site of infection [9], [10]. TLRs have been identified in many cells throughout the eye, including retinal pigment epithelial cells, astrocytes, corneal epithelium, iris epithelium, and Muller cells [11], [12]. Studies have demonstrated the importance of TLR-mediated recognition of ocular pathogens during bacterial keratitis [13]–[17], but analysis of the role of TLRs in modulating posterior segment inflammation during bacterial infection is lacking.
Bacillus possesses known ligands for recognition via TLR2 such as peptidoglycan and lipotechoic acid. Bacillus anthracis has been shown to stimulate signaling through TLR2 [18]. Genetically, B. anthracis and B. cereus are close relatives and are considered monophyletic clones [19]. With the exception of the anthrax toxin, B. anthracis and B. cereus synthesize a similar complement of toxins and enzymes. Therefore, the similarities of B. cereus and B. anthracis may also extend to that of the cell wall, and their ligands may be recognized by TLRs in a similar fashion.
Because TLRs are integral to the initial recognition of organisms during infection, we hypothesized that the uniquely explosive immune response observed during B. cereus endophthalmitis is directly influenced by TLR2. We tested this hypothesis by comparing the pathogenesis of experimental B. cereus endophthalmitis in TLR2-deficient mice with that of infection in wild type mice.
Results
TLR2 expression in the retina during B. cereus endophthalmitis
Studies have reported increased expression of TLR2 in response to infection [20]–[22] and inflammation [23]–[25], while others have reported decreased or attenuated expression [26]–[28]. Numerous in vitro studies document the activation of TLR2 by Gram-positive ligands, but these models may not represent the in vivo environment during infection. We therefore analyzed the expression of TLR2 in retinas of wild type mice during the course of infection (Figure 1). Quantitative real-time PCR of TLR2 mRNA in retinas demonstrated no change in expression during infection.
Figure 1. TLR2 expression in the retina during B. cereus endophthalmitis.
C57BL/6J mouse eyes were injected with 100 CFU B. cereus and retinas were harvested at 0, 0.5, 1, 2, 4, and 8 h postinfection. (A) No significant change in retinal TLR2 mRNA expression was detected during infection. Values are mean ± SD of N≥4 retinas per time point (P≤0.05, 0 h postinfection compared with all other time points). (B) TLR2 PCR of wild type and TLR2-/- strains used in this study. Reactions using primers for genotyping [41] or real-time PCR (Methods) are shown.
Effect of TLR2 deficiency on intraocular growth of B. cereus
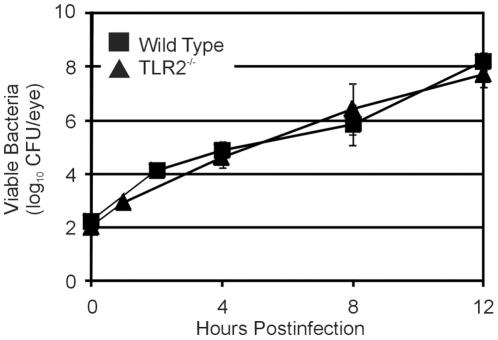
The intraocular growth rates of B. cereus were analyzed in infected eyes of wild type and TLR2-/- mice (Figure 2). The growth rates of B. cereus in TLR2-/- eyes were similar to that of wild type eyes when compared at 4, 8 and 12 h postinfection (P≥0.162). Growth rates reached 108 CFU/eye by 12 h in wild type and TLR2-/- mice. Ramadan et al. [29] previously showed that B. cereus grew to similar numbers in eyes of wild type mice of a similar background. This result demonstrates that the TLR2 functional deficiency does not affect the intraocular growth rates of B. cereus during endophthalmitis.
Figure 2. Bacterial growth during experimental B. cereus endophthalmitis.
C57BL/6J wild type and TLR2-/- mouse eyes were injected with 100 CFU B. cereus. Eyes were harvested, homogenized, and analyzed for bacterial growth. B. cereus grew to similar concentrations in infected eyes of TLR2-/- mice and wild type mice (P≥0.05 at all time points). Values represent the mean±SEM of N≥8 eyes per time point for at least 2 separate experiments.
Effect of TLR2 deficiency on retinal function during B. cereus endophthalmitis
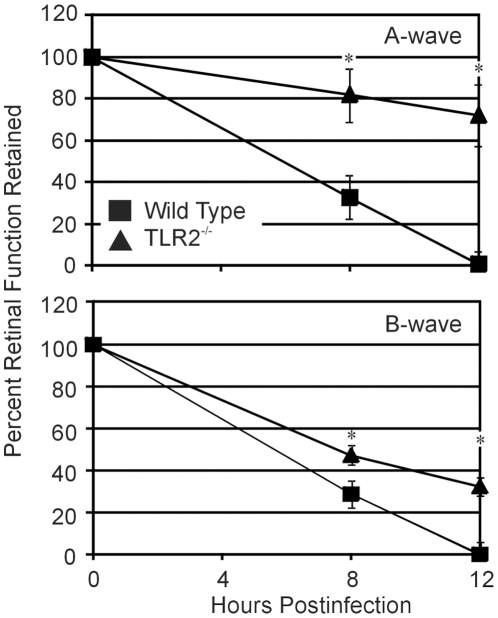
Analysis of retinal function loss during infection is summarized in Figure 3. We observed significantly slower retinal function declines in infected TLR2-/- eyes compared to that of infected wild type eyes. Reductions in A-wave function in TLR2-/- eyes were significantly less than that of wild type eyes at 8 and 12 h (P≤0.004). The loss of B-wave function in infected eyes of TLR2-/- mice was also significantly less than that of infected eyes of wild type mice at 8 and 12 h (P≤0.023). However, retinal function loss was considerable regardless of the TLR2 background of the infected eyes. These results suggest that while the TLR2 functional deficiency altered the speed at which retinal function was lost, the absence of a functional receptor ultimately had a minimal effect on overall vision loss in this model.
Figure 3. Retinal function analysis during B. cereus endophthalmitis.
C57BL/6J wild type and TLR2-/- mouse eyes were injected with 100 CFU B. cereus. Retinal function was assessed by electroretinography. At 8 and 12 h postinfection, A- and B-wave amplitudes retained were significantly lower in infected wild type eyes than in infected TLR2-/- eyes. By 12 h, retinal function was abolished in infected eyes of wild type mice, and significant function loss was seen in infected eyes of TLR2-/-. Values represent the mean±SEM of N = 8 eyes per time point for at least 2 separate experiments. *P≤0.05.
Effect of TLR2 deficiency on intraocular inflammation during B. cereus endophthalmitis
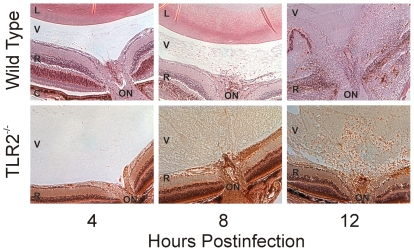
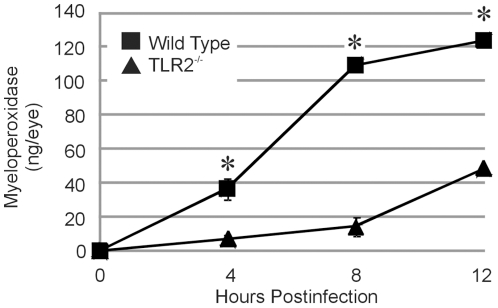
Figure 4 depicts a histological comparison of posterior segment inflammation in wild type and TLR2-/- mice during B. cereus endophthalmitis. The images are centered at the optic nerve/retinal interface and include the vitreous. At 4 h, fibrin infiltrate and transient PMN were seen in the vitreous of infected wild type eyes. At the same time, the vitreous of eyes of TLR2-/- mice were relatively clear and similar to that of mock-injected control eyes. At 8 h, PMN were seen in close proximity to the optic nerve head and significant fibrin infiltrate was observed throughout the vitreous in infected wild type eyes. Comparatively, there were very few PMN visible in the posterior segment of infected TLR2-/- mice. At this time, significant fibrin infiltrate was observed in the vitreous of infected TLR2-/- eyes, and retinas appeared to be intact. At 12 h, retinas of wild type mice were significantly disrupted and retinal layers were indistinguishable. Significant infiltration of PMN, fibrin infiltrate, and complete loss of retinal architecture was observed in infected wild type eyes at 12 h. At the same time, eyes of TLR2-/- mice demonstrated minimal disruption of retinal structure and infiltration of PMN into the vitreous from the optic nerve, similar to that seen in wild type eyes at 8 h. These results suggested that retinal disruption and PMN influx into the posterior segment was delayed in eyes of TLR2-/- mice. Myeloperoxidase (MPO) activities of infiltrating PMN are summarized in Figure 5. Significantly greater MPO activity was detected in infected eyes of wild type mice compared to that of infected eyes of TLR2-/- mice at 4, 8, and 12 h (P≤0.0001). Similar levels of MPO were detected in infected TLR2-/- eyes at 12 h and wild type eyes at 4 h (P = 0.0528). These data correlated with the histology data, further demonstrating that the delay in posterior segment inflammation resulted from the absence of functional TLR2.
Figure 4. Retinal histology of B. cereus endophthalmitis.
Wild type and TLR2-/- mouse eyes were injected with 100 CFU B. cereus. Eyes were harvested and processed for hematoxylin and eosin staining. Infected TLR2-/- had significantly less inflammation than infected eyes of wild type mice. Sections are representative of 4 eyes per group. L, lens; V, vitreous; R, retina; C, choroid; ON, optic nerve head. Magnification, 40X.
Figure 5. Infiltration of PMN into mouse eyes during B. cereus endophthalmitis.
C57BL/6J wild type and TLR2-/- mouse eyes were injected with 100 CFU B. cereus. PMN infiltration was estimated by quantifying MPO in whole eyes by sandwich ELISA. MPO concentrations were significantly higher in infected wild type eyes than in infected TLR2-/- eyes (*P≤0.05), suggesting greater numbers of PMN in infected wild type eyes than in infected TLR2-/- eyes. Values represent the mean±SEM for N≥4 per group for at least 2 separate experiments.
Effect of TLR2 deficiency on proinflammatory cytokines/chemokines during B. cereus endophthalmitis
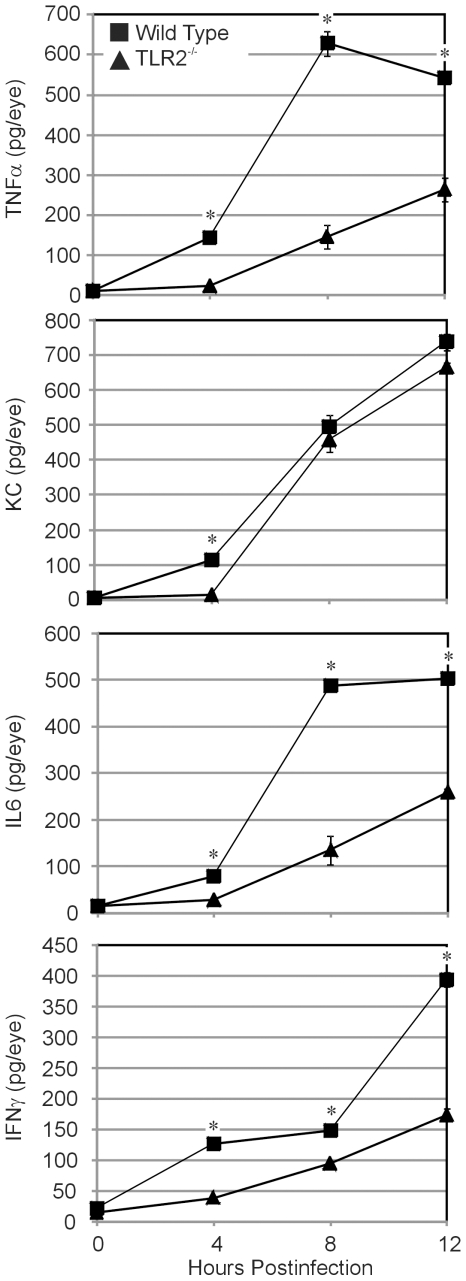
Levels of cytokines and chemokines in B. cereus-infected eyes are summarized in Figure 6. Infected TLR2-/- eyes had significantly less TNFα,IL6, and IFNγ at 4, 8, and 12 h postinfection as compared to infected wild type eyes (P≤0.0005). KC values were also greater in infected wild type eyes compared to that of infected TLR2-/- eyes at 4 h only (P≤0.0001). These results, together with the histology and myeloperoxidase activity data, confirmed the diminished intraocular inflammation in infected TLR2-/- eyes during experimental B. cereus endophthalmitis.
Figure 6. Proinflamatory cytokine and chemokine expression during experimental B. cereus endophthalmitis.
C57BL/6J wild type and TLR2-/- mouse eyes were injected with 100 CFU B. cereus. Ocular proinflammatory cytokines and chemokines were analyzed by sandwich ELISA. Overall, greater levels of TNFα, KC, IL-6, and INFγ were synthesized in infected eyes of wild type mice compared with that of infected eyes of TLR2-/- mice. Values represent the mean±SEM for N≥6 per group for at least 2 separate experiments.
Discussion
The innate immune response is the first line of defense against an invading pathogen such as B. cereus [9], [10], [30]. Without that initial line of defense, especially in an immune-privileged environment such as the eye, pathogens would freely replicate, produce toxic factors, and damage tissue. Retinal tissue damage is irreversible, resulting in vision loss. This study unambiguously demonstrated that during B. cereus endophthalmitis, TLR2 directly influenced the severity of intraocular inflammation. The absence of a functional TLR2, shown in several other models to be essential for recognition of Gram-positive pathogens, altered the expression of proinflammatory cytokines and chemokines, resulting in delayed recruitment of PMN into the eye. This effect significantly limited intraocular inflammation, but had only a minimal effect on loss of retinal function during infection.
The absence of a functional TLR2 did not affect the growth of B. cereus in the intraocular environment. We previously reported that intraocular inflammation in TNFα-deficient mice was limited, resulting in a larger bacterial load and faster retinal function loss during endophthalmitis [29]. In the present study, intraocular bacterial loads were similar regardless of the presence of a functional TLR2, and despite the relative lack of inflammation that would otherwise serve to limit bacterial growth. These results suggest that the limited inflammation observed in TLR2-/- eyes may have been sufficient enough to arrest the degree of uninhibited growth seen in TNFα-deficient eyes. It appears that a threshold of inflammation must be present in the eye to control bacterial growth.
Similar bacterial growth rates in eyes of wild type and TLR2-/- mice may also indicate that similar levels of toxins were produced in these eyes. Throughout the course of B. cereus endophthalmitis, several toxins are produced, including hemolysins, phospholipases, enterotoxins and proteases [4]. Yet, there was a delay in retinal function loss in TLR2-/- eyes and eventually, TLR2-/- eyes lost significant function. Since intraocular bacterial growth (and presumably toxin production) was similar regardless of TLR2 genetic background, the differences observed in function loss may be attributed to differences in intraocular inflammation. We have previously shown that metabolically inactive B. cereus causes an inflammatory influx resulting in some retinal function loss [3]. Inflammation may therefore contribute to some aspect of retinal function loss, potentially disrupting the biochemical processes associated with the phototransduction cascade as inflammatory cells enter the retina or as retinal or inflammatory cells synthesize cytokines/chemokines that affect these processes. The roles of specific B. cereus cell wall components in the explosive intraocular response are being investigated.
The primary difference observed between endophthalmitis in wild type versus TLR2-/- mice was the lack of significant posterior segment inflammation. In eyes of TLR2-/- mice, proinflammatory cytokine/chemokine synthesis was arrested, resulting in delayed recruitment of PMN into the retina and vitreous. This limited inflammation was similar to that observed in this infection model in TNFα-/- mice. When subjected to similar intravitreal challenge with B. cereus, eyes of TNFα-/- mice had significantly less MPO and proinflammatory cytokines during the course of infection compared to that of eyes of wild type mice [29]. Retinal histology also showed preserved retinal architecture in TLR2-/- mice compared to that of wild type mice. The lack of PMN recruitment in both TNFα-/- and TLR2-/- mice suggest that the lack of TNFα affected the recruitment of PMN into the eye in this model. The contribution of other important proinflammatory mediators to intraocular inflammation are being investigated.
Kumar et al. analyzed the efficacy of the synthetic TLR2 ligand, Pam3Cys, in altering the outcome of S. aureus endophthalmitis [31]. In that study, upregulation of TLR2 by Pam3Cys prior to infection led to reduced numbers of intraocular staphylococci and preservation of retinal function, compared to untreated infected control mice. Retinal TLR2 was upregulated upon intravitreal challenge with Pam3Cys, but downregulated upon intravitreal challenge with S. aureus. From this data, it is not clear at what time during experimental S. aureus infection TLR2 initiated the immune response. However, pre-infection challenge with Pam3Cys clearly altered the TLR2 response to S. aureus intraocular challenge, diminishing inflammation, reducing bacterial load, and preserving retinal function in this model. In our study, absence of TLR2 led to decreased inflammation, but no decrease in bacterial load and significant retinal function loss. Taken together, these results suggest that the mechanisms of the intraocular TLR2-mediated immune response to B. cereus and S. aureus are quite different. We [3] reported that the kinetics and degrees of intraocular inflammation caused by B. cereus, S. aureus, and E. faecalis were highly variable, suggesting that the intraocular immune response is organism-dependent. This is clearly the case with relatively avirulent organisms such as S. epidermidis, which causes infection and inflammation in the eye only at high concentrations [32], [33]. Suggested development of therapeutics that target pattern recognition receptors must therefore not only account for organism-dependent differences in recognition, but also realistic timing of administration for different infection scenarios.
In this study, retinal TLR2 mRNA expression did not increase during infection. Downregulation of TLR2 in response to infection has been reported during experimental S. aureus endophthalmitis [31]. Hyporesponsiveness to lipoteichoic acid has been suggested as a potential mechanism of limiting inflammation-induced damage [34]–[37]. Although TLR2 appears to be essential for initial bacterial recognition and rapid inflammation during B. cereus endophthalmitis, upregulation of TLR2 may not be necessary once the organisms are recognized and the inflammatory cascade has begun. Early mediation of the TLR2 pathway activated at the time of infection may potentially limit TLR2 signaling, unnecessary inflammation, and further damage. The fact that intraocular inflammation, although delayed, occurs at all in the absence of TLR2 suggests that pathogen recognition and the resulting response occurs by a redundant mechanism. As indicated earlier, other TLRs have been detected in cells throughout the eye [11], [12] and have been shown to be important in ocular infection [13]–[17]. We also found diminished inflammation in B. cereus-infected eyes of TLR4-/- mice, results similar to that observed in infected TLR2-/- eyes (Novosad and Callegan, unpublished work). B. cereus does not possess the classic TLR4 ligand, LPS. B. cereus does, however, secrete cereolysin O, a cholesterol-dependent cytolysin (CDC) similar in sequence and structure to that of other CDCs demonstrated to interact with TLR4 [38]–[40]. If both TLR2 and TLR4 respond similarly in the eye to infection with B. cereus, this may account for the unusually robust inflammation observed during this disease. Current studies are analyzing TLR4-B. cereus interactions in the eye and the triggers involved in these interactions.
The present results unequivocally demonstrated that a lack of functional TLR2 significantly altered the intraocular inflammatory response to B. cereus endophthalmitis. Because B. cereus is a rapidly blinding infection, therapeutics designed to delay its pathogenicity could prove to be an invaluable tool. Further understanding of the mechanisms by which TLR2 and other components of innate immunity respond to B. cereus and other organisms in the eye could facilitate the development of new therapeutic regimens to hamper inflammation and prevent vision loss during this blinding infection.
Materials and Methods
Experimental Bacillus cereus Endophthalmitis
Animals were used following institutional guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Wild type C57BL/6J mice (male 6–8 weeks of age; Jackson Laboratories, Bar Harbor, ME) and homozygous TLR 2-/- mice [41] were used for infection studies. These mice were backcrossed onto a C57BL/6J background for 5–8 generations. Genotypes were verified using primer sets and PCR conditions developed for these knockouts [41]. Each mouse was anesthetized with a combination of ketamine (85 mg/kg body weight; Bionichepharma, LLC., St. Lake Forrest, IL) and xylazine (14 mg/kg body weight; Rompun; Bayer Corp., Shawnee Mission, KS). Each eye was topically anesthetized prior to intravitreal injections and analysis of retinal function by electroretinography (0.5% proparacaine HCl; Ophthetic; Allergan, Hormigueros, Puerto Rico). Experimental endophthalmitis was induced in mice as previously described with B. cereus strain ATCC 14579 (American Type Culture Collection [ATCC], Manassas, VA) [7], [8], [29]. Briefly, 0.5 µL of brain heart infusion media containing approximately 100 colony forming units (CFU) of B. cereus were injected into the midvitreous. The contralateral eye was not injected (absolute control).
Electroretinography (ERG)
Electroretinography (ERG) is used to analyze retinal function and physiology. The retinal response is stimulated by a transient flash of light and results from a chain of electrical responses in the form of graded potentials evoked in each layer of the retina. The response consists of an A-wave measured in a negative amplitude below zero, followed by a B-wave measured from the trough of the A-wave to the highest positive value above zero. The leading edge of the A-wave provides a direct measure of photoreceptor activity, while the B-wave represents the action of Muller cells, bipolar cells, and second order neurons (amacrine and ganglion cells). Scotopic A- and B-wave amplitudes were recorded for each experimental (infected) eye compared with its fellow control (uninfected) eye (UTAS3000; LKC Technologies, Inc., Gaithersburg, MD). The percentage of retinal function retained was calculated as follows 100 – {[1 – (experimental A-wave amplitude/control A-wave amplitude)] x 100} or 100 – {[1 – (experimental B-wave amplitude/control B-wave amplitude)] x100} [7], [8], [29]. Scotopic ERGs were performed at 8 and 12 h postinfection (N = 8 eyes per group per time point, mean±standard error of the mean [SEM]). Scotopic ERGs were not performed earlier than 8 h because of the extended dark adaptation time needed for the mouse retina.
Histology
Whole eyes were harvested at 0, 4, 8, or 12 h postinfection and incubated in an 85/15 paraformaldehyde/alcohol fixative for 24 h at room temperature. Whole eyes were then exchanged into 70% ethanol for 24 h and then embedded in paraffin. Sections were deparaffinized and stained in Harris hematoxylin solution for 8 min, counterstained in eosin-phloxine B solution for 30 sec, dehydrated through two changes of 95% alcohol and cleared in two changes of xylene. Images are representative of 4 eyes per group at each time point.
Bacterial Quantitation
Bacteria were quantified in whole eyes as previously described [7], [8], [29]. Briefly, whole eyes were homogenized with 1-mm sterile glass beads (BioSpec Products, Inc., Bartlesville, OK) in 400 µL PBS and then 10-fold track diluted onto BHI agar. Values represent the mean±SEM for N≥8 eyes per time point.
Myeloperoxidase (MPO)
Polymorphonuclear leukocytes (PMN) contain myeloperoxidase, a peroxidase enzyme stored in azurophilic granules. Previous studies have shown that PMN are the primary cell type entering the eye during the first 12 h of B. cereus endophthalmitis [8]. Estimating PMN influx into the eye was achieved by quantifying myeloperoxidase in whole eyes by sandwich ELISA (Mouse MPO ELISA Test Kit; Cell Sciences, Canton, MA), as previously described [29]. Negative controls included noninfected eyes. Eyes were analyzed for MPO activity at 4, 8, or 12 h postinfection. Results are reported as MPO ng/eye±SEM for N≥4 eyes per group per time point.
Quantitation of TLR2 in Retinas
Eyes were collected and retinas dissected from each eye cup for quantitation of TLR2 by real-time PCR and Western blot. One retina was used for each real-time PCR reaction, with N≥4 retinas analyzed per group in duplicate. Primer efficiencies were verified by performing real time RT-PCR on a standard curve created with cDNA produced from qPCR total reference RNA (BioRad, Hercules CA). All primers were designed using Ensembl and Primer 3. Primers used for TLR2 were forward 5'-ATGCTTCGTTGTTCCCTGTGTTGC-3’ and reverse 5’-AACAAAGTGGTTGTCGCCTGCTTC-3'. The 2−ΔΔCT standard curve method was used to evaluate the relative expression level of TLR2 in infected eyes of wild type mice. Quantitative real-time PCR was performed (iCycler iQ, BioRad) according to the manufacturer’s instructions. Briefly, the thermal cycling conditions were 40 cycles of 55°C for 30 sec and 95°C for 1 min. The cycle threshold (CT) was set for the target gene, where all amplicons were in the exponential phase of amplification. Data were analyzed using the relative standard curve method. All target CT values reported by the iCycler software were normalized to the endogenous control, β-actin (target mean input – endogenous control mean input = target N). The resulting CT value was then normalized to the untreated wild type control (target N/ control N = relative fold difference in target expression). A greater than 2-fold change in mRNA expression was considered significant.
Quantitation of Cytokines and Chemokines
Ocular proinflammatory cytokines and chemokines were quantified as previously described [7], [8], [30]. Harvested eyes were mixed with a protease inhibitor cocktail (Triton X-100, 0.5 M EDTA, 10 mM sodium orthovanadate [Sigma] and Protease Inhibitor [Calbiochem, La Jolla, CA] in PBS, pH 7.4) and homogenized with glass beads. Commercial ELISA kits (Quantikine; R&D Systems, Minneapolis, MN) were used to analyze the levels of KC (IL-8), TNFα, IL-6, and IFNγ in accordance with the manufacturer’s instructions. Eyes were analyzed at 4, 8, or 12 h postinfection. Cytokine and chemokine concentrations were interpolated from standard curves. The lower limits of detection for each assay are as follows: KC, 2 pg/ml; TNFα, 5 pg/ml; IL6, 2 pg/ml; IFNγ, 2 pg/ml. Values are expressed as mean±SEM for N≥6 eyes per time point.
Statistics
If not stated otherwise, results were the arithmetic means±standard errors of the mean (SEM) of all of the samples in the same experimental group. A two-tailed Student t test was used to determine the statistical significance of the data. Wilcoxon’s rank sum test was used for statistical comparison between groups. Statistical significance was determined at P < 0.05.
Acknowledgments
The authors thank Nanette Wheatley (Dean McGee Eye Institute) and Mark Dittmar (Dean A. McGee Eye Institute Animal Research Facility) for their invaluable technical assistance, Eric Pearlman (Case Western Reserve University, Cleveland OH) for providing offspring of the Akira TLR2-/- mice [41], Paula Pierce (Excalibur Pathology, Moore, OK) for histology expertise, and Jonathan Hunt and Drs. Phil Coburn and Martin-Paul Agbaga (Oklahoma University Health Sciences Center) for their helpful comments and assistance with confirmatory experiments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by National Institutes of Health (NIH) Grant R01EY012985 (to MCC, http://www.nih.gov/). The authors' research is also supported in part by NIH Grants P30EY12191 (NIH CORE grant to Robert E. Anderson, OUHSC), Research Resources Grant P20RR17703 (NCRR COBRE grant to Robert E. Anderson, OUHSC), and an unrestricted grant to the Dean A. McGee Eye Institute from Research to Prevent Blindness. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Callegan MC, Gilmore MS, Gregory M, Ramadan RT, Wiskur BJ, et al. Bacterial endophthalmitis: therapeutic challenges and host-pathogen interaction. Prog Retin Eye Res. 2007;26(2):189–203. doi: 10.1016/j.preteyeres.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David DB, Kirkby GR, Noble BA. Bacillus cereus endophthalmitis. Br J Ophthalmol. 1994;78(7):577–580. doi: 10.1136/bjo.78.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callegan MC, Booth MC, Jett BD, Gilmore MS. Pathogenesis of Gram-positive bacterial endophthalmitis. Infect Immun. 1999;67(7):3348–3356. doi: 10.1128/iai.67.7.3348-3356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beecher DJ, Pulido JS, Barney NP, Wong AC. Extracellular virulence factors in Bacillus cereus endophthalmitis: methods and implication of involvement of hemolysin BL. Infect Immun. 1995;63(2):632–639. doi: 10.1128/iai.63.2.632-639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callegan MC, Kane ST, Cochran DC, Gilmore MS, Gominet M, et al. Relationship of plcR-regulated factors to Bacillus endophthalmitis virulence. Infect Immun. 2003;71(6):3116–3124. doi: 10.1128/IAI.71.6.3116-3124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callegan MC, Kane ST, Cochran DC, Novosad B, Gilmore MS, et al. Bacillus endophthalmitis: roles of bacterial toxins and motility during infection. Invest Ophthalmol Vis Sci. 2005;46(9):3233–3238. doi: 10.1167/iovs.05-0410. [DOI] [PubMed] [Google Scholar]
- 7.Moyer AL, Ramadan RT, Novosad BD, Astley R, Callegan MC. Bacillus cereus-induced permeability of the blood ocular barrier during experimental endophthalmitis. Invest Ophthalmol Vis Sci. 2009;50(8):3783–8793. doi: 10.1167/iovs.08-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramadan RT, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res. 2006;31(11):955–965. doi: 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Beutler BA. TLRs and innate immunity. Blood. 2009;113(7):1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006;90(1):103–108. doi: 10.1136/bjo.2005.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chui JJ, Li MW, Di Girolamo N, Chang JH, McCluskey PJ, et al. Iris pigment epithelial cells express a functional lipopolysaccharide receptor complex. Invest Ophthalmol Vis Sci. 2010;51(5):2558–2567. doi: 10.1167/iovs.09-3923. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Hise AG, Kalsow CM, Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect Immun. 2006;74(9):5325–5332. doi: 10.1128/IAI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Du W, McClellan SA, Barrett RP, Hazlett LD. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2006;47(11):4910–4916. doi: 10.1167/iovs.06-0537. [DOI] [PubMed] [Google Scholar]
- 15.Jin X, Lin Z, Xie X. The delayed response of Toll-like receptors may relate to Pseudomonas aeruginosa keratitis exacerbating rapidly at the early stages of infection. Eur J Clin Microbiol Infect Dis. 2010;29(2):231–238. doi: 10.1007/s10096-009-0844-z. [DOI] [PubMed] [Google Scholar]
- 16.Yuan X, Wilhelmus KR. Toll-like receptors involved in the pathogenesis of experimental Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2009;51(4):2094–2100. doi: 10.1167/iovs.09-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang X, Du W, Barrett RP, Hazlett LD. ST2 is essential for Th2 responsiveness and resistance to Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2007;48(10):4626–4633. doi: 10.1167/iovs.07-0316. [DOI] [PubMed] [Google Scholar]
- 18.Hughes MA, Green CS, Lowchyj L, Lee GM, Grippe VK, et al. MyD88-dependant signaling contributes to protection following Bacillus anthracis spore challenge of mice: implications for Toll-like receptor signaling. Infec Immun. 2005;73(11):7535–7540. doi: 10.1128/IAI.73.11.7535-7540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilas-Bôas GT, Peruca AP, Arantes OM. Biology and taxonomy of Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensis. Can J Microbiol. 2007;53(6):673–687. doi: 10.1139/W07-029. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong L, Medford AR, Hunter KJ, Uppington KM, Millar AB. Differential expression of Toll-like receptor (TLR)-2 and TLR-4 on monocytes in human sepsis. Clin Exp Immunol. 2004;136(2):312–319. doi: 10.1111/j.1365-2249.2004.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burrough ER, Diverde KD, Sahin O, Plummer PJ, Zhang Q, et al. Vet Pathol. In press; 2010. Expression of Toll-like receptors 2 and 4 in subplacental trophoblasts From guinea pigs (Cavia porcellus) following infection with Campylobacter jejuni. [DOI] [PubMed] [Google Scholar]
- 22.Wang JP, Zhang Y, Wei X, Li J, Nan XP, et al. Circulating Toll-like receptor (TLR) 2, TLR4, and regulatory T cells in patients with chronic hepatitis C. APMIS. 2010;118(4):261–270. doi: 10.1111/j.1600-0463.2010.02586.x. [DOI] [PubMed] [Google Scholar]
- 23.Hei Z, Chi X, Cheng N, Luo G, Li S. Upregulation of TLR2/4 expression in mononuclear cells in postoperative systemic inflammatory response syndrome after liver transplantation. 519589Mediators Inflamm. 2010;2010 doi: 10.1155/2010/519589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F, Yang N, Zhang L, Tan H, Huang B, et al. Increased expression of Toll-like receptor 2 in rat diabetic nephropathy. Am J Nephrol. 2010;32(2):179–186. doi: 10.1159/000317023. [DOI] [PubMed] [Google Scholar]
- 25.Tu XK, Yang WZ, Shi SS, Wang CH, Zhang GL, et al. Spatio-temporal distribution of inflammatory reaction and expression of TLR2/4 signaling pathway in rat brain following permanent focal cerebral ischemia. Neurochem Res. 2010;35(8):1147–1155. doi: 10.1007/s11064-010-0167-6. [DOI] [PubMed] [Google Scholar]
- 26.Pimentel-Nunes P, Roncon-Albuquerque R, Jr, Gonçalves N, Fernandes-Cerqueira C, Cardoso H, et al. Attenuation of toll-like receptor 2-mediated innate immune response in patients with alcoholic chronic liver disease. Liver Int. 2010;30(7):1003–1011. doi: 10.1111/j.1478-3231.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- 27.Schaaf B, Luitjens K, Goldmann T, van Bremen T, Sayk F, et al. Mortality in human sepsis is associated with downregulation of Toll-like receptor 2 and CD14 expression on blood monocytes. Diagn Pathol. 2009;4:12. doi: 10.1186/1746-1596-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumura N, Takeyama Y, Ueda T, Yasuda T, Shinzeki M, et al. Decreased expression of Toll-like receptor 2 and 4 on macrophages in experimental severe acute pancreatitis. Kobe J Med Sci. 2007;53(5):219–227. [PubMed] [Google Scholar]
- 29.Ramadan RT, Moyer AL, Callegan MC. A role for tumor necrosis factor-alpha in experimental Bacillus cereus endophthalmitis pathogenesis. Invest Ophthalmol Vis Sci. 2008;49(10):4482–4489. doi: 10.1167/iovs.08-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netea MG, van der Graaf C, Van der Meer JW, Kullberg BJ. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J Leukoc Biol. 2004;75(5):749–755. doi: 10.1189/jlb.1103543. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, Singh CN, Glybina IV, Mahmound TH, Yu FS. Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis. J Infect Dis. 2010;2010(2):255–263. doi: 10.1086/649589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brozou CG, Karabatakis V, Giannousis M, Mandraveli K, Karkavelas G, et al. The efficacy of intravitreal povidone iodine application in experimental Staphylococcus epidermidis endophthalmitis. Ophthalmic Res. 2009;41(4):181–185. doi: 10.1159/000217721. [DOI] [PubMed] [Google Scholar]
- 33.Hosseini H, Mehryar M, Eghtedari M, Ahmadi F, Nowroozizadeh S. Effect of intravitreal triamcinolone acetonide in the treatment of experimental Staphylococcus epidermidis endophthalmitis. Curr Eye Res. 2009;34(2):145–151. doi: 10.1080/02713680802585938. [DOI] [PubMed] [Google Scholar]
- 34.Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J Immunol. 2001;166(8):5161–5167. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- 35.Jacinto R, Hartung T, McCall C, Li L. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance: distinct alterations in IL-1 receptor-associated kinase. J. Immunol. 2002;168(12):6136–6141. doi: 10.4049/jimmunol.168.12.6136. [DOI] [PubMed] [Google Scholar]
- 36.Li CH, Wang JH, Redmond HP. Bacterial lipoprotein-induced self-tolerance and cross-tolerance to LPS are associated with reduced IRAK-1 expression and MyD88-IRAK complex formation. J Leukoc Biol. 2006;79(4):867–875. doi: 10.1189/jlb.0905505. [DOI] [PubMed] [Google Scholar]
- 37.Kim HG, Lee SY, Kim NR, Ko MY, Lee JM, et al. Inhibitory effects of Lactobacillus plantarum lipoteichoic acid (LTA) on Staphylococcus aureus LTA-induced tumor necrosis factor-alpha production. J Microbiol Biotechnol. 2008;18(6):1191–1196. [PubMed] [Google Scholar]
- 38.Park JM, Ng VH, Maeda S, Rest RF, Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J Exp Med. 2004;200(12):1647–1655. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, et al. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect Immun. 2005;73(10):6479–6487. doi: 10.1128/IAI.73.10.6479-6487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito Y, Kawamura I, Kohda C, Tsuchiya K, Nomura T, et al. Seeligeriolysin O, a protein toxin of Listeria seeligeri, stimulates macrophage cytokine production via Toll-like receptors in a profile different from that induced by other bacterial ligands. Int Immunol. 2005;17(12):1597–1606. doi: 10.1093/intimm/dxh341. [DOI] [PubMed] [Google Scholar]
- 41.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]