Abstract
Purpose
Fulvestrant is known to promote the degradation of the estrogen receptor (ER) in the nucleus. However, fulvestrant also promotes the aggregation of the newly synthesized ER in the cytoplasm. Accumulation of protein aggregates leads to cell death but this effect is limited as a result of their elimination by the proteasome. We tested whether combining fulvestrant with the proteasome inhibitor, bortezomib, could enhance the accumulation of ER aggregates and cause apoptotic cell death.
Experimental Design
The rate of aggregation of the ER was monitored in ER+ breast cancer cells lines, T47D, ZR-75.1, BT474, MDA-MB-361, MCF-7, fulvestrant resistance MCF-7, and tamoxifen-resistant T47D-cyclin D1 cells. Activation of the unfolded protein response, apoptosis, and metabolic rate were also monitored in these cell lines following treatment with fulvestrant, bortezomib, or bortezomib in combination with fulvestrant.
Results
We found that bortezomib enhances the fulvestrant-mediated aggregation of the ER in the cytoplasm without blocking the degradation of the ER in the nucleus. Further, these aggregates activate a sustained unfolded protein response leading to apoptotic cell death. Further, we show that the combination induced tumor regression in a breast cancer mouse model of tamoxifen resistance.
Conclusions
Adding bortezomib to fulvestrant enhances its efficacy by taking advantage of the unique ability of fulvestrant to promote cytoplasmic aggregates of the ER. As this effect of fulvestrant is independent of the transcriptional activity of the ER, these results suggest that this novel combination may be effective in breast cancers that are ER+ but estrogen independent.
Introduction
As 70% of breast cancers express the estrogen receptor (ER), endocrine therapy targeting the ER represents a major therapeutic tool. In addition to tamoxifen and aromatase inhibitors, fulvestrant is a third drug, which acts by promoting the proteasomal degradation of the ER (1, 2).
However, a second effect of fulvestrant is the aggregation of the newly synthesized ER in the cytoplasm (3–5). Such aggregation is not observed following treatment with tamoxifen and occurs following the disappearance of the ER in the nucleus (5). Despite these findings, the current view of the mode of action of fulvestrant only considers its effect on the degradation of the ER in the nucleus.
Accumulation of protein aggregates is cytotoxic. Elimination of protein aggregates can be achieved by 2 pathways: degradation by the proteasome or by autophagy (6). On one hand, macroautophagy and proteasomal degradation of protein aggregates limit the toxicity of protein aggregates. On the other hand, when protein aggregates become insoluble they can lead to the inhibition of the proteasome and the activation of the unfolded protein response (UPR). The UPR is a complex cellular response that can lead to either cytoprotective or proapoptotic responses (7). The outcome of the activation of the UPR is dependent on the intensity of the stress signal, with a mild stress leading to the activation of cytoprotective responses, whereas intense stress leads to apoptosis (7).
In neurodegenerative diseases, the toxicity of protein aggregates involves the activation of a proapoptotic UPR (8, 9). Therefore, these studies offer a precedent for the notion that cytoplasmic protein aggregates promote apoptosis via the activation of the UPR.
Interestingly, resistance to fulvestrant is associated with the activation of a cytoprotective UPR (10, 11). These observations raise the possibility that the ER aggregates formed following treatment with fulvestrant alone may generate a weak signal that activates such cytoprotective UPR. If so, drugs able to amplify these aggregates may allow the conversion to a proapoptotic UPR. However, this possibility was never tested.
Bortezomib is a Food and Drug Administration–approved proteasome inhibitor (12) shown to activate the UPR (13). Therefore, we initiated this study to test whether the addition of bortezomib to fulvestrant may enhance the efficacy of fulvestrant by amplifying the ER aggregates and inducing a proapoptotic UPR.
Materials and Methods
Cell culture, reagent, and transfection
T47D and ZR-75.1 cells were grown in RPMI medium supplemented with 10% FBS, insulin (5 mg/mL), and antibiotics (Life Technologies, Inc.). MCF-7, MCF-7-F40, BT474, MDA-MB-361, MDA-MB-453, and MDA-MB-157 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and antibiotics. For depletion of estrogen, cells were cultured in phenol red–free RPMI 1640 or DMEM supplemented with 10% charcoal/dextran-treated FBS (HyClone), insulin (5 mg/mL), and antibiotics before the treatments. Bortezomib was dissolved in saline solution at a dose of 2.6 mmol/L as a stock solution (Millennium). Fulvestrant (Tocris) was dissolved in 100% ethanol. Transient transfections for green fluorescent protein (GFP)-ER were conducted by the Amaxa nucleofection technology according to the manufacture’s instructions.
MTT assay
MTT (Sigma) assays were carried out as described previously (14) by using the indicated drugs.
Western blot analysis
Western blot analyses were carried out as described previously (14). Using the following antibodies: rabbit anti-ER antibody (G-20; Santa Cruz), mouse anti-BiP (DB Biosciences), rabbit anti-LC3 antibody (PM036; MBL International), rabbit anti-cleaved caspase 3 antibody (Cell Signaling), rabbit anti-cleaved caspase 9 antibody (Cell Signaling), or mouse anti-tubulin antibody(University of Iowa).
Immunofluorescence staining
T47D cells were transfected with GFP-ER and ubiquitin by Amaxa nucleofection. Staining was done by using the rabbit anti-ubiquitin antibody (Sigma Aldrich) and anti-mouse Alexa Fluor 594 (Molecular probes) as described previously (14).
Proteasomal activity assay
10ug of T47D cell extracts treated with or without fulvestrant and/or bortezomib were incubated for 2.5 hours at 37°C in 100 µL assay buffer (50 mmol/L Tris-HCl, pH 7.5) with 10 mmol/L fluorogenic substrate Suc-LLVY-AMC (CALBIOCHEM). Release of free hydrolyzed 7-amino-4-methylcoumarin (AMC) groups was measured by using an ISS Counter with an excitation filter of 380 nm and an emission filter of 460 nm (PerkinElmer).
Xenograft Implantation and Measurement of Tumor Size
Xenografts were established as described previously (14). Bortezomib (0.05 mg/kg body weight) was given by tail vein injection twice weekly. Fulvestrant (5 mg) dissolved in 100% ethanol and diluted in peanut oil was injected subcutaneously, weekly. Our protocol was approved by the animal ethic committee at Mount Sinai School of Medicine.
Results
Bortezomib does not block the fulvestrant-mediated degradation of the ER but enhances the cytoplasmic aggregation of the ER by fulvestrant
Fulvestrant promotes the proteasome-dependent degradation of the ER in the nucleus (2, 15, 16). Therefore, as expected, we found that treatment with fulvestrant alone led to a reduction in the ER level in the nucleus (Fig. 1A and B) and these effects of fulvestrant were inhibited when cells were incubated in the presence of the proteasome inhibitors, LLnL and MG132 (Fig. 1A and B). In contrast, incubation in the presence of the proteasome inhibitor bortezomib did not rescue the level of the ER to the level of untreated cells by Western blot analysis (Fig. 1A) and did not prevent the elimination of the ER from the nucleus by treatment with fulvestrant (Fig. 1B). As bortezomib inhibits only 1 catalytic activity of the proteasome, whereas LLnL and MG132 inhibit all 3 catalytic activities, this result indicates that the remaining catalytic activities are sufficient to promote the degradation of the ER in presence of bortezomib and fulvestrant.
Figure 1.
The fulvestrant-mediated cytoplasmic ER aggregates are amplified by bortezomib. A, T47D cells were pretreated with or without 15 nmol/L of bortezomib, LLnL, or MG132 for 30 minutes, then treated with or without 0.1 µmol/L of fulvestrant for 2 hours. ER levels were determined by Western blotting. B, immunofluorescence of GFP-ER in T47D cells treated with or without fulvestrant (Ful) and/or bortezomib (Bor), LLnL, or MG132 for 15 hours. C, T47D cells were pretreated with or without 15 nmol/L of bortezomib for 30 minutes, then treated with or without 0.1 µmol/L of fulvestrant for additional 2 hours. The levels of ER in the soluble and insoluble fractions were determined by Western blotting. D, T47D cells were pretreated with or without 15 nmol/L of bortezomib for 30 minutes, then treated with or without 0.1 µmol/L of fulvestrant for 1, 2, 3, or 4 hours. The levels of endogenous ER in the insoluble fractions were determined by Western blotting, whereas the localization of endogenous ER was determined by immunofluorescence (IF) at the 4 hours treatment time point. DAPI, 4′,6-diamidino-2-phenylindole.
We confirmed that fulvestrant leads to the accumulation of the newly synthesized GFP-ER in the cytoplasm (Fig. 1B) and found that in the presence of bortezomib and fulvestrant, the staining became localized into foci (Fig. 1B). Accumulation of ER aggregates in the cytoplasm was confirmed by immunofluorescence of endogenous ER (Fig. 1D). Further, the cytoplasmic accumulation of endogenous ER corresponded to the appearance of the ER in the insoluble fraction (Fig. 1C). However, detection of the ER in the insoluble fraction was abolished after 3 hours (Fig. 1D) by Western blotting, whereas the ER aggregates remained detectable after 4 hours by immunofluorescence (Fig. 1D) suggesting that they may become further modified.
These results revealed that bortezomib does not inhibit the elimination of the ER in the nucleus but enhances the accumulation of ER aggregates in the cytoplasm.
The cytoplasmic aggregates of the ER are ubiquitinated and inhibit the activity of the proteasome
Aggregation of proteins results from their misfolding. Misfolded proteins are eliminated by ubiquitin–protea-some pathway (6). We therefore tested whether the ER aggregates observed following fulvestrant and bortezomib treatment are ubiquitinated. T47D cells were transfected with both GFP-ER and ubiquitin and then treated with fulvestrant and bortezomib. We found that although ubiquitin localized throughout the cells, as expected, the intensity of the ubiquitin staining was stronger in the area where the ER aggregates were detected (Fig. 2A), suggesting that the aggregates are ubiquitinated. As ubiquitination results in a shift in molecular weight chains, this finding explains the disappearance of ER by Western blot analysis at a time point where the staining persists by immunofluorescence (Fig. 1D).
Figure 2.
Insoluble ER aggregates colocalize with ubiquitin. A, cells transiently transfected with GFP-ER were treated with fulvestrant for 12 hours. Immunofluorescence was done by using anti-ubiquitin antibody. The representative pictures are shown. B, the chymotrypsin-like activity of the proteasome was measured by using the fluorogenic substrate Suc-LLVY-AMC as described in Materials and Methods. C, T47D cells were transiently transfected with Myc-ubiquitin (Myc-Ub) for 24 hours and subsequently treated with either 15 nmol/L bortezomib, 3 µmol/L fulvestrant, or both for 2 days. After 2 days, total amount of polyubiquitinated (poly-Ub) proteins in the extracts were determined by Western blot analysis. D, diagram of the lid and catalytic core of the proteasome. B, bortezomib; F, fulvestrant; C, control.
We next addressed the effect of endogenous ER aggregates on the activity of the proteasome. The lid of the proteasome contains ubiquitin receptor and unfoldases, which allow translocation of substrates into the catalytic core. We reasoned that ER aggregates may exceed the unfolding capacity of the lid and as a result prevent their translocation. As the proteasome is required for the continual turnover of proteins, the binding to the lid in absence of translocation may block the entrance of the catalytic core and therefore indirectly inhibit the proteasome. To test this possibility, we first measured the chymotrypsin activity of the proteasome by using a fluorogenic peptide. As expected, we found that bortezomib alone was very efficient (80% inhibition of cleavage) at blocking cleavage of this peptide, because bortezomib is an inhibitor of the chymotrypsin-like activity (Fig. 2B). However, we also found that fulvestrant alone inhibits the chymotrypsin-like activity by 20% (Fig. 2B) suggesting that the accumulation of endogenous ER aggregates following fulvestrant are sufficient to partially limit the assess of the peptide to the enzyme. Combining fulvestrant and bortezomib led to an additive inhibition of the chymotrypsin-like activity (Fig. 2B).
Second, we assessed the ability of fulvestrant, bortezomib, or both to inhibit the elimination of polyubiquitinated proteins in general. We found that the baseline level of ubiquitination was not significantly affected by bortezomib alone (Fig. 2C). Treatment with fulvestrant alone led to the mild accumulation of high molecular weight polyubiquitinated proteins, suggesting that unlike bortezomib, the inhibition of the proteasome as a result of the formation of ER aggregates is not specific to any catalytic activity but acts by blocking the entrance of the catalytic core. However, treatment with both fulvestrant and bortezomib led to a drastic accumulation of high molecular weight polyubiquitinated proteins (Fig. 2C). These results indicate that combining the specific inhibition of the chymotrypsin-like activity and the inhibition of translocation of polyubiquitinated proteins represent a double lock on the activity of the proteasome (Fig. 2D).
The fulvestrant–bortezomib combination induces the unfolded protein response and apoptosis
Inhibition of the proteasome activates the UPR, which is characterized by elevated level of the chaperone BiP (7). Therefore, we analyzed the level of BiP by Western blotting in T47D cells. We found that, as expected, bortezomib led to an elevation in the level of BiP (Fig. 3A) and, further, fulvestrant alone also led to an elevation in the levels of BiP, a result that is consistent with the ability of fulvestrant alone to partially inhibit the proteasome (Fig. 2B, D). However, BiP levels were drastically more elevated following treatment with both drugs (Fig. 3A).
Figure 3.
Bortezomib–fulvestrant combination activates the UPR and apoptosis. A, T47D cells were treated with 15 nmol/L bortezomib (B) or 3 µmol/L fulvestrant (F) or both for 2 days and the level of BiP determined by Western blot analysis. Tubulin was used as the loading control (C). B, T47D cells were treated with 15 nmol/L bortezomib or 3 µmol/L fulvestrant or both for 2 days and the levels of bid, bcl-2, procaspase 8, cleaved caspase 9, and cleaved caspase 3 were determined by Western blot analysis. C, 47D cells were treated with increasing concentrations of fulvestrant (Ful) and/or bortezomib for 2 days. The percentage of inhibition in metabolic rate after treatment was determined by MTT assay. Results are presented as the mean from 1 independent experiment carried out in triplicate. D, ZR-75.1 cells were treated with increasing concentration of fulvestrant and/or bortezomib for 2 days. The percentage of inhibition in metabolic rate after treatment was determined by MTT assay. Results are presented as the mean from 1 independent experiment carried out in triplicate. E, BT474 cells were treated with 3 µmol/L fulvestrant and increasing concentrations of bortezomib alone or with fulvestrant for 2 days. The percentage of inhibition in metabolic rate after treatment was determined by MTT assay. Results are presented as the mean from 1 independent experiment carried out in triplicate. F, Western blot analysis of the ER in T47D, ZR-75.1, BT474, and MDA-MB-361 cells.
To determine the outcome of the activation of the UPR by fulvestrant, bortezomib, or both, we next analyzed a panel of apoptotic markers in T47D cells. We found that fulvestrant alone did not affect the levels of bid, bcl-2, or procaspase 8, nor did it led to the cleavage and activation of caspases 3 and 9 compared with the untreated cells (Fig. 3B), suggesting that fulvestrant alone generates a mild stress, which activates a cytoprotective outcome of the UPR. Treatment with bortezomib alone led to a reduction in bid, bcl-2, and procaspase 8, as well as the detection of cleaved caspases 3 and 9 (Fig. 3B); however, these effects were drastically more severe on treatment with both fulvestrant and bortezomib (Fig 3B). These results suggest that the addition of bortezomib to fulvestrant results in a strong stress signal that activates a proapoptotic UPR.
To determine the effect of the combination, we next carried out an MTT assay on T47D and ZR-75.1 cells treated with bortezomib alone or in combination with fulvestrant. We found that although 12.5 nmol/L bortezomib led to a 30% (Fig. 3C) and 50% (Fig. 3D) reduction in metabolic rate, respectively, addition of fulvestrant reduced the metabolic rates by 60% in T47D cells (Fig. 3C) and 73% in ZR-75.1 cells (Fig. 3D). One-way ANOVA test also revealed that there is statistically significant difference when fulvestrant is combined with higher concentration of bortezomib in both T47D cells (10 and 12.5 nmol/L of bortezomib for P values of 0.0005 and 0.01, respectively) and ZR-75.1 cells (10 and 12.5 nmol/L of bortezomib for P values of 0.001 and 0.03, respectively).
The sensitivity to the fulvestrant–bortezomib was further tested in 2 additional ER+ cell lines, BT474 and MDA-MB-361. We found that BT474 cells were insensitive to both 3 µmol/L fulvestrant alone and 10, 30, and 50 nmol/L bortezomib alone (Fig. 3E). However, when 3 µmol/L fulvestrant was combined to 30 and 50 nmol/L bortezomib, a reduction in metabolic rate was observed and was statistically significant with P values of 0.001 and 0.0001 (Fig. 3E). However, MDA-MB-361 cells were not affected by either drug alone or in combination (data not shown). Considering that the levels of the ER is drastically low in BT474 and MDA-MB-361 cells compared with T47D and ZR-75.1 cells (Fig. 3F), the requirement for increased dose of bortezomib is in fact not surprising. Further, the observation that the level of the ER is even lower in MDA-MB-361 than in BT474 cells, also offers an explanation for the insensitivity of MDA-MB-361 cells.
These results and the fact that fulvestrant acts on the ER predicts that ER− cell lines should be resistant to the fulvestrant–bortezomib combination. To test this possibility the ER− cell lines, MDA-MB-453 and MDA-MD-157, were treated the combination. We found that in both cases, the combination had no significant effect compared with bortezomib alone (Supplementary Data) indicating that the efficacy of the combination is dependent on the expression of the ER.
Collectively, these results suggest that the inhibition of the proteasome that results from treatment with the combination leads to the activation of a proapoptotic UPR.
Autophagy confers resistance to the fulvestrant–bortezomib combination
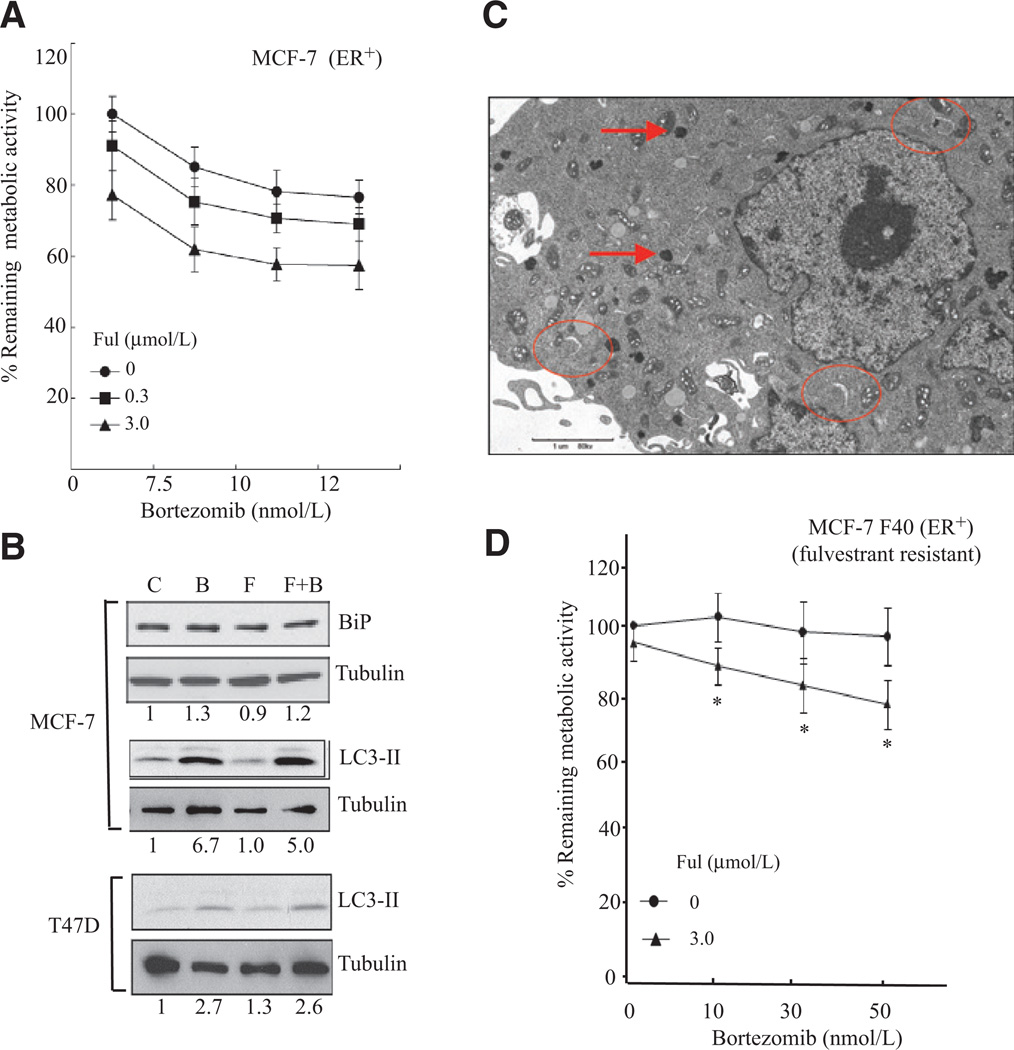
The sensitivity of the ER+ breast cancer cell line MCF-7 was also tested. Although MCF-7 cells were found to be sensitive to fulvestrant alone, the addition of bortezomib did not significantly increased the sensitivity (Fig. 4A). Further, we found that unlike T47D cells (Fig. 3A) BiP was unaffected (Fig. 4B), indicating that the UPR is not activated in MCF-7 cells.
Figure 4.
Resistance to the fulvestrant–bortezomib in ER+ cells correlates with the induction of autophagy. A, the ER+ cell line MCF-7 was treated with increasing doses of bortezomib in absence of fulvestrant (Ful) or in presence of 0.3 and 3 µmol/L fulvestrant for 2 days. Metabolic rate was determined by MTT assay. B, MCF-7 cells were treated with 15 nmol/L of bortezomib (B), 3 µmol/L fulvestrant (F), or both for 2 days and the level of BiP and the autophagy marker LC3-II determined by Western blot analysis. Tubulin was used as a loading control (C). T47D cells were treated the same as MCF-7 cells and the activation of autophagy determined by monitoring the level of LC3-II by Western blot analysis. C, MCF-7 cells were treated with 15 nmol/L of bortezomib and 3 µmol/L fulvestrant for 2 days and the cells fixed for electron microscopy. D, MCF-7-F40 cells were treated with 3 µmol/L fulvestrant alone, increasing doses of bortezomib alone, or in combination with fulvestrant and their metabolic rate determined by MTT assay 2 days after treatment.
We next tested whether autophagy is activated by using the elevation of LC3-II levels as a marker. We found that bortezomib, but not fulvestrant, leads to the elevation in LC3-II in MCF-7 cells (Fig. 4B, middle), whereas there was a minimal elevation in LC3-II levels in T47D cells (Fig. 4B, bottom). Induction of autophagy was confirmed by the detection of arc shape membranes and late autophagosomes by electron microscopy (Fig. 4C). As autophagy represents a mechanism of resistance to proteasome inhibition (17), we concluded that macroautophagy causes resistance to the combination in MCF-7 cells.
We next aimed at defining the sensitivity of fulvestrant-resistant cells to the combination. The MCF-7-F40 line (18) was obtained and tested for its sensitivity. We found that these cells were resistant to both 3 µmol/L fulvestrant alone or increasing doses of bortezomib (Fig. 4D). However, treatment with 3 µmol/L fulvestrant in combination with 30 and 50nmol/L bortezomib reduced their metabolic rate significantly with P values of 0.0008 and 0.0001. Therefore, this result suggests that the fulvestrant–bortezomib combination can restore sensitivity in otherwise fulvestrant-resistant cells.
The bortezomib–fulvestrant combination promotes regression of tamoxifen-resistant breast cancer xenografts
Overexpression of cyclin D1 is associated with resistance to tamoxifen and we confirmed this observation by using T47D-cyclin D1 cells, which overexpress cyclin D1 (14). We tested whether T47D-cyclin D1 cells are sensitive to the fulvestrant–bortezomib combination and found that these cells were the most sensitive, with a 90% decrease in metabolic rate (Fig. 5A). One-way ANOVA test also revealed that there is statistically significant difference when fulvestrant is combined with bortezomib (P values of 0.004, 0.0005, and 0.00045 for 7.5, 10, and 12.5 nmol/L of bortezomib, respectively).
Figure 5.
Tamoxifen-resistant cells are sensitive to the fulvestrant–bortezomib combination. A, the ER+ cell lines T47D-cyclin D1 were treated with increasing doses of bortezomib in absence of fulvestrant (Ful) or in presence of 0.3 and 3 µmol/L fulvestrant for 2 days. Metabolic rate was determined by MTT assay. B, 4 groups of 8 nude mice were injected with 1 × 107 T47D-cyclin D1 cells and tumors allowed to grow for 3 weeks. At week 3, mice were treated with vehicle (control), bortezomib alone (Bor), fulvestrant alone (Ful), or bortezomib plus fulvestrant (F + B). Tumor growth was determined over a period of 7 weeks. The average tumor volume in each group was adjusted to 100 mm3 to allow for a direct comparison of the 4 groups. Statistical difference in tumor volumes between groups was determined by repeated measures ANOVA. C, T47D cells were transfected with the indicated plasmids for 24 hours and the incubated the cells in the presence of 3 µmol/L fulvestrant and 12 nmol/L bortezomib for 1 hour. D, model of the effect of the fulvestrant–bortezomib combination.
The sensitivity of T47D-cyclin D1 cells was further tested by using a xenograft model. Tumors were allowed to growth for 3 weeks and mice were then randomized to 1 of following 4 groups: (i) control, (ii) fulvestrant alone, (iii) bortezomib alone, and (iv) fulvestrant–bortezomib combination. We found that both fulvestrant and bortezomib led to a reduction in the growth rate of the tumors compared with tumors in the untreated group (Fig. 5B). Repeated measures ANOVA statistical analysis revealed P values of 0.031 for control group versus bortezomib group and of 0.043 for the control group versus fulvestrant group. However, tumor volumes were found to regress in the fulvestrant–bortezomib group. Repeated measures ANOVA statistical analysis revealed P values of 0.0019 for control group versus fulvestrant + bortezomib group. Therefore, these results indicate that the fulvestrant–bortezomib combination is effective in this model of tamoxifen resistance.
Activating mutations in the ER have been identified in ER+ but tamoxifen-resistant breast cancer patients (19). However, because the fulvestrant–bortezomib approach relies on the ability to promote toxic aggregates of the ER, we reasoned that mutants ER might be more prone to aggregation as they are misfolded. To test this possibility, we created 2 mutants, Y537N and Y537S, by site directed mutagenesis, transfected these plasmids in T47D cells and incubated the cells in the presence of fulvestrant and bortezomib for 1 hour. We found that although no aggregation of the ER is observed at this time point in cells expressing wild-type ER, aggregation in the cytoplasm is readily detectable in cells expressing mutants ER (Fig. 5C). Therefore, these results support the notion that ER+ but estrogen-independent breast cancers, due to mutation in the ER, maybe sensitive to the fulvestrant–bortezomib combination.
Discussion
Figure 5D shows a model that summarizes the effect of fulvestrant and bortezomib. First, fulvestrant promotes the degradation of the ER in the nucleus, blocking its ability to promote transcription. Second, fulvestrant causes the aggregation of newly synthesized ER in the cytoplasm, which causes inhibition of the proteasome by clogging the lid. Third, bortezomib inhibits the proteasome and increases the ER aggregates formed by fulvestrant. The excessive accumulation of ER aggregates then causes activation of a proapoptotic UPR.
The ability of fulvestrant to promote the degradation of the ER is currently considered the only mechanism by which fulvestrant prevents the growth of ER+ breast cancer cells. Although the ability of fulvestrant to promote the aggregation of the ER in the cytoplasm has been described by several groups (3–5), whether these aggregates play a role in the mode of action of fulvestrant had never been investigated previously. Our data show that these cytoplasmic aggregates of the ER lead to a mild inhibition of the proteasome and the induction of the UPR without the activation of apoptosis. Therefore, fulvestrant activates a cytoprotective UPR, which suggests that the aggregates do not contribute to the mode of action of fulvestrant to prevent the growth of ER+ breast cancer cells. In fact, these observations raise the possibility that the induction of a cytoprotective UPR by fulvestrant may contribute to resistance to fulvestrant. In agreement with this possibility, splicing of XBP1 and activation of NF-κB, 2 cytoprotective pathways downstream of the activation of the UPR have been identified as mechanisms of resistance to fulvestrant (10, 11, 20–22). These results imply that the efficacy of fulvestrant may be limited by its ability to activate these cytoprotective responses.
However, our data also suggest that by adding bortezomib, the activation of a cytoprotective UPR by fulvestrant is converted into a proapoptotic UPR. As bortezomib is known to stabilize the inhibitor of NF-κB and therefore block the activity of NF-κB (23) and that bortezomib was also reported to block the splicing of XBP-1 (24), these observations suggest the switch from a cytoprotective UPR with fulvestrant alone to a proapoptotic UPR with the fulvestrant–bortezomib combination.
Therefore, we propose that bortezomib enhances the efficacy of fulvestrant by acting at 2 distinct points: (i) the enhancement of the fulvestrant-mediated aggregation of the ER and (ii) the inhibition of NF-κB activity and XBP-1 splicing. This unique mode of action represents a strong scientific rational for the design of a clinical trial testing the superiority of the fulvestrant–bortezomib combination over fulvestrant alone in ER+ breast cancer patients that have recurred on prior endocrine therapy.
However, our data also show that although MCF-7 cells were the most sensitive to fulvestrant alone, addition of bortezomib did not significantly increased the efficacy of fulvestrant in these cells. This observation is in agreement with the induction of autophagy rather than the UPR in these cells. This result is consistent with the observations that autophagy contributes to the efficacy of fulvestrant in these cells (25) and induces resistance to bortezomib (17, 26). Therefore, autophagy may be a mechanism by which bortezomib does not enhance the efficacy of fulvestrant.
Our data suggest that the fulvestrant–bortezomib combination works best in tamoxifen-resistant models. The observation that cyclin D1–overexpressing cancer cells are more sensitive than nonoverexpressing cells is likely because of the fact that the threshold of induction of apoptosis by bortezomib is lower in these cells (14). Further, our data raise the possibility that mutations affecting the ER may serve to sensitize cells further to this novel combination by exploiting their altered conformation. If so, ER+ but estrogen-independent cells that are resistant to all form of classical endocrine therapy approach are predicted to be sensitive to the fulvestrant–bortezomib combination.
As for the fulvestrant-resistant MCF-7-F40 cells, we found that they are also resistant to bortezomib suggesting that these cells are very different from MCF-7 cells. This possibility supports the recent hypothesis that antiestrogen resistance does not arise from acquired mutation but rather from selection of a small preexisting population of cells that are intrinsically resistant (18). As we found that addition of higher dose of bortezomib in combination with fulvestrant was able to reverse resistance in these cells, we propose that the fulvestrant-resistant cells may simply have an increased capacity of induction of cytoprotective UPR and that in this context higher dose of bortezomib is required to convert this response to a proapoptotic UPR. Alternatively, high doses of bortezomib were recently shown to cause the complete repression of the transcription of the ER (27). Therefore, by promoting the degradation of the ER by fulvestrant and the inhibition of its transcription by bortezomib, the combination may completely abolish ER signaling in these cells.
In addition, we found that even in cells in which the expression of the ER is very low such as that observed in BT474 cells, the fulvestrant–bortezomib combination was effective although higher doses of bortezomib (30–50 nmol/L) were required. Considering that the serum levels of bortezomib reaches 509 µmol/L (12) after injection and gradually decreases, these higher doses are easily achievable clinically.
In summary, the ability of bortezomib to enhance the efficacy of fulvestrant by at least 2 different mechanisms makes this combination even more attractive.
Translational Relevance.
Endocrine therapy is the most frequent treatment of estrogen receptor (ER)-positive breast cancers. Endocrine therapy acts by repressing the transcriptional activity of the ER. One major difficulty with this approach is that it allows for the selection of mutations that results in the growth of cells that have adapted to low estrogen conditions and causes recurrence. Results presented here suggest that the efficacy of endocrine therapy can be enhanced by using the unique ability of fulvestrant to cause the aggregation of the ER. We describe that by combining fulvestrant to the proteasome inhibitor, bortezomib, the aggregation of the ER results in apoptosis and tumor regression. Fulvestrant is currently approved in postmenopausal women with breast cancers that have recurred on other endocrine therapies. The result presented in this study served as the basis for a phase II randomized trial comparing fulvestrant alone or in combination with bortezomib in this patient population.
Supplementary Material
Acknowledgments
We thank Shachar Shimonovich for technical assistance.
Grant Support
This work was supported by a grant from the Samuel Waxman Cancer Research Foundation and from the Chemotherapy Foundation to D. Germain. L. Papa is supported by NIH/National Cancer Institute training Grant T32 CA78207.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Buzdar AU, Robertson JF. Fulvestrant: pharmacologic profile versus existing endocrine agents for the treatment of breast cancer. Ann Pharmacother. 2006;40:1572–1583. doi: 10.1345/aph.1G401. [DOI] [PubMed] [Google Scholar]
- 2.Stenoien DL, Patel K, Mancini MG, Dutertre M, Smith CL, O’Malley BW, et al. FRAP reveals that mobility of oestrogen receptor-alpha is ligand- and proteasome-dependent. Nat Cell Biol. 2001;3:15–23. doi: 10.1038/35050515. [DOI] [PubMed] [Google Scholar]
- 3.Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106:1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- 4.Htun H, Holth LT, Walker D, Davie JR, Hager GL. Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor. Mol Biol Cell. 1999;10:471–486. doi: 10.1091/mbc.10.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pick H, Jankevics H, Vogel H. Distribution plasticity of the human estrogen receptor alpha in live cells: distinct imaging of consecutively expressed receptors. J Mol Biol. 2007;374:1213–1223. doi: 10.1016/j.jmb.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy. 2008;4:141–150. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- 7.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, et al. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 10.Davies MP, Barraclough DL, Stewart C, Joyce KA, Eccles RM, Barraclough R, et al. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. Int J Cancer. 2008;123:85–88. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- 11.Gu Z, Lee RY, Skaar TC, Bouker KB, Welch JN, Lu J, et al. Association of interferon regulatory factor-1, nucleophosmin, nuclear factor-kappaB, and cyclic AMP response element binding with acquired resistance to Faslodex (ICI 182,780) Cancer Res. 2002;62:3428–3437. [PubMed] [Google Scholar]
- 12.Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, et al. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin Cancer Res. 2004;10:3954–3964. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- 13.Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005;65:3828–3836. doi: 10.1158/0008-5472.CAN-04-3684. [DOI] [PubMed] [Google Scholar]
- 14.Ishii Y, Waxman S, Germain D. Tamoxifen stimulates the growth of cyclin D1-overexpressing breast cancer cells by promoting the activation of signal transducer and activator of transcription 3. Cancer Res. 2008;68:852–860. doi: 10.1158/0008-5472.CAN-07-2879. [DOI] [PubMed] [Google Scholar]
- 15.Callige M, Kieffer I, Richard-Foy H. CSN5/Jab1 is involved in ligand-dependent degradation of estrogen receptor {alpha} by the proteasome. Mol Cell Biol. 2005;25:4349–4358. doi: 10.1128/MCB.25.11.4349-4358.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callige M, Richard-Foy H. Ligand-induced estrogen receptor alpha degradation by the proteasome: new actors? Nucl Recept Signal. 2006;4:e004. doi: 10.1621/nrs.04004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milani M, Rzymski T, Mellor HR, Pike L, Bottini A, Generali D, et al. The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res. 2009;69:4415–4423. doi: 10.1158/0008-5472.CAN-08-2839. [DOI] [PubMed] [Google Scholar]
- 18.Coser KR, Wittner BS, Rosenthal NF, Collins SC, Melas A, Smith SL, et al. Antiestrogen-resistant subclones of MCF-7 human breast cancer cells are derived from a common monoclonal drug-resistant progenitor. Proc Natl Acad Sci U S A. 2009;106:14536–14541. doi: 10.1073/pnas.0907560106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25:869–898. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 20.Gomez BP, Riggins RB, Shajahan AN, Klimach U, Wang A, Crawford AC, et al. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J. 2007;21:4013–4027. doi: 10.1096/fj.06-7990com. [DOI] [PubMed] [Google Scholar]
- 21.Pratt MA, Bishop TE, White D, Yasvinski G, Menard M, Niu MY, et al. Estrogen withdrawal-induced NF-kappaB activity and bcl-3 expression in breast cancer cells: roles in growth and hormone independence. Mol Cell Biol. 2003;23:6887–6900. doi: 10.1128/MCB.23.19.6887-6900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riggins RB, Zwart A, Nehra R, Clarke R. The nuclear factor kappa B inhibitor parthenolide restores ICI 182,780 (Faslodex; fulvestrant)-induced apoptosis in antiestrogen-resistant breast cancer cells. Mol Cancer Ther. 2005;4:33–41. [PubMed] [Google Scholar]
- 23.McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–179. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke R, Shajahan AN, Riggins RB, Cho Y, Crawford A, Xuan J, et al. Gene network signaling in hormone responsiveness modifies apoptosis and autophagy in breast cancer cells. J Steroid Biochem Mol Biol. 2009;114:8–20. doi: 10.1016/j.jsbmb.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu K, Dunner K, Jr, McConkey DJ. Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene. 2009;29:451–462. doi: 10.1038/onc.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers GL, Ellison-Zelski SJ, Casa AJ, Lee AV, Alarid ET. Proteasome inhibition represses ERalpha gene expression in ER+ cells: a new link between proteasome activity and estrogen signaling in breast cancer. Oncogene. 2009;29:1509–1518. doi: 10.1038/onc.2009.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.