Abstract
Background
Molecular and cellular events that resulted in leukemia development are well characterized but initial engraftment and proliferation of leukemic cells in bone marrow and early modifications of the bone marrow microenvironment induced by engrafted leukemic cells remain to be clarified.
Design and Methods
After retro-orbital injection of 1,000 leukemic cells expressing Mixed Lineage Leukemia-Eleven Nineteen Leukemia fusion protein in non-conditioned syngenic mice, kinetics of leukemic burden and alterations of femoral hematopoietic populations were followed using an in vivo confocal imaging sytem and flow cytometry.
Results
Three days after injection, 5% of leukemic cells were found in femurs. Little proliferation of engrafted leukemic cells could then be detected for more than two weeks while the number of femoral leukemic cells remained stable. Twenty days after injection, leukemic cells preferentially proliferated in femoral diaphysis where they formed clusters on the surface of blood vessels and bone. B220+ lymphoid cells were found near these leukemic cell clusters and this association is correlated with a decreased number of femoral B220+IgM+ cells. Increasing the number of injected leukemic cells or conditioning recipient mice with γ-irradiation resulted in leukemic cell development in diaphysis and knee. Competition experiments indicate that proliferation but not engraftment is a rate-limiting factor of leukemic cells spreading in diaphysis. Finally, 30 days after injection leukemia developed.
Conclusions
After retro-orbital injection of 1,000 leukemic cells expressing Mixed Lineage Leukemia-Eleven Nineteen Leukemia into syngenic mice, leukemic cell burden preferentially initiates in femoral diaphysis and is preceded by changes of femoral B-lymphoid populations.
Keywords: MLL-ENL, leukemic cells, in vivo imaging, hematopoiesis
Introduction
Cell migration, homing and proliferation occur during solid tumor and metastasis formation and during leukemogenesis. After migration, solid tumor cells or leukemic cells often find an appropriate niche in bone marrow and there is increasing evidence to indicate that the bone marrow microenvironment plays a critical role in the development and progression of malignancies evolving in the bone marrow. Cues from bone marrow microenvironment can affect lineage determination of transformed hematopoietic cells, as exemplified by human CD34+ cord blood cells transformed with the MLL-AF9 oncogene. These cells can develop into acute lymphoblastic leukemia or acute biphenotypic leukemia when transplanted into NOD/SCID/β2 microglobulin null mice, but develop only into acute myeloid leukemia when transplanted in NOD/SCID mice that express the human cytokines SCF, GM-CSF and IL-3.1 On the other hand, tumor development in the bone marrow can affect the regulation of normal hematopoiesis and/or alters hematopoietic stem cell (HSC) niches. In a mouse xenograft model of Nalm-6 pre-B acute lymphoblastic leukemia, leukemic cells can alter the bone marrow niches and impact normal hematopoietic progenitor cell interactions with the microenvironment, reducing the hematopoietic progenitor cell pool2 while likely favoring leukemic cell development. In a syngenic Notch-induced T-cell leukemia model, leukemia development was also associated with progressive suppression of hematopoiesis. But in this model, leukemic mice have more quiescent HSCs and more cycling progenitors leading to suppression of hematopoiesis by exhaustion of hematopoietic progenitors.3 Taken together, these results highlight the constant cross talk between bone marrow microenvironment, hematopoiesis and leukemia development.
One of the most common chromosomal breakpoint regions associated with human leukemias occurs at chromosome 11q23. This results in balanced translocations that fused the Mixed Lineage Leukemia (MLL) gene, in frame, with many partner genes in both human acute myeloid leukemia (AML)1,4 and human acute lymphoid leukemia (ALL).5 The Mixed Lineage Leukemia-Eleven Nineteen Leukemia (MLL-ENL) translocation is found in human B-lineage ALLs and myelomonocytic AMLs.6–8 Transplantation of mouse hematopoietic progenitors transduced by a retrovirus that can express MLL-ENL mRNA consistently generates AML in mice6 demonstrating the powerful transforming capabilities of the fusion gene alone once it is expressed in a susceptible cell background. Recently, MEF2C, a member of the myocyte enhancer factor 2 (MEF2) transcription factor, has been shown to regulate homing and invasiveness of MLL-ENL leukemic cells without contributing to the establishment or maintenance of leukemic stem cells generated by MLL-ENL fusion protein9 indicating the relevance of this leukemia model to study leukemic cell invasion of bone marrow.
Initial engraftment and proliferation of leukemic cells in normal bone marrow and early modifications of bone marrow microenvironment induced by engrafted leukemic cells have not been well characterized due to the problems in studying, in vivo at the cellular level, the initial events that occur during bone marrow burden by leukemic cells. Techniques derived from optical microscopy2,10–14 have shown the presence of leukemic cells in the bone marrow niches11 but these observations have been made after injection of a high number of leukemic cells and/or use of irradiated or immunodeficient mice.2,14–15 Moreover, the spreading of leukemic cells in long bones could not be followed in vivo at the cellular level as very few imaging systems are able to follow labeled cells in long bones.16–17 We recently developed an in vivo imaging system that consists of a confocal endoscope which can detect, in live mice, a single fluorescent cell in the cavity of long bones and at the surface of different organs.16 We used this imaging system to study the early steps of hematopoietic reconstitution by hematopoietic stem cells in long bones after lethal irradiation.16 We now used this system to investigate the early steps of leukemia development inside the femur. After retro-orbital injection of 1,000 GFP+ MLL-ENL murine leukemic cells into non-conditioned syngenic mice, we studied the kinetics and topology of leukemic cell burden in the femoral bone marrow. We also studied endogenous and exogenous regulations of leukemia development in the femur and identified possible cross talks between leukemic cells and normal hematopoietic progenitors.
Design and Methods
Mice and leukemic cell injection
We housed 129/Sv mice (8–10 weeks old) in the CEA/DSV/iRCM animal facility. Animal care was in accordance with French Government procedures (Services Vétérinaires de la Santé et de la Production Animale, Ministère de l’Agriculture, France). Murine leukemic cells that express the human MLL-ENL fusion protein4,6 were transduced with a lentivirus expressing GFP (MND-GFP lentivirus)18 and amplified in vivo in syngenic 129/Sv mice (Table 1). Mice with more than 95% of leukemic cells in their bone marrow were used to obtain leukemic cells. Non-conditioned 129/Sv mice were anesthetized by isoflurane gas inhalation and indicated numbers of syngenic murine GFP+ or CFSE-labeled leukemic cells16 were given by retro-orbital injection. Conditioning by 3.5 Gy γ-irradiation was performed using a 60Co irradiator.
Table 1.
In vivo MLL-ENL leukemic cell expansion.
In vivo imaging
Fiberoptic imaging system was used as previously described.16 Video data were acquired with the CellVizio 488 and analyzed with Image Cell software (Mauna Kea TechnologiesTM, France). Details of the visualization method and data analysis are shown in the Online Supplementary Design and Methods and Video 1.
Flow cytometry analysis of leukemic and hematopoietic cells
To assess GFP+ or fluorescent labeled-leukemic cell distribution within the femur, animals were euthanized at different time points. The femurs were removed, cleaned and cut in 3 sections: knee, diaphysis and femoral head. Cutting points were always positioned 4 mm from the two ends of the femur. Bone marrow of each compartment was flushed with phosphate buffered saline. After red blood cell lysis with Ammonium Chloride Solution (STEMCELL Technologies, Vancouver, BC, Canada) cells were counted (Online Supplementary Table S1) and analyzed by cytometry for GFP, CFSE or CellTrace violet fluoresence. For in vivo cell proliferation analyses, MLL-ENL leukemic cells were stained with 0.5mM of CFSE or CellTrace violet according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). CFSE or CellTrace violet staining was quantified by FACS analysis before injection and the labeled leukemic cells were then injected into non-conditioned 129/Sv mice.
Hematopoietic cells from each femoral compartment were immunophenotyped using anti-CD3-APC, -Gr-1-APC, -CD11b-PE (BD Pharmingen, San Diego, CA, USA) and anti-IgM-APC, -B220-PE-Cy7 (eBioscience, San Diego, CA, USA) antibodies. Cytometry data were acquired on a CYAN cytometer (Dako, Glostrup, Denmark) and analyzed with the FlowJo software (Tree Star).
Hematopoietic cell transplantation
YFP+ hematopoietic cells, obtained from 8–10 week old 129/S (7AC5/EYFP) mice by flushing bone marrow from femur and tibia with phosphate buffered saline were centrifuged. The pellet was resuspended in Hanks balanced salt solution (HBSS) supplemented with 10% fetal calf serum (FCS). YFP+ hematopoietic cells were stained with biotinylated anti-Lin antibody cocktail (Miltenyi Biotec, Bergisch Gladbach, Germany), anti–Sca-1-PECy7 and anti–CD117 (cKit)-APC (BD Biosciences) antibodies for 30 min at 4°C. The biotinylated antibodies were developed with streptavidin (SA)–eFluor 450 (eBioscience). Lin-Sca+c-Kit+ (KLS) YFP+ hematopoietic cells were sorted on an Influx Cell Sorter (BD Biosciences). Retro-orbital transplantation of 10,000 KLS was performed in non-conditioned 129/Sv recipient mice. At indicated times, the femoral bone marrow underwent FACS analysis as described above.
Immunohistochemistry
Femurs were removed, cleaned, put in a 4% formol solution for 72 h at room temperature and then kept at 4°C. Decalcification of femoral bones was performed by electrolysis with a TPE30 Decalcifer solution (Sakura Finetek, The Netherlands) for 3 h. Femurs were then immediately oriented to produce a longitudinal 4 μm-depth slice when cut with the Nitrom HM340E (Sakura) and placed in paraffin. The wax was cleared and slides were placed in the Retriever 2100 (Proteogenix, Oberhausbergen, France) to unmask antigens, treated with 5% BSA for 30 min, and incubated with anti-EGFP antibody (Abcam, Cambridge, UK) overnight at 4°C. A secondary impress anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA) was added on slides and revealed with 3,3’-Diaminobenzidine (DAB) (Vector Laboratories). Slides were incubated with anti-CD45R/B220 antibody (eBioscience) overnight at 4°C for 2-color staining, revealed with secondary anti-rat biotinylated antibody and Vector-VIP (Vector Laboratories). After hematoxyline counter-coloration, cover-slides were mounted using a Eukitt mounting medium and slides were stored in the dark. Pictures were taken with a microscope equipped with an Olympus optical camera.
Statistical analysis
Statistical analysis was performed by two-tailed t-test (Graphpad inStat Software).
Results
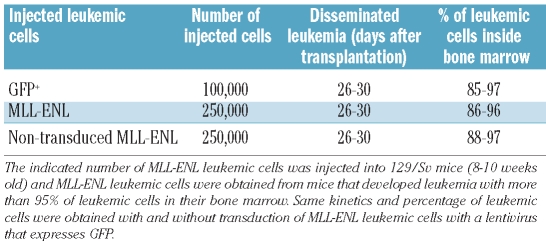
To investigate the early steps of leukemia development, we transplanted 1,000 GFP+ MLL-ENL leukemic cells into non-conditioned 129/Sv mice by retro-orbital injection. Development of leukemic cells in the femur was studied by in vivo confocal endoscopy16 over 30 days following the injection of cells, i.e. before the complete dissemination of the leukemia. Three days after injection, 5% of the GFP+ leukemic cells could be detected in the two femurs. There was no great increase in this number of GFP+ leukemic cells for more than 17 days (Figure 1A). Twenty to 25 days after injection, an increased number of GFP+ leukemic cells could be observed in femoral diaphysis but not epiphyses (Figure 1A, Online Supplementary Video 2). Thirty days after injection, GFP+ leukemic cells invaded the femurs (Figure 1A) and were detected in liver and spleen (Figure 1A). GFP+ leukemic cells were only detected in the bloodstream at 25 days (data not shown) and less than 200 GFP+ leukemic cells were detected in the spleen at Day 25 (data not shown; Figure 1A) suggesting that femurs were the sites where leukemic cells could initially proliferate. Quantification of the frames that contained leukemic GFP+ cells 17–30 days after injection showed a higher number of frames containing GFP+ cells in femoral diaphysis at Days 20 and 25 post injection (Figure 1B). Quantification of the total number of GFP+ leukemic cells by FACS analysis showed 3 times more leukemic cells in diaphysis compared to knee at Day 17 (Figure 1C). This ratio increased at Days 20 and 25 (10 and 20 times more GFP+ leukemic cells in diaphysis than knee) indicating a preferential proliferation of GFP+ leukemic cells in diaphysis (Figure 1C). At Day 30, a similar number of GFP+ leukemic cells could be detected in diaphysis and knee indicating dissemination of these leukemic cells in femurs (Figure 1C). Similar results were obtained when 1,000 GFP+ leukemic cells were injected into non-conditioned 129/Sv mice through the jugular vein (Online Supplementary Figure S1A) indicating leukemic cell distribution/engraftment efficiency was not dependent on injection site.
Figure 1.
Kinetics of femoral leukemia development after injection of 1,000 MLL-ENL leukemic cells into non-conditioned 129/Sv mice. One thousand GFP+ MLL-ENL leukemic cells were intravenously injected into non-conditioned 129/Sv syngenic mice. The data are representative of 5 individual experiments with 4 mice per day of acquisition. (A) Left panel: pictures represent frames of 20s to 35s videos acquired over the length of the femur and show GFP+ leukemic cells in the knee, diaphysis and femoral head areas 3, 6, 13, 17, 20, 25 and 30 days after injection (scale bar 20 μm). Right panel: twenty, 25 and 30 days after injection of GFP+ leukemic cells, videos of spleen and liver were performed to track GFP+ cells. Pictures represent frames of 20s to 35s videos acquired at the surface of these organs and show the GFP+ leukemic cells observed. (B) The graphics shown below represent the fluorescence intensity (RFU, Relative Fluorescence Units) detected across the femur by the 10,000 optical fibers per individual video frame. The vertical strokes indicate different positions of the microprobe tip in the femoral cavity. Only RFU in excess of a value of 75 is considered a positive signal. (C) Histograms show the total number of GFP+ leukemic cells for each femoral compartment at 17, 20, 25 and 30 days after injection. These numbers were obtained by multiplying cell numbers of the three femoral compartments by the percentage of GFP+ leukemic cells of each femoral compartment determined by flow cytometry and represent mean ± SEM (n=20). (D) Histograms show the total number of YFP+ hematopoietic cells for each femoral compartment at 5, 15 and 25 days after transplantation of 10,000 YFP+ KLS hematopoietic cells in non-conditioned 129/Sv recipient mice. These numbers were obtained by multiplying cell numbers of the three femoral compartments by the percentage of YFP+ hematopoietic cells of each femoral compartment determined by flow cytometry and represent mean ± SEM (n=5). (E) Fifteen days after transplantation of 10,000 YFP+ KLS hematopoietic cells, percentages of immature (B220+IgM−) and mature B cells (B220+IgM+) and monocytes/granulocytes (CD11b+Gr1+, CD11b+Gr1−, CD11b−Gr1+) were determined in each femoral compartment (n=5).
To study if the development of leukemia in diaphysis was due to the leukemic origin of the cells or to the absence of conditioning, we transplanted, by retro-orbital injection, non-conditioned 129/Sv mice with 10,000 YFP+ c-kit+, lin−, Sca+ (KLS) cells enriched in hematopoietic stem cells (HSCs). Five days after transplantation, YFP+ hematopoietic cells could be detected in knee and diaphysis (Figure 1D). The increase in number of YFP+ hematopoietic cells was similar in knee, diaphysis and femoral head 15 days after transplantation. This number decreased 25 days after transplantation and no YFP+ hematopoietic cells could be detected 30 days after transplantation (Figure 1D; data not shown). Increasing the number of YFP+ KLS injected cells up to 25,000 did not change engraftment kinetics and, as for 10,000 YFP+ KLS injected cells, no YFP+ hematopoietic cells could be detected 30 days after transplantation (Online Supplementary Figure S1B; data not shown). Qualitative analyses of hematopoietic cells present 15 days after transplantation showed mostly mature myeloid cells with similar percentages in the three femoral compartments (Figure 1E) whereas no KLS cells could be detected (Online Supplementary Figure S1B). These results indicated that transplantation of KLS cells into non-conditioned 129/Sv mice did not mimic the preferential engraftment and initial proliferation of MLL-ENL leukemic cells in the femoral diaphysis.
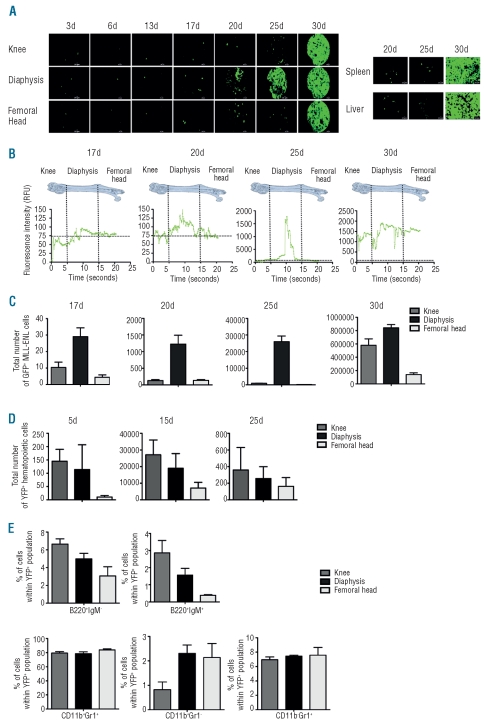
To characterize parameters that regulate leukemic cell development in femurs, we first determined the effects of increasing the number of injected leukemic cells on the development of leukemia. Compared to injection of 1,000 leukemic cells, injection of 5,000 (resp. 10,000) leukemic cells resulted in a 10-, 7-, 37- and 5- (resp. 20-, 10-, 66- and 7-) fold increase in the numbers of leukemic cells at Days 17, 20, 25 and 30 (Figure 2A). Thus, increasing the number of injected leukemic cells resulted in a burst of proliferation of leukemic cells between 20 and 25 days after injection. When 5,000 (resp. 10,000) leukemic cells were injected, 350 (resp. 800) leukemic cells were detected in diaphysis and 100 (resp. 250) leukemic cells were detected in knee 17 days after injection (Figure 2B). Twenty days after injection, the ratio of leukemic cells in diaphysis versus knee (3 and 2.5 for 5,000 or 10,000 leukemic cells injected, respectively) was similar to that observed 17 days after injection indicating the same proliferation of leukemic cells in diaphysis and knee and/or migration of leukemic cells from diaphysis to knee (Figure 2B). Compared to the number of leukemic cells found 20 days after injection of 1,000 leukemic cells, a 17- (resp. 30) fold increase in the number of leukemic cells in knee was found when 5,000 (resp. 10,000) leukemic cells were injected whereas a 6- (resp. 8) fold increased number of leukemic cells in diaphysis was found when 5,000 (resp. 10,000) leukemic cells were injected (Figure 2B). These results show that increasing the number of injected leukemic cells resulted in proliferation and/or spreading of leukemic cells in knee. Twenty-five days after injection of 5,000 (resp. 10,000) compared to 1,000 leukemic cells, a 300- (resp. 750) and 25- (resp. 37) fold increase in numbers of leukemic cells were found in knee and diaphysis, respectively (Figure 2B) whereas 30 days after injection, these ratios fell to 5 (Figure 2B) indicating an early burst of proliferation of leukemic cells when 5,000 or 10,000 leukemic cells were injected. Similar results were obtained when 10,000 GFP+ leukemic cells were injected into non-conditioned 129/Sv mice through the jugular vein (Online Supplementary Figure S1C). Altogether, these results show that increasing the number of injected leukemic cells resulted in leukemia development in knee and diaphysis, and faster initial leukemia development kinetics.
Figure 2.
Femoral leukemia development after injection of 1,000, 5,000 or 10,000 MLL-ENL leukemic cells into non-conditioned or after injection of 1,000 MLL-ENL leukemic cells into 3.5 Gy irradiated 129/Sv mice. One thousand, 5,000 or 10,000 GFP+ MLL-ENL leukemic cells were injected intravenously into non-conditioned 129/Sv syngenic mice (A and B). (A) Histograms show the total number of GFP+ leukemic cells found in the 2 femurs at 17, 20, 25 and 30 days after injection. These numbers were obtained by multiplying total bone marrow cell number by the percentage of GFP+ leukemic cells determined by flow cytometry and represent mean ± SEM (n=5 for each indicated day). (B) Histograms show the total number of GFP+ leukemic cells for each femoral compartment at 17, 20, 25 and 30 days after injection and represent mean ± SEM (n=5 for each indicated day). (C) One thousand GFP+ MLL-ENL leukemic cells were intravenously injected into non-conditioned (control) or conditioned (3.5Gy) 129/Sv syngenic recipient mice. Histograms show the total number of GFP+ leukemic cells for each femoral compartment at 17, 20, 25 and 30 days after injection and represent mean ± SEM (n=5 for each indicated day).
The role of the microenvironment was studied by conditioning transplanted mice using a 3.5Gy γ-irradiation. When 1,000 GFP+ leukemic cells were injected into mice previously irradiated at 3.5Gy, 200 (resp. 100) leukemic cells were detected in knee (resp. diaphysis) 17 days after injection indicating that sub-lethal irradiation at 3.5Gy did modify the number and localization of leukemic cells in femurs (Figure 2C). Twenty days after injection, the number of leukemic cells in diaphysis was 2-fold higher than in knee indicating preferential proliferation of leukemic cells in diaphysis (Figure 2C). In addition, an invariable 3-fold higher number of leukemic cells in diaphysis at Days 17, 20, 25 and 30 post injection was observed in irradiated mice compared to non-irradiated mice indicating a similar proliferation of engrafted leukemic cells in irradiated and non-irradiated recipient mice (Figure 2C). This preferential proliferation of the engrafted leukemic cells in diaphysis was observed using different doses of sublethal irradiation (data not shown). These results indicate that irradiation could alter microenvironment of knee and diaphysis that become more permissive to leukemic cell engraftment but did not modify the preferential proliferation of the engrafted leukemic cells in diaphysis.
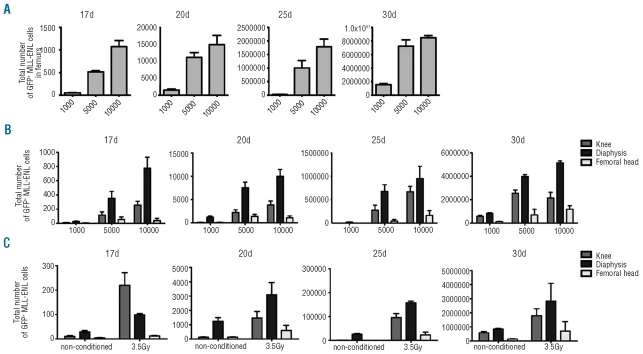
The kinetics of MLL-ENL leukemia development in femurs indicated a possible latency period of two weeks with no or low proliferation of a limited number of GFP+ leukemic cells or an active proliferation/apoptosis of leukemic cells during this period. To detect proliferation of engrafted leukemic cells during the latency period, MLL-ENL leukemic cells were labeled with 5-carboxyfluorescein diacetate succinimidyl ester (CFSE) before retro-orbital injection. CFSE dye did not impair the ability of labeled leukemic cells to develop leukemia one month after injection of 1,000 CFSE-labeled MLL-ENL leukemic cells (data not shown). Imaging of femurs 5 and 15 days after injection of 1,000 CFSE-labeled MLL-ENL leukemic cells showed a similar number of CFSE+ cells compared to the number of GFP+ leukemic cells found 5 and 15 days after injection of 1,000 GFP+ MLL-ENL leukemic cells indicating little proliferation of leukemic cells during this period (Online Supplementary Figure S2A). FACS analyses of total femoral bone marrow supported the imaging data and also showed a 1.5 increase in the total number of CFSE+ leukemic cells in the knee and femoral head during this 2-week period and a 1.2-fold decrease of CFSE+ leukemic cells in diaphysis indicating a possible migration of the CFSE-labeled leukemic cells from diaphysis to epiphyses (Online Supplementary Figure S2B). To detect proliferation of GFP+ leukemic cells, these cells were labeled before injection with CellTrace violet dye. This can be distinguished from GFP fluorescence by FACS analysis. Five days after injection of 1,000 violet-labeled GFP+ MLL-ENL leukemic cells, violet labeled leukemic cells could only be detected in diaphysis and quantification of CellTrace violet fluorescence intensities indicated that most of the leukemic cells in diaphysis had undergone 2 divisions (Figure 3). Ten days after injection, proliferation of injected leukemic cells could be detected in diaphysis whereas leukemic cells detected in the knee and the femoral head have a very weak if any CellTrace violet intensity (Figure 3) and a similar pattern of CellTrace violet fluorescence was detected fifteen days after injection (Figure 3) suggesting initial engraftment and proliferation of leukemic cells in diaphysis and spreading of proliferative leukemic cells from diaphysis to epiphyses. Twenty days after injection, i.e. when a burst of leukemic cells occurred, no violet positive cells could be detected in knee, diaphysis or femoral head (Figure 3). Together with those obtained with GFP+ leukemic cells, these results indicated that during the 15 days after injection MLL-ENL leukemic cells have limited proliferation capacity mostly in femoral diaphysis and might spread inside the femoral cavity.
Figure 3.
Kinetics of proliferation of engrafted MLL-ENL leukemic cells after CFSE labeling. Flow cytometric analysis of CellTrace violet labeled GFP+ leukemic cells detected in knee, diaphysis and femoral head of non-conditioned 129/Sv mice injected with 1,000 CellTrace violet labeled GFP+ MLL-ENL leukemic cells. Upper panels: FACS analyses of GFP and violet fluorescence 5, 10, 15 and 20 days after injection. Lower panels: mean fluorescence of violet labeled GFP+ leukemic cells (black line) or unlabeled GFP+ leukemic cells (dash line) 5, 10, 15 and 20 days after injection. The data are representative of 3 experiments with 5 mice per day of acquisition.
We performed competition experiments by injection of 1,000 GFP+ MLL-ENL leukemic cells into non-conditioned 129/Sv mice challenged, three days later, by a second injection of 9,000 non-labeled MLL-ENL competitor leukemic cells (Online Supplementary Figure S3). Twenty days after injection of GFP+ leukemic cells, i.e. 17 days after injection of non-labeled leukemic cells, the number, fluorescence intensity mean and preferential location in diaphysis of GFP+ leukemic cells were similar to those observed in the absence of competitor leukemic cells indicating that initial homing and engraftment of the GFP+ leukemic cells were not disturbed by the competitor leukemic cells (Online Supplementary Figure S3; Figure 1C). Five days later, i.e. when leukemic cells proliferated, the presence of competitor leukemic cells resulted in 3 times less GFP+ leukemic cells detected in diaphysis together with a diminished fluorescence mean (Online Supplementary Figure S3; Figure 1C; Online Supplementary Video 3) indicating that some limiting factors might delay but not abolish proliferation of competitor leukemic cells. Thirty days after injection of GFP+ leukemic cells, i.e. 27 days after injection of non-labeled leukemic cells, 10 times less GFP+ leukemic cells were found in diaphysis than after injection of 1,000 MLL-ENL GFP+ leukemic cells without competitor leukemic cells (Online Supplementary Figure S3; and Figure 1C). As expected, co-injection of 1,000 GFP+ MLL-ENL leukemic cells and 9,000 non-labeled MLL-ENL competitor leukemic cells at Day 0 resulted in an average of 10 times dilution of GFP+ leukemic cells in knee and diaphysis after Day 20, i.e. when cells proliferated (data not shown). Altogether, these results strengthened the preferential development of leukemia in diaphysis when 1,000 leukemic cells are injected and suggested there are some limiting factors that might regulate proliferation of leukemic cells.
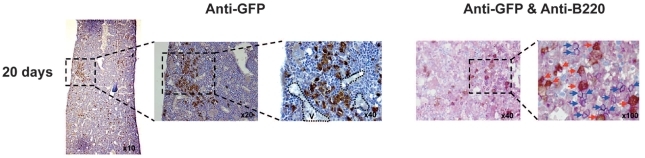
Bone marrow cellularity was unchanged during the latency period of two weeks (data not shown) indicating that leukemic cells did not quantitatively modify hematopoiesis during this period. To precisely locate GFP+ MLL-ENL leukemic cells within diaphysis, we performed imunohistochemistry using an anti-GFP antibody. Twenty days after injection of 1,000 GFP+ MLL-ENL leukemic cells, GFP+ cells were mainly found in diaphysis (Figure 4) where they seemed to spread from clusters of GFP+ leukemic cells edging blood vessels and in contact with the bone (Figure 4) indicating a possible bone marrow microenvironment permissive for MLL-ENL leukemia development. No GFP+ leukemic cells were observed in knee and femoral head (Online Supplementary Figure S4). Labeling of hematopoietic mature cells that could be associated with these clustered leukemic cells revealed mainly B220+ cells in the vicinity of GFP+ clusters (Figure 4; data not shown). To find any physiological consequences of this co-localization, FACS analysis of KLS and mature bone marrow cells was performed 5, 10 and 15 days after injection of 1,000 GFP+ MLL-ENL leukemic cells. This analysis showed a decrease in number of mature B cells (B220+IgM+) starting at Day 10 and reaching a 2-fold decrease at Day 15 in knee, diaphysis and femoral head (Online Supplementary Figure S5) together with an unchanged number of KLS (data not shown), T cells (CD3+), monocytes/granulocytes (CD11b+Gr1+) and immature B cells (B220+IgM−) (Online Supplementary Figure S5). Interestingly, increasing the number of injected MLL-ENL leukemic cells did not modify the kinetics and rate of reduction of mature B cells (data not shown). These results suggest that leukemic cells might induce change in femoral hematopoietic populations and/or might borrow hematopoietic cell niches probably associated with the establishment of a microenvironment favorable for their development.
Figure 4.
Changes in femoral hematopoietic populations during MLL-ENL leukemia development. Immunohistochemistry of GFP+ leukemic cells (brown cells) in diaphysis of 129/Sv mice 20 days after injection of 1,000 GFP+ leukemic cells. The three pictures show a leukemic cluster enlarged 10, 20 and 40 times, respectively (discontinued black line). Vessels (V) are delimited by black dot lines.
Discussion
The predominant growth of leukemic cells over the normal hematopoietic cells is a common feature shared by most types of leukemias and results in suppression of normal hematopoiesis as disease develops. This predominant growth depends on cell intrinsic factors, such as genetic events that are necessary and sufficient to generate development of leukemias, and cell extrinsic factors, such as leukemia established in locations that are favorable for its growth. MLL-rearranged leukemias represent about 10% of all leukemia cases and more than 70 different MLL fusion partner genes have been characterized.19 The MLL-ENL fusion oncogene is a major cell intrinsic factor of leukemic cells as it can transform mouse or human HSCs or hematopoietic progenitors. This resulted in acute lymphoid leukemia when human hematopoietic progenitor cells expressing MLL-ENL were transplanted into immuno-deficient mice5 or in acute myeloid leukemia when mouse HSCs or myeloid progenitors expressing MLL-ENL were transplanted into syngenic and SCID recipient mice.20–21 MLL-ENL induced acute myeloid leukemia can be serially transplanted in vivo.6
We used injection of 1,000 MLL-ENL leukemic cells into non-conditioned syngenic mice to model leukemia relapse that can occur when migrating residual leukemic cells engrafted bone marrow although leukemia relapse could also be accounted for by leukemic cells that were already embedded in the bone marrow. We used an in vivo fiberoptic confocal endoscope that can detect a single fluorescent cell in the cavity of the long bones of live mice and on the surface of different organs16 to study the early steps of leukemia development after injection of 1,000 GFP+ MLL-ENL leukemic cells into non-conditioned syngenic mice. As early as three days after injection, few GFP+ MLL-ENL leukemic cells could be detected in knee, diaphysis and femoral head indicating no preferential initial homing of these leukemic cells in the different parts of the femur. This homogeneous initial homing was followed by a latency period of 15 days with little proliferation of the engrafted leukemic cells and a preferential proliferation in the diaphysis 17–25 days after injection. This preferential initial leukemia development in diaphysis was due to the leukemic origin of the cells as transplantation of normal hematopoietic cells enriched in HSCs (KLS hematopoietic cells) resulted in similar homing and differentiation of the transplanted cells in knee and diaphysis. Finally, increasing the number of injected leukemic cells or conditioning recipient mice by γ-irradiation resulted in an initial leukemia development in knee and diaphysis. This indicates a possible saturation of the niches present in diaphysis that might provide a permissive microenvironment for leukemia development and/or migration of leukemic cells from the diaphysis to the knee. It could also indicate a γ-irradiation mediated modification of the knee microenvironment that would become more permissive for leukemia development. The effect of γ-irradiation on femoral leukemic cell localization contrasted with the specific recruitment of KLSCD34− hematopoietic cells enriched in HSCs in the femoral head but not in the femoral diaphysis or knee when KLSCD34− hematopoietic cells were transplanted into lethally irradiated mice.16 This indicates a different type of engraftment of normal and leukemic cells in femurs. Finally, even when 200 times more leukemic cells were injected into mice (Table 1), leukemia developed between 26 and 30 days after injection, i.e. not much before leukemia development after injection of 1,000 leukemic cells. Leukemic cells found in all organs studied was the parameter used to score leukemia development and we did not perform any survival curve due to ethical considerations. Thus, whether increasing the number of injected leukemic cells resulted in differential survival is unknown.
Co-injection of 1,000 GFP+ MLL-ENL leukemic cells with 9,000 competitive non-labeled MLL-ENL leukemic cells changed the femoral distribution of the co-injected GFP+ MLL-ENL leukemic cells that were observed in diaphysis and knee. However, this co-injection did not modify the kinetics of GFP+ MLL-ENL leukemic cell development in diaphysis when compared to leukemia development after injection of 1,000 GFP+ MLL-ENL leukemic cells. In contrast, injecting 9,000 competitive non-labeled MLL-ENL leukemic cells three days after a first injection of 1,000 GFP+ MLL-ENL leukemic cells resulted in an unaffected proliferation of the GFP+ MLL-ENL leukemic cells in diaphysis until Day 20 post injection with little proliferation in knee. This time point corresponds to 17 days after injection of the 9,000 competitive non-labeled MLL-ENL leukemic cells; a time point at which little proliferation could be observed, as verified when 10,000 MLL-ENL leukemic cells were injected. These results indicate a time regulated development of leukemia in femurs with no effects of engrafted leukemic cells on proliferating leukemic cells during the latency period. The increase in GFP+ MLL-ENL leukemic cells was much greater 20–30 days after injection than in the presence of competitive MLL-ENL leukemic cells. These competitive cells are highly proliferative during this period and might inhibit proliferation of previously engrafted leukemic cells. Thus, early and late stages of leukemia development might require different bone marrow environmental cues and might have different actions on surrounding hematopoietic or stromal cells; a phenomenon previously observed in a Notch1-induced leukemia model.3
Twenty days after injection of 1,000 GFP+ MLL-ENL leukemic cells, clusters of MLL-ENL leukemic cells were seen near blood vessels at the diaphysis endosteum. These microdomains are reminiscent of bone marrow niches and could create a favorable environment for development and spreading of leukemic cells.12 These vascularized microdomains are similar to those where B cells have been observed22 suggesting a possible competition between leukemic cells and B-cell progenitors for specialized niches. This leukemic cell localization correlated with a gradual decrease in the number of B220+IgM+ mature B cells together with an unaltered number of B220+IgM− immature B cells before any high proliferation of the engrafted leukemic cells. Inflammation has been shown to specifically reduce bone marrow stem cell factor (SCF) and CXCL12 to levels that do not support B-lymphopoiesis but remain sufficient for continued granulopoiesis.23 This decreased B-lymphopoiesis gradually liberates bone marrow niches to be occupied by myeloid cells.23 Our study indicated that a similar phenomenon might occur during the early stages of the development of a myeloid leukemia. Leukemic cells might be located near B220+IgM− immature B cells and might impair the differentiation of these immature B cells into B220+IgM+ mature B cells.
This study raises questions on the nature of the initial events that occur during leukemia development. The number of injected leukemic cells had no impact on the latency period. This was always followed by an intense proliferation of the engrafted leukemic cells indicating that, during this period, leukemic cells might modify the bone marrow microenvironment to create or colonize niches where they can proliferate and/or might increase vascularization by inducing neoangiogenesis.24 Focusing on these changes might offer new approaches to prevent leukemia relapse.
Acknowledgments
The authors would like to thank J. Rebouillat for technical support (Mauna Kea Technologies) and Bertrand Tavitian and Frédéric Ducongé for the development of the confocal imaging system. We are grateful to the staff of the iRCM animal colony for excellent support in mouse studies.
Footnotes
Funding: AJR is supported by fellowships from the CEA. This project was supported by grants from La Fondation de France (UB:032145/N.:2009002630), ARC (3527), Inserm and CEA/DSV. The LRTS team is recognized by the “Ligue contre le Cancer”.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wihelm JS, et al. Micro-environment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13(6):483–95. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322(5909):1861–5. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 3.Hu X, Shen H, Tian C, Yu H, Zheng G, XuFeng R, et al. Kinetics of normal hematopoietic stem and progenitor cells in a Notch1-induced leukemia model. Blood. 2009;114(18):3783–92. doi: 10.1182/blood-2009-06-227843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somervaille TCP, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–68. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Barabé F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316(5824):600–4. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- 6.Lavau C, Szilvassy SJ, Slany R, Cleary ML. Immortalization and leukemic transformation of a myelomonocytic precursor by retro-virally transduced HRX-ENL. EMBO J. 1997;16(14):4226–37. doi: 10.1093/emboj/16.14.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Luo RT, Mi S, Sun M, Chen P, Bao J, et al. Consistent deregulation of gene expression between human and Murine MLL rearrangement leukemias. Cancer Res. 2009;69(3):1109–16. doi: 10.1158/0008-5472.CAN-08-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horton SJ, Walf-Vorderwülbecke V, Chatters SJ, Sebire NJ, de Boer J, Williams O. Acute myeloid leukemia induced by MLL-ENL is cured by oncogene ablation despite acquisition of complex genetic abnormalities. Blood. 2009;113(20):4922–9. doi: 10.1182/blood-2008-07-170480. [DOI] [PubMed] [Google Scholar]
- 9.Schwieger M, Schüler A, Forster M, Engelmann A, Arnold MA, Delwel R, et al. Homing and invasiveness of MLL/ENL leukemic cells is regulated by MEF2C. Blood. 2009;114(12):2476–88. doi: 10.1182/blood-2008-05-158196. [DOI] [PubMed] [Google Scholar]
- 10.Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457(7225):92–6. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–70. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 12.Sipkins DA, Wei X, Wu JW, Runnels JM, Côté D, Means TK, et al. In vivo imaging of specialized bone marrow endothelial micro-domains for tumour engraftment. Nature. 2005;435(7044):969–73. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Askenasy N, Farkas DL. Optical imaging of PKH-labeled hematopoietic cells in recipient bone marrow in vivo. Stem Cells. 2002;20(6):501–13. doi: 10.1634/stemcells.20-6-501. [DOI] [PubMed] [Google Scholar]
- 14.Ailles LE, Gerhard B, Kawagoe H, Hogge DE. Growth characteristics of acute myelogenous leukemia progenitors that initiate malignant hematopoiesis in nonobese diabetic/severe combined immunodeficient mice. Blood. 1999;94(5):1761–72. [PubMed] [Google Scholar]
- 15.Dick JE, Lapidot T. Biology of normal and acute myeloid leukemia stem cells. Int Journal Hematol. 2005;82(5):389–96. doi: 10.1532/IJH97.05144. [DOI] [PubMed] [Google Scholar]
- 16.Lewandowski D, Barroca V, Ducongé F, Bayer J, Van Nhieu JT, Pestourie C, et al. In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood. 2010;115(3):443–52. doi: 10.1182/blood-2009-05-222711. [DOI] [PubMed] [Google Scholar]
- 17.Köhler A, Schmithorst, Filippi MD, Ryan MA, Daria D, Gunzer M, et al. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood. 2009;114(2):290–8. doi: 10.1182/blood-2008-12-195644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunet de la Grange P, Armstrong F, Duval V, Rouyez MC, Goardon N, Romeo PH, et al. Low SCL/TAL1 expression reveals its major role in adult hematopoietic myeloid progenitors and stem cells. Blood. 2006;108(9):2998–3004. doi: 10.1182/blood-2006-05-022988. [DOI] [PubMed] [Google Scholar]
- 19.Meyer C, Kowarz E, Hofmann J, Renneville A, Zuna J, Trka J, et al. New insights to the MLL recombinome of acute leukemias. Leukemia. 2009;23(8):1490–9. doi: 10.1038/leu.2009.33. [DOI] [PubMed] [Google Scholar]
- 20.Cozzio A, Passegué E, Ayton PM, Karsunky H, Cleary ML, Weissman IL. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17(24):3029–35. doi: 10.1101/gad.1143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.So CW, Karsunky H, Passegué E, Cozzio A, Weissman IL, Cleary ML. MLL-GAS7 transforms multipotent hematopoietic progenitors and induces mixed lineage leukemias in mice. Cancer Cell. 2003;3(2):161–71. doi: 10.1016/s1535-6108(03)00019-9. [DOI] [PubMed] [Google Scholar]
- 22.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6(2):107–16. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 23.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201(11):1771–80. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayala F, Dewar R, Kieran M, Kalluri R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23(12):2233–41. doi: 10.1038/leu.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]