Abstract
Background
Primary immune thrombocytopenia is a bleeding diathesis with an unknown etiology in predisposed individuals with immune disturbances. Although it is claimed that children and adults differ in clinical and laboratory aspects, few data exist to corroborate this observation. Our objective was to assess comparative data from children and adults with newly diagnosed immune thrombocytopenia.
Design and Methods
Clinical and laboratory data of 1,784 children and 340 adults were extracted from the Pediatric and Adult Registry on Chronic Immune Thrombocytopenia. The registry represents a prospective cohort of children and adults with newly diagnosed immune thrombocytopenia. Participating investigators registered their patients immediately after the diagnosis using a web based data transfer. Children aged under 16 years were compared with adults aged 16 years and over with descriptive statistical analyses.
Results
The presenting mean platelet count of children and adults was 18.1 and 25.4×109/L. Signs of bleeding were reported in 24% of children and in 23% of adults, and intracranial hemorrhage in 10 of 1,784 children and in 6 of 340 adults. Co-morbidity was observed in 3.9% of children and in 30% of adults. Bone marrow aspiration and laboratory tests (antinuclear antibodies, human immunodeficiency and hepatitis C virus) were performed more frequently in adults. Children and adults were followed with a ‘watch and wait’ strategy in 20% and in 29%, respectively. Immunoglobulins were used more frequently in children and corticosteroids in adults.
Conclusions
Comparative data of children and adults with newly diagnosed immune thrombocytopenia revealed similarities in presenting platelet counts and in bleeding, whereas differences occurred in co-morbidity, diagnostic procedures and therapy.
Keywords: immune thrombocytopenia, newly diagnosed, similarities, differences, comparative data
Introduction
Immune thrombocytopenia (ITP), formerly known as idiopathic or immune thrombocytopenic purpura, is an acquired bleeding diathesis resulting from premature platelet destruction, reduced platelet production or a combination of both.1,2 Primary ITP is defined as isolated thrombocytopenia in the absence of an identified etiology or illness. Secondary ITP assumes the presence of a concurrent underlying disorder responsible for disturbed immune function leading to thrombocytopenia. The list of such disorders is extensive and may include autoimmune diseases (e.g. systemic lupus erythematosus or antiphospholipid syndrome), lymphoproliferative disorders, and chronic infections (e.g. Helicobacter pylori, human immunodeficiency virus or hepatitis C virus) in addition to many others.3 ITP occurs across all age groups. The estimated incidence in children is approximately 1.9 to 6.4 cases per 100,000 per year and for adults 3.3 per 100,000 per year.4 Retrospective data, consensus statements, expert opinion and textbooks suggest that childhood and adult onset ITP have distinctly different laboratory findings and clinical features.5–9
Due to the rarity of ITP, and the paucity of prospective clinical trial data, the Intercontinental Cooperative ITP Study Group (ICIS) was founded in 1997 by an international panel of hematologists with the goal of establishing a worldwide network of physicians and researchers to collect data to better define the natural history of childhood ITP, in addition to questions concerning diagnosis and therapy, including early predictors of chronic ITP (www.itpbasel.ch). In 2002, ICIS established the Pediatric and Adult Registry on Chronic ITP (PARC ITP). This international, multi-center registry was designed to collect prospective laboratory and clinical data from children and adults with newly diagnosed ITP and to follow them continuously over time. The information in the database will be used to compare the laboratory and clinical features of pediatric and adult ITP, to analyze the heterogeneity and natural history of the disorder across the different age groups, to validate its diagnosis and management, and to identify new patient selection criteria for future trials.
This investigation is restricted to the query of the PARC ITP Registry assessing clinical findings at the time of initial diagnosis, with the aim of comparing the differences between children and adults with ITP. The findings suggest that the differences observed between the two age groups are smaller than expected.
Design and Methods
Registry design
The structure of the Registry is similar to its predecessors; ICIS Registry I10 and ICIS Registry II.11 Patient registration is based on voluntary participation by physicians involved in the management of patients with ITP worldwide. Information about the Registry was made available on the internet (www.itpbasel.ch), at scientific conferences and symposia, meetings organized by ICIS, and publication of regular newsletters and journal supplements. The ICIS questionnaires are case based, designed to collect prospective clinical and laboratory data at first presentation and throughout the course of ITP, and intended to generate hypotheses with the potential to add side-studies to the main database. The PARC-ITP Registry opened in May 2004 to obtain prospective data on the clinical presentations, natural history, and clinical course of children older than two months of age and adults with newly diagnosed ITP. Data was submitted electronically directly to the ICIS coordinating office in Basel, Switzerland via the internet to a secure, password protected access site (www.parc-itp.net), which was developed and is actively managed by the ICIS coordinating office. Data was compiled and stored using Microsoft Access 2003. The registry protocol received ethical approval by the Swiss Ethics Committee in Basel, Switzerland (reference EKBB 235/03) and by the local internal review boards and ethics committees of participating institutions, as per local requirements.
Registration process
Patients could be registered if the clinical impression of the local treating physician was that the diagnosis was consistent with ITP and the platelet count was less than 150×109/L. The study protocol included the practice guidelines which were published at that time.6,7,12 However, diagnosis, clinical criteria, and treatment decisions were left to the participating physician. A platelet count of less than 100×109/L was not used, as the newer definitions of ITP3 were published later.
Questionnaires
The questionnaire included demographic information, date of diagnosis, blood counts at diagnosis, family history of thrombocytopenia, co-morbid conditions (none, hypertension, diabetes, gastrointestinal disease, thyroid disease, cancer, alcohol abuse, cardiovascular disease and splenomegaly), concurrent medications (none, platelet antagonists, vitamin K antagonists, other anticoagulants, and anti-inflammatory agents), bleeding symptoms, details of laboratory diagnosis, transfusions, splenectomy, and medicinal treatments (intravenous immunoglobulins, corticosteroids, anti-D immunoglobulin). Details of laboratory diagnosis included complete blood counts, whether bone marrow aspiration was performed, and serology for antiphospholipid, antinuclear, and platelet associated antibodies, HIV, hepatitis C and Helicobacter pylori (positive or negative test results). The information on methodology for each test was not collected.
Statistical analysis
Descriptive statistical analyses were used to characterize comparisons of children (age <16 years) with adults (age ≥ 16 years). PARC-ITP represents a registry with prospective observation without protocol based diagnostic or therapeutic interventions. As it reflects the actual clinical practice of a multinational group of volunteer investigators there is no mechanism guaranteed to provide a balance of the investigated parameters, thus limiting the value of formal statistical tests. To evaluate the differences in the medians between the two age groups, the Mann-Whitney U test was used when the data was assumed not to be normally distributed. Fisher’s exact test was used for classified, qualitative variables. Data were analyzed with STATA, Version 10. Due to the characteristics of this analysis, statistical inferences were not made and any P value should only be interpreted as an indication that the differences between the observed values in each age group reach a level of significance. The data is presented in this way to enable identification of clinical or laboratory features which may differ in children and adults, and to identify therapies which may be preferred in one group or the other.
Results
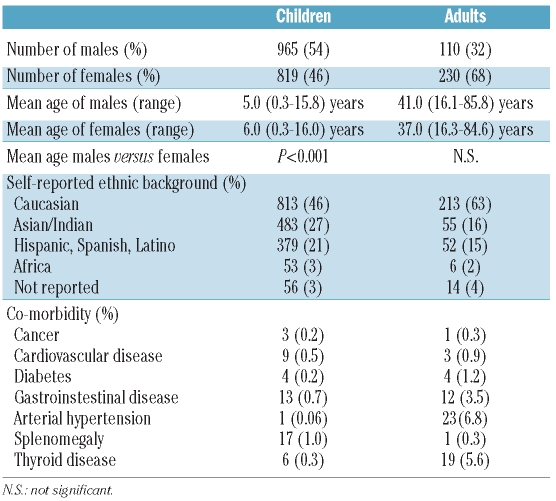
At the time of this query, data was provided by 84 investigators representing 74 participating sites in 31 countries (see Acknowledgements). A total of 2,124 patients with primary ITP were registered in the PARC-ITP Registry between May 1 2004 and March 20 2009. The number of patients enrolled varied by participating site: more than 20 patients were enrolled by 29 investigators, 11–20 patients by 10, 6–10 patients by 11, and less than 5 patients by 15. Patients were divided into two groups according to age. Children were defined as patients between the age of three months to 16 years, and adults by an age of 16 years or over. There were 1,784 (84%) children and 340 (16%) adults. The mean age of children was 5.4 years (range 0.3–16 years) and that of adults was 39.0 years (range 16.1–85.8 years). See Table 1 for patients’ characteristics.
Table 1.
Patients’ characteristics.
Self-reported ethnic background of the patients is presented in Table 1. The percentage of adults was higher in Caucasians and smaller in the other groups. In children, the male:female ratio was in favor of males, with female patients being older (P=0.001). In adults, there were more females, with no difference in age between males and females.
Co-morbidity was observed in 69 of 1,784 children (3.9%) and in 102 of 340 adults (30%) (P<0.0001). More than one co-morbid condition was reported in one child and in 33 adults. Splenomegaly was reported in 17 children and one adult, challenging the diagnosis of primary ITP. Further details of the co-morbid conditions were not requested. Patients with a co-morbid condition are described in Table 1. Diabetes, gastrointestinal disease, arterial hypertension and thyroid disease occurred more often in adults. Cancer, cardiovascular disease, and splenomegaly were observed in both age groups at similar frequencies.
Concurrently used medications at the time of diagnosis were found more commonly in adults than children. Nineteen of 1,784 children were taking anti-inflammatory drugs at the time of diagnosis of ITP. None were taking platelet antagonists, vitamin K antagonists, or other anticoagulants. In the 340 adults, 17 were prescribed anti-inflammatory medications, 11 platelet antagonists, 4 vitamin K antagonists, and 2 were taking other anticoagulants.
A family history of thrombocytopenia was identified in 42 of 1,784 children (2%) and in 9 of 340 adults (3%).
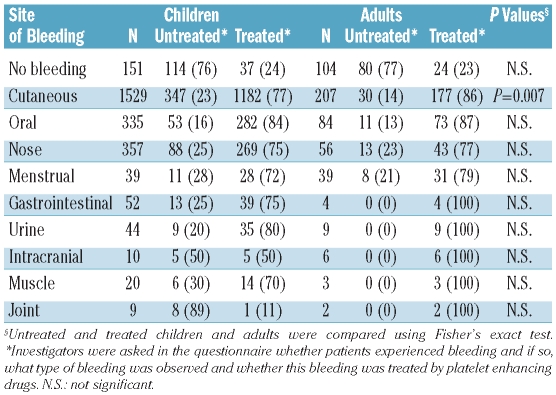
Clinical signs of bleeding were assessed by location (no symptoms, cutaneous, oral cavity, nose, menstrual, gastrointestinal, urine, intracranial, muscle, and joint) and if the specific bleeding event required medicinal treatment (intravenous immunoglobulins, corticosteroids, anti-D or combination therapy) (Table 2). Bleeding manifestations (any site) were observed more frequently in children (1,629 patients, 91%) than in adults (236 patients, 69%) (P<0.0001).
Table 2.
Bleeding signs and treatment of children and adults (number with percentage in parentheses).
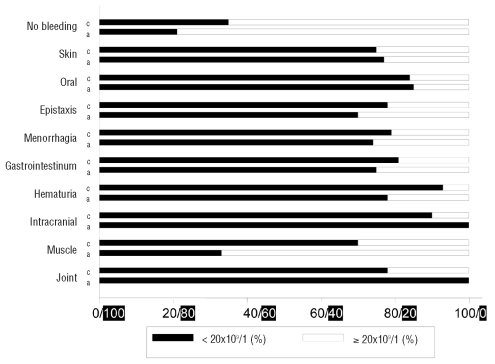
A similar percentage of children and adults who had no reported signs of bleeding received some form of platelet enhancing treatment; 24% (37 of 151 children) and 23% (24 of 104 adults). Intracranial hemorrhage occurred in 10 of 1,784 children (0.6%) and in 6 of 340 adults (1.8%) (not significant). The mean age of children with intracranial hemorrhage was five years and that of adults was 60 years. The outcome of these patients remains unknown. When bleeding signs were analyzed in patients with a presenting platelet count less than or equal/higher than 20×109/L. a similar occurrence of bleeding was observed, except for muscle bleeds, which occurred more frequently at lower platelet counts (Figure 1).
Figure 1.
Black bars (from left, 0%, to the right, 100%) indicate patients with a presenting platelet count of <20x109/L, and white bars (from right, 0%, to the left, 100%) patients with a presenting platelet count of ≥20x109/L. c: children, a: adults.
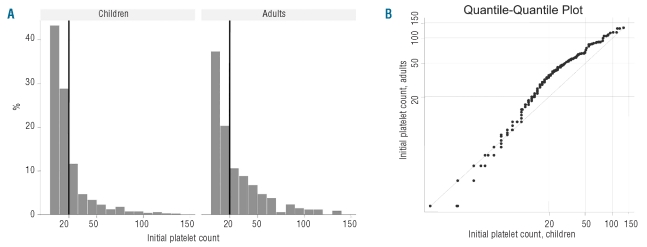
The mean platelet count at diagnosis was 18.1 (range 0–137)×109/L for children and 25.4 (range 0–133) ×109/L for adults. The distribution of the platelet count is shown in Figure 2A. A platelet count of less than 20×109/L was found more often in children (1,286 of 1,784 children, 79%) than in adults (186 of 340 adult patients, 58%) (P<0.0001). To compare similarity or dissimilarity of the platelet count distribution between children and adults, a quantile-quantile plot was calculated (Figure 2B). This suggests a strong similarity of platelet counts in the low range (platelet count 0–15×109/L) of both age groups and slightly higher platelet counts in adults compared to pediatric patients above this value.
Figure 2.
(A) Platelet count of children and adults at the time of ITP diagnosis. The vertical line indicates a platelet count of 20x109/L. (B) Platelet counts of children and adults at the time of the diagnosis of ITP are plotted as quantile-quantile plot to compare the distribution of platelet counts of children and adults. A logarithmic scale was chosen in order to highlight platelets <50x109/L. With equal size of the number of observations (N) for children and adults, each data set is ranked from the smallest to the highest value. This gives N pairs (x,y) from (xmin/ymin) to (xmax/ymax) and each pair is a point in the quantile-quantile plot. If the two distributions are similar, the points occur approximately on the diagonal of the plot. For unequal size of the two data sets interpolation to the same quantile is used.
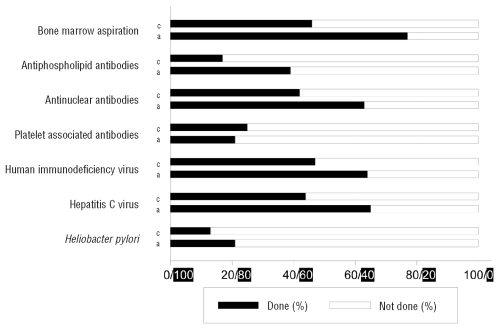
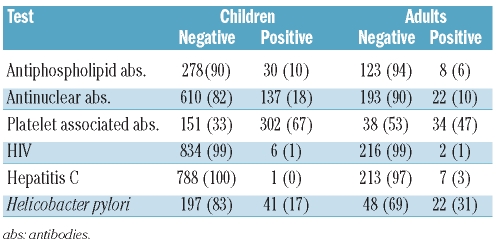
The choice of diagnostic procedure and frequency of testing differed among children and adults (Figure 3). Bone marrow examination, antinuclear antibody, HIV and hepatitis C testing were performed more frequently (P<0.0001) in adults than in children. Bone marrow examinations were performed more frequently in patients treated with corticosteroids in contrast to other treatments in both children (78%) and adults (85%). Antiphospholipid antibody, platelet associated antibody and Helicobacter pylori testing were performed in less than 50% of patients in both age groups. Laboratory test results (children vs. adults) were similar for HIV (1% vs. 1%), while testing for HCV (0% vs. 3%) and Helicobacter pylori (17% vs. 31%) was positive more often in adults (Table 3). Antinuclear (18% vs. 10%) and platelet associated antibodies (67% vs. 47%) were more frequently positive in children than in adults, whereas antiphospholipid antibodies were present in children and in adults at a similar frequency (10% vs. 6%).
Figure 3.
Black bars (from left, 0%, to the right, 100%) indicate the percentage of patients in whom a given laboratory test was performed and white bars (from right, 0%, to the left, 100%) indicate the percentage of patients in whom that given test was not performed. Test results are presented in Table 3. c: children, a: adults.
Table 3.
Laboratory test results at first presentation of children and adults (%)
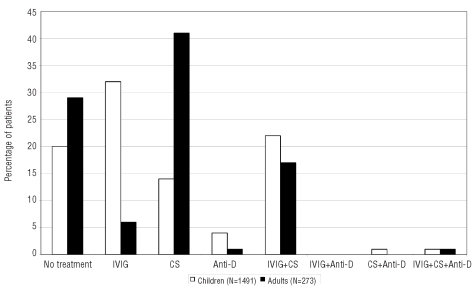
Management of children and adults is summarized in Figure 4. Immunoglobulins were used more frequently in children, whereas adults received corticosteroids more frequently.
Figure 4.
Percentage of children (white bars) and adults (black bars) is shown according to their initial management: observation without active treatment, intravenous immunoglobulins (IVIG), corticosteroids (CS), anti-D antibodies (anti-D).
A sub-group analysis of children and adults who did not receive any platelet enhancing drug treatment at first presentation identified 364 children (20%) and 100 adults (29%). The mean age of this sub-group was 6.2 years in children and 38.8 years in adults. Bleeding manifestations (any site) occurred in 250 (69%) children and in 20 (20%) adults (P<0.001). If data are analyzed in patients with a presenting platelet count of less than 20×109/L, bleeding occurred in 98 children and in 3 adults. Intracranial hemorrhage was not observed in this sub-group. Overall co-morbidity was found in 16 (4%) children and in 34 (34%) adults (P<0.001). The presenting mean platelet count was 45.5×109/L in children and 57.9×109/L in adults (not significant).
Discussion
Clinical and laboratory characteristics of children and adults with newly diagnosed ITP have not been studied systematically. Clinical impression and the published literature suggest that there are differences in the clinical and laboratory aspects of pediatric and adult ITP,6,8 with variable disease presentations, clinical outcomes, and responses to treatment. Knowledge of the similarities and differences that may exist have the potential to affect clinical practice and provide a starting point for further study of the underlying etiologies and pathophysiological causes in each age group. The PARC-ITP Registry is the first prospective collection of such clinical data, and the first which allows for direct comparison of the comparative clinical and laboratory characteristics of ITP in children and adults. In this analysis of the PARC-ITP database, the clinical and laboratory findings at initial presentation were studied and compared.
At the time of this analysis, the majority of the subjects in the Registry were children. The first ICIS registries were exclusively pediatric; consequently there was greater early involvement by pediatric investigators. However, the pro-portion of adult data being submitted is increasing with time, and it is expected that later queries should reflect a greater balance. Although the adult data represent only a fraction of the total, 340 subjects is a large enough sample size to make valid comparisons to the much larger pediatric population. Our data support and confirm some previously reported observations, including the predominance of males in the pediatric age group, whereas female gender dominates among adult patients.13–15 This analysis suggests that autoimmune disorders, which are associated with female gender,16 may be an explanation for the greater number of adult women with ITP. Reasons for the observed high proportion of males in childhood ITP remain unknown.10,14–15
An important finding of this analysis is the huge overlap in the clinical and laboratory features of ITP at first diagnosis among children and adults. Similarities are observed in presenting platelet counts, incidence and type of bleeding when platelet counts are less than 20×109/L, and reported family history of thrombocytopenia. Although bleeding occurred more frequently in children than in adults, the decision to treat was similar for both age groups. The severity of bleeding events cannot be compared since bleeding scores were not requested.
Not unexpectedly, concurrent use of medication and co-morbid disorders were reported more often for adults. In both adults and children, gastrointestinal disease was the most common associated co-morbid condition. Of note, 17 children were reported to have splenomegaly. The presence of co-morbidities, splenomegaly, and positive family history of ITP among children and adults points to the heterogeneity of ITP and suggests that the classification into primary and secondary ITP needs critical reconsideration.
With the exception of the high incidence of co-morbid conditions in adults, greatest dissimilarities between pediatric and adult ITP were different approaches to diagnostic evaluation and treatment. Adult patients had laboratory testing more often than children (Figure 3). Surprisingly, a similar proportion of children and adults were observed without active treatment, in contrast to previously published reports.6,8 When treatment was prescribed, intravenous immunoglobulins and anti-D were used more frequently in children, while corticosteroids dominated in adults.
A registry is a scientific tool with advantages and disadvantages. As a consequence the data collected by the PARC-ITP do have some limitations. A great advantage of a registry is the ability to use it collaboratively, in a multinational setting, to collect data from a large number of patients with rare disorders in a relatively short interval of time. The information gathered can then be used to develop scientific questions for further study, and the database adapted to focus on specific areas of study based on new ideas or important findings. It also serves as a valuable tool to develop better definitions of inclusion and exclusion criteria for clinical study by clarifying the characteristics of the population to be evaluated. Although these benefits are well suited to the study of ITP, there are some disadvantages. These include the large numbers of different individuals entering data, reducing inter-rater reliability and data quality, as well as the inability to directly compare the data to that collected in a clinical trial. This Registry was case based and was dependent upon voluntary contributions from investigators with very different clinical practices across 31 countries. Many factors may have an impact on this. PARC-ITP has worked to maximize data quality with regular assessment of information being submitted, ongoing refinement of the software, scheduled interim analyses, and knowledge of participating investigators and their sites.
In summary, it is increasingly recognized that ITP represents a spectrum of disorders, all having in common the immune mediated destruction or impaired production of platelets resulting in thrombocytopenia. Different pediatric and adult ITP have overlapping clinical characteristics and the differences have been emphasized. The results of this analysis suggest that the differences at initial presentation may be less than previously recognized. Children and adults have comparable initial platelet counts, bleeding symptoms with platelet counts less than 20×109/L, and similar likelihood to be treated for bleeding. The PARC-ITP Registry data may serve as a source of information to understand the demographic, clinical and laboratory aspects of ITP, and to serve as a base for designing future clinical trials.
Acknowledgments
We thank all patients who continue to participate in the PARC-ITP Registry and the staff of the participating institutions. We are indebted to Verena Stahel, Caroline Asal Martin and Monika Imbach for data administration and secretarial work. We thank all investigators of the PARC-ITP Registry who support ICIS and generously provided us with their patient data.
Appendix
The list of participating physicians (Intercontinental Cooperative ITP Study Group (ICIS) investigators) is as follows:
Argentina: Donato Hugo, Elena Graciela, Espina Bibiana, Graciera Alfonso, Kohan Regina, Lavergne Marta, Picón Armando Oscar, Pierdominici Marta, Rapetti Maria Cristina, Riccheri Cecilia, Schvartzman Gabriel. Austria: Minkov Milen, Trebo Monika. Belarus: Uglova Tatjana. Cambodia: Devenish Robyn, Sophâl Chean. Canada: Blanchette Victor, Cham Bonnie, Eisenstat David, Israels Sara, Klaassen Robert, Lee David, Lillicrap David, McCusker Patricia, Silva Mariana, Yanofsky Rochelle. China: Wu Runhui, Yu Ziqiang. Croatia: Roganovic Jelena. Denmark: Kjaersgaard Mimi. Ecuador: Sghirla Juan. Egypt: AL-Tonbary Youssef, Elalfy Mohsen, Fouda Ashraf, Khadiga Yehia. France: Lutz Patrick. Germany: Erkel Joseph, Groth Renate, Holzhauer Susanne, Hummler Markus, Janssen Gisela, Meyer Oliver, Salama Abdulgabar, Schütz Barbara, Taube Tillmann, von Stackelberg Arend. Greece: Aronis Sophie, Platokouki Helen. Iran: Faranoush Mohammad. Israel: Koren Ariel, Levin Carina, Revel-Vilk Shoshana, Shraga Aviner, Tamary Hannah, Yacobovich Joanne. Italy: Cattaneo Marco, Fortuna Stefania, Ruggeri Marco. Japan: Bessho Fumio. Korea South: Choe Byung-Kyu, Kim Heung-Sik, Kwon Ki-Young, Park Sang-Kyu. Netherlands: Koene Harry. Pakistan: Adil Salman Naseem, Alidina Amin, Fadoo Zehra, Farrukh Ali, Kakepoto Ghulam Nabi, Khurshid Mohammad, Naz Naveen, Sajida Ali Muhammad, Shaikh Mohammed Usman, Vaziri Irfan. Poland: Niewiadomska Edyta, Zawilska Krystyna. Russia: Abdukadyrov Kudrat, Chistyakova Vera, Pshenichnaya Ksenia. Serbia & Montenegro: Colovic Milica, Elezovic Ivo, Todorovic Milena, Vukovic Suvajdzic Nada. South Africa: Wainwright Linda. Sri Lanka: Vidyatilake Sudharma. Switzerland: Brazzola Pierluigi, Gratwohl Alois, Kühne Thomas, Rischewski Johannes, Tichelli André. Thailand: Chuansumrit Ampaiwan. Turkey: Aydinok Yesim, Balkan Can, Kavakli Kaan. United Kingdom: Grainger John. USA: Bennett Carolyn, Bergstrom Steven, Bottner Wayne A, Boudreaux Jeanne, Chitlur Meera, Cole Craig, Ettinger Robert S, Farnen John P, Felgenhauer Judy, Go Ronald, Green David, Guerrera Michael, Inoue Susumu, Jacobs Shana, Lockhart Sharon, Luchtman-Jones Lori, Michaels Lisa, Neier Michelle, Nugent Diane, Onwuzurike Nkechi, Ramdas Jagadeesh, Reynolds Frank, Shaffer Linda, Sharp James C, Sprehe Michael R, Stout Linda A, Tancabelic Jakica, Tarantino Michael D, Wells Donlad T, Zakarija Anaadriana.
Footnotes
Funding: the registry is supported in part by grants from the ITP Foundation Darien, CT, USA, Eduard Waeffler-Ludwig Stiftung, Basel, and University Children’s Hospital Basel, University of Basel, Switzerland.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Cines DB, McMillan R. Pathogenesis of chronic immune thrombocytopenic purpura. Curr Op Hematol. 2007;14(5):511–4. doi: 10.1097/MOH.0b013e3282ba5552. [DOI] [PubMed] [Google Scholar]
- 2.Imbach P, Lazarus AH, Kühne T. Intravenous immunoglobulins induce potentially synergistic immunomodulations in autoimmune disorders. Vox Sanguinis. 2010;98(3):385–94. doi: 10.1111/j.1423-0410.2009.01264.x. [DOI] [PubMed] [Google Scholar]
- 3.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura (ITP) of adults and children. Report from an international working group. Blood. 2009;113(11):2386–93. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 4.Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol. 2009;85(3):174–80. doi: 10.1002/ajh.21616. [DOI] [PubMed] [Google Scholar]
- 5.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346(13):995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 6.George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for The American Society of Hematology. Blood. 1996;88(1):3–40. [PubMed] [Google Scholar]
- 7.British Committee for Standards in Haematology General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol. 2003;120(4):574–96. doi: 10.1046/j.1365-2141.2003.04131.x. [DOI] [PubMed] [Google Scholar]
- 8.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–86. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 9.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 10.Kühne T, Imbach P, Bolton-Maggs PHB, Berchtold W, Blanchette V, Buchanan GR for the Intercontinental Childhood ITP Study Group. Newly diagnosed idiopathic thrombocytopenic purpura in childhood: an observational study. Lancet. 2001;358:2122–5. doi: 10.1016/S0140-6736(01)07219-1. [DOI] [PubMed] [Google Scholar]
- 11.Neunert CE, Buchanan GR, Imbach P, Bolton-Maggs PHB, Bennett CM, Neufeld EJ, et al. Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood. 2008;112(10):4003–8. doi: 10.1182/blood-2008-03-138487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eden OB, Lilleyman JS. Guidelines for management of idiopathic thrombocytopenic purpura. The British Paediatric Haematology Group. Arch Dis Child. 1992;67(8):1056–8. doi: 10.1136/adc.67.8.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kühne T, Buchanan GR, Zimmerman S, Michaels LA, Kohan R, Berchtold W, et al. for the Intercontinental Childhood ITP Study Group. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from The Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143(5):605–8. doi: 10.1067/s0022-3476(03)00535-3. [DOI] [PubMed] [Google Scholar]
- 14.Sutor AH, Harms A, Kaufmehl K. Acute immune thrombocytopenia (ITP) in childhood: retrospective and prospective survey in Germany. Semin Thromb Hemost. 2001;27(3):253–67. doi: 10.1055/s-2001-15255. [DOI] [PubMed] [Google Scholar]
- 15.Shirahata A, Fujisawa K, Ishii E, Ohta S, Sako M, Takahashi Y, et al. A nationwide survey of newly diagnosed childhood idiopathic thrombocytopenic purpura in Japan. J Pediatr Hematol Oncol. 2009;31(1):27–32. doi: 10.1097/MPH.0b013e318190d44e. [DOI] [PubMed] [Google Scholar]
- 16.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10(8):594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]