Abstract
Background
Recovery of thymopoiesis after allogeneic hematopoietic stem cell transplantation is considered pivotal for full immune competence. However, it is still unclear to what extent insufficient recovery of thymopoiesis predicts for subsequent opportunistic infections and non-relapse mortality.
Design and Methods
A detailed survey of all post-engraftment infectious complications, non-relapse mortality and overall survival during long-term follow-up was performed in 83 recipients of allogeneic stem cell grafts after myeloablative conditioning. Recovery of thymopoiesis was assessed using analysis of signal joint T-cell receptor rearrangement excision circles. The impact of recovery of thymopoiesis at 2, 6, 9 and 12 months post-transplantation on clinical outcome beyond those time points was evaluated by univariate and multivariate Cox regression analyses.
Results
The cumulative incidence of severe infections at 12 months after transplantation was 66% with a median number of 1.64 severe infectious episodes per patient. Patients in whom thymopoiesis did not recover were at significantly higher risk of severe infections according to multivariable analysis. Hazard ratios indicated 3- and 9-fold increases in severe infections at 6 and 12 months, respectively. Impaired recovery of thymopoiesis also translated into a higher risk of non-relapse mortality and outweighed pre-transplant risk factors including age, donor type, and disease risk-status.
Conclusions
These results indicate that patients who fail to recover thymopoiesis after allogeneic hematopoietic stem cell transplantation are at very high risk of severe infections and adverse clinical outcome.
Keywords: thymopoiesis, opportunistic infections, transplantation outcome, TREC
Introduction
Immune reconstitution after allogeneic hematopoietic stem cell transplantation (HSCT) is a complex process involving various components of the innate and adaptive immune system.1,2 While early reconstitution is mainly characterized by restoration of mucosal barriers, neutrophil recovery and natural killer (NK)-cell recovery, reconstitution in the later post-transplant period is dominated by recovery of newly developed T cells. Two main pathways of T-cell regeneration contribute to post-transplant T-cell recovery: thymopoiesis and peripheral expansion of mature T cells.3,4 Thymopoiesis provides a de novo pool of naïve T cells which is essential for diverse T-cell receptor (TCR) repertoire formation and sustained long-term immunity. It has been shown that recovery of thymopoiesis after allogeneic HSCT is compromised in older patients, in recipients with reduced pre-transplant thymic function, and in patients suffering from acute or chronic graft-versus-host disease (GvHD).5–13 However, it is unclear to what extent lack of recovery of thymopoiesis itself predicts for subsequent opportunistic infections and overall non-relapse mortality. We, therefore, prospectively monitored recovery of thymopoiesis following allogeneic HSCT, recording all post-engraftment opportunistic infections during follow-up, and addressed the question of whether and, if so, to what extent patients without sufficient thymic recovery are at higher risk of infections and adverse outcome.
Design and Methods
Patients and grafts
Eighty-three patients undergoing myeloablative allogeneic HSCT at the Erasmus MC/Daniel den Hoed Cancer Center from either a matched related (n=50) or unrelated donor (n=33) were included in this study. HLA-typing included high-resolution typing for HLA-A, B, C, DRB1, DQB1 and DPB1. Patients and donors were considered matched if an 8/8 match for A,B,C, DRB1 at the allele level was obtained; according to this definition, 27 (82%) unrelated donor-recipient pairs were considered allele-matched (Table 1). Patients were considered “standard risk” in case of a diagnosis of acute myeloid leukemia in first complete remission, acute lymphoblastic leukemia in first complete remission, chronic myeloid leukemia in first chronic phase, or untreated aplastic anemia. All other diagnoses were considered “high risk.” The institutional review board approved the protocols and all patients and donors provided informed consent. Bone marrow or mobilized peripheral blood progenitor cells were harvested as described elsewhere.14 T-cell depletion was performed predominantly by selection of CD34+ cells. CD34+ cells were positively selected using an immunoadsorption biotin-avidin column (Ceprate SCsystem; CellPro, Bothell, WA, USA) or by immunomagnetic cell selection using the CliniMACS system (CliniMACS, Miltenyi Biotec, Bergisch Gladbach, Germany). If the CD34+-selection procedure resulted in less than 1×105 CD3+ T cells/kg, the graft was supplemented to contain an intended minimum of 1–2×105 CD3+ T cells/kg.15 Transplantations were performed between January 1998 and December 2001.
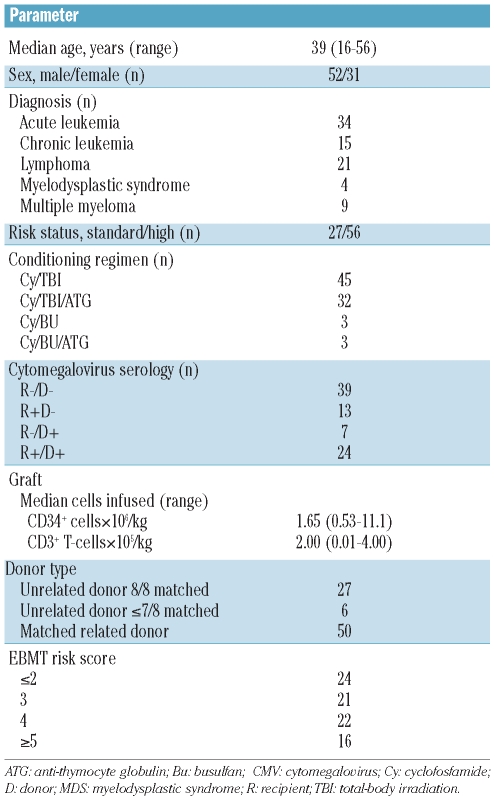
Table 1.
Characteristics of the patients and grafts (n=83).
Conditioning and supportive care
Conditioning and GvHD prophylaxis were performed as described previously.15 All patients received prophylactic ciprofloxacin (500 mg orally twice daily) and prophylactic fluconazole (200 mg once daily) as infection prevention until neutrophil recovery. Trimethoprim-sulfamethoxazole (480 mg once daily) was administered for the prevention of infections with Pneumocystis carinii from neutrophil recovery (> 0.5×109/L) until at least 6 months after transplantation or prolonged in the case of chronic GvHD. All patients received prophylactic acyclovir from transplantation until cessation of cyclosporine. GvHD was graded and treated as described previously.15
Infections
All infections diagnosed following transplantation were evaluated and scored by grade, localization, and causative micro-organism according to the NCI Common Toxicity Criteria (CTC) as described previously.15 CTC grade 3–4 infections were defined as severe (CTC grade 3) to life-threatening (CTC grade 4) infections with the need for admission and intravenous treatment. Culture-documented bacteremia, viremia, or fungemia were considered definite infections even without signs or symptoms of an infection. Clinical infection was defined as symptoms and signs consistent with an infection, but without microbiological proof. A chronic infection was scored as a single infection. A recurrent infection was scored as multiple infections only if episodes were clearly separated by an asymptomatic period of longer than 4 weeks. A polymicrobial infection of one organ or several adjacent organs was considered as a single infection. Death associated with a definite infection was defined as findings consistent with an infection and detection of the pathogen in an autopsy specimen, or death after a definite infection that was considered causative, either directly (e.g., pneumonia) or indirectly (e.g., sepsis with subsequent adult respiratory distress syndrome). Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) reactivation were diagnosed and treated as described previously.15
Immune monitoring
Peripheral blood absolute lymphocyte counts were calculated using the clinical laboratory leukocyte count and percentage of lymphocytes (leukocyte count × % lymphocytes) at 1, 2, 3, 6, 9, 12, 18 and 24 months following transplantation.
Lymphocyte subsets
Absolute numbers of peripheral blood CD3+, CD4+ and CD8+ T lymphocytes and CD3− CD16/56+ NK cells were determined by a four-color single platform flow cytometric assay using a “dual-anchor” gating strategy at serial time-points (months 2, 3, 6, 9, 12, 18, and 24) following transplantation. By inclusion of a calibrated number of fluorescent beads (Flow-Count beads; Beckman-Coulter, Miami, USA) in a lyseno-wash technique, the assay allows for direct calculation of absolute numbers of labeled cells per microliter of blood according to the ratio between beads and labeled cells. The monoclonal antibodies used for flow cytometric analysis were fluorescein isothiocyanate (FITC)-labeled CD3, phycoerythrin (PE)-labeled CD8, PE-labeled CD16/CD56, peridinyl cholorophyllin (PerCP)-labeled CD45, and allophycocyanin (APC)-labeled CD4 (BD Biosciences). Flow cytometric analysis was performed using a FACSCalibur (BD Biosciences). List mode data were collected and analyzed using CELLQuest software (BD Biosciences). At some time-points only mononuclear cells were available for FACS analysis. Absolute blood counts of lymphocyte subsets were then calculated using clinical laboratory leukocyte count + differential as: Abssubset = (ALC + AMC) × %subset /100, where ALC = absolute lymphocyte count, AMC = absolute monocyte count, and %subset = percent subset cells among all mononuclear cells.
Quantification of signal joint T-cell receptor rearrangment excision circles
The frequency of signal joint T-cell receptor rearrangment excision circle (sjTREC)-positive cells per 105 CD3+ T cells (sjTREC/105 CD3+ T cells) was determined in patients at 2, 3, 6, 9, 12, 18 and 24 months after allogeneic HSCT and in healthy volunteers [n=22; median age, 39 years (range, 20–54 years)]. Peripheral blood mononuclear cells were isolated by Ficoll/Hypaque density gradient centrifugation, cryopreserved with 10% dimethyl sulfoxide and stored until testing. Following thawing, DNA was purified from the cells using the QIAamp DNA mini kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. sjTREC were quantified by a previously described 5’ nuclease based real-time quantitative polymerase chain reaction (RQ-PCR) assay using the ABI PRISM 7700 sequence detector (Applied Biosystems, Foster City, CA, USA)16 modified for human samples. Sequences of the primers and probe were used as described previously17 for the detection of sjTREC: forward primer 5′- CCATGCTGACACCTCTGGTT -3′, reverse primer 5′- TCGTGAGAACGGTGAATGAAG -3′and probe FAM-5′-CACGGTGATGCATAGGCA CCTGC -3′-TAMRA. To compensate for variations in input DNA we used the constant gene segment of the TCRA gene (Cα) as an endogenous reference gene. Sequences for the detection of the TCRA gene (Cα) were: forward primer 5′- CCTGATCCTCTTGTCCCACAG -3′, reverse primer 5′- GGATTTAGAGTCTCTCAGCTGGTACA -3′ and probe JOE-5′- ATCCAGAACCCTGACCCTGCCG -3′-TAMRA. The frequency of sjTREC in CD3+ T lymphocytes could be calculated by normalizing the sjTREC RQ-PCR to the Cα RQ-PCR, on the assumption that the total number of nucleated cells in the peripheral blood is represented by the CD45+ cell subset. The ratio of CD45/CD3 cells in peripheral blood mononuclear cells was determined by flow cytometry. The frequency of peripheral blood sjTREC is not only influenced by thymic output, but also by post-rearrangement expansion.9 SjTREC content (sjTREC/mL blood) is not influenced by peripheral expansion and may, therefore, be a better estimate of thymic output.18 sjTREC content (sjTREC/mL blood) was calculated by multiplying the sjTREC frequency (sjTREC/105 CD3+ T cells) by the absolute numbers of CD3+ T cells/mL blood.
Statistical considerations
Endpoints of the study included hematologic recovery, acute GvHD grades 2–4, chronic GvHD (limited + extensive as well as extensive alone), infections of CTC grade 3 or more or grade 4 alone, overall survival, and non-relapse mortality. Time to hematologic recovery (neutrophils >0.5×109/L and platelets >50×109/L) was measured from the date of transplantation. Time to develop acute GvHD grades 2–4 was calculated from transplantation until occurrence of acute GvHD (by definition until day 100). Time to develop chronic GvHD was only calculated for patients who survived at least 100 days after transplantation. Death without having suffered from chronic GvHD was considered a competing risk. Patients without having suffered from chronic GvHD and still alive at the date of last contact were then censored. Time to infections of CTC grade 3 or more, or grade 4 alone, was determined from the date of transplantation, and relapse before or death without such infections were considered competing risks. Overall survival was calculated from the date of transplantation until death. Patients still alive at the date of last contact were then censored. Causes of death were classified as relapse-related mortality or non-relapse mortality, and these were considered competing risks. Distribution of EBMT risk score, taking age, stage of disease, donor type, gender and time to transplantation into account, was determined as described elsewhere19 and presented as categories of 0–2, 3, 4 and 5–7. Overall survival was calculated using the actuarial method of Kaplan and Meier, and a Kaplan-Meier curve was generated to illustrate survival. For all other time-to-event endpoints, cumulative incidences were calculated taking into account the competing risks, and cumulative incidence curves were generated. Univariate and multivariate Cox regression analyses were performed to evaluate the impact of lymphocyte (subset) recovery and sjTREC content or frequency at 2, 6, 9 and 12 months on clinical outcome beyond those time points. Hazard ratios (HR) and corresponding 95% confidence intervals (95% CI) were determined, and P values were calculated using the likelihood ratio test. Patients with a failure due to a competing risks were censored in the Cox regression analyses. The immunological and sjTREC data at those time points were also compared to those from 22 healthy donors, using the Wilcoxon rank-sum test. All reported P values are two-sided, and a significance level α = 0.05 was used.
Results
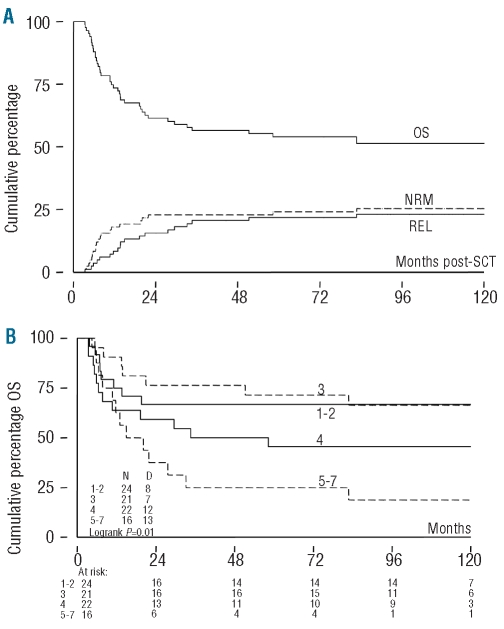
The patients’ characteristics are presented in Table 1. The median age of the patients was 39 years (range, 16–56 years). Fifty-six of 83 patients (67%) were classified as having high-risk disease. Bone marrow or mobilized peripheral blood was used as the stem cell source in 67 and 16 patients, respectively. Fifty patients (60%) received a stem cell graft from a matched related donor and 33 (40%) from an unrelated donor. All grafts were partially depleted of T cells, predominantly by CD34+ selection. The median number of CD34+ cells infused was 1.65×106/kg (range, 0.53–11.1) while the median number of CD3+ T cells infused was 2.00×105/kg (range, 0.01–4.00). After a median follow-up of 118 months (range, 24–139 months), 43 of 83 patients were still alive (Figure 1A). A decrease in overall survival was observed with an increasing EBMT risk score (Figure 1B). Death was due to causes other than relapse in 21 (25%) patients and to relapse in 19 (23%) patients (Figure 1A). Infectious complications were the cause of 17 out of the 21 deaths (81%) and GvHD contributed to 11 out of these 21 deaths (53%). By day 100 post-transplant, 48 of 83 patients (58%) had developed grade II–IV acute GvHD and 12% had developed grade III–IV acute GvHD. At 1 year after transplantation 27 patients (33%) had experienced limited (n=13) or extensive (n=14) chronic GvHD. At 2 years after transplantation 35% of recipients had experienced limited (n=14) or extensive (n=14) chronic GvHD.
Figure 1.
Outcome. (A) Cumulative percentage of overall survival (OS), non-relapse mortality (NRM), and relapse mortality (REL) in months following allogeneic HSCT; OS, NRM and REL (which are competing risks) sum up to 100% at all time points. (B) Cumulative percentage of OS stratified by EBMT risk score in months following allogeneic HSCT (log rank: P=0.01).
Infections
The total number of severe (CTC grade 3 or 4) infections were recorded in detail in surviving recipients up to 4 years after allogeneic HSCT. Between days 30 and 365, the rate of infections was 0.64 per 100 patient-days, 66% of patients had experienced at least one severe infection during that time period and an average of 1.64 episodes of severe infections occurred per patient (range, 0–7). Between 12 and 24 months post-transplantation, the rate of severe infections was 0.16 infections per 100 patient-days. Only three severe bacterial infections were reported between 2 and 4 years after transplantation (rate: 0.01 infections/100 patient-days). One or more causative micro-organisms were identified in 69% of reported infections. The most important infectious agents were viruses followed by bacteria and fungi (data not shown). The cumulative incidence of severe infections (CTC grade 3 or 4) was comparable between recipients who did or did not receive antithymocyte globulin as part of the conditioning regimen.
Immune recovery
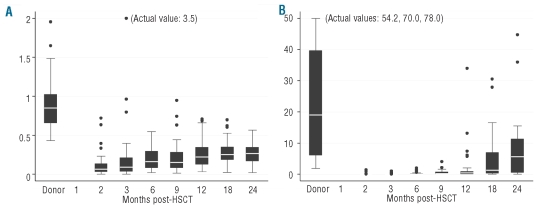
Neutrophil recovery (absolute neutrophil count >0.5×109/L) occurred at a median of 18 days after transplantation. Lymphocyte recovery was assessed at 2, 3, 6, 9, 12, 18 and 24 months post-transplantation and compared to pre-transplantation values in healthy sibling donors. NK cell numbers recovered rapidly and returned to within the normal range between 2 and 3 months post-transplantation. CD8+ T-cell numbers normalized between 12 and 18 months post-transplantation. In contrast, CD4+ T-cell recovery was extremely slow (Figure 2A). The median CD4+ T-cell numbers exceeded 200/μL blood only by 12 months post-transplantation.
Figure 2.
Immune reconstitution. Box plots are shown of (A) CD4+ T-cell recovery (CD4+ T cells/μL blood) and (B) sjTREC+ T-cell recovery (sjTREC+ cells/mL blood) in recipients in time following allogeneic HSCT and values in normal donor controls. Each box shows the median, quartiles, and extreme values. Outliers are represented by •.
The frequency of sjTREC+ T cells as well as the total sjTREC+ cell content were measured in patients at successive time-points following transplantation and in healthy donors. In healthy donors, the median frequency of sjTREC+ T cells was 1.205/105 CD3+ T cells (range, 147–3.962) and the median sjTREC+ cell content measured 19.044/mL blood (range, 1.873–77.952). Both sjTREC frequency and sjTREC content showed an aged-dependent decrease in healthy donors (Spearman’s rank correlation: r=−0.66, P<0.001), as has been described previously. Although the percentage of patients with detectable sjTREC+ T cells increased over time following transplantation, sjTREC+ T cells were only detected in 17% of patients at 2 months, in 61% at 9 months and in 83% at 24 months post-transplantation. The median sjTREC+ cell content measured was 0 (range, 0–1,392) at 2 months, 208 (range: 0–3,991) at 9 months and 5,629 (range: 0–44,664)/mL blood at 24 months post-transplantation (Figure 2B). Even at 24 months post-transplantation, the sjTREC+ cell content had not recovered to normal values (P=0.001). Of note, sjTREC values did not correlate with CD4+ or CD3+ T-cell numbers at any time point evaluated (data not shown). sjTREC+ T-cell frequencies and content were strongly associated both in healthy donors and in patients at all time-points evaluated (r=0.93–1.00). We evaluated whether pre-transplant variables would affect sjTREC+ T-cell recovery. Higher recipient age was associated with impaired recovery of sjTREC+ T cells, either estimated by sjTREC+ T-cell frequency or by sjTREC+ content. In addition, donor age was also related with post-transplant recovery. Donor and recipient age were highly correlated (r=0.64, P<0.0001). No differences were observed in sjTREC+ T-cell recovery between recipients of grafts from matched related or unrelated donors, peripheral blood versus bone marrow as the stem cell source, high versus low CD34+ cell dosage or high-risk versus standard-risk disease. In addition, CMV serostatus, preceding receipt of antithymocyte globulin, and prior history of acute GVHD were not different between patients who did or did not have sjTREC+ T-cell recovery at 6 or 12 months post-transplantation. More specifically, CMV-seropositive recipients who received grafts from CMV-seronegative donors were not at higher risk of insufficient recovery of sjTREC+ T cells as compared to recipients with a more favorable constellation of CMV serostatus. However, more recipients who had experienced extensive chronic GVHD at any time during the first year post-transplantation failed to recover thymopoiesis at 12 months as compared to patients without that history (5/10 versus 2/23, P=0.02).
Predictive impact of recovery of thymopoiesis
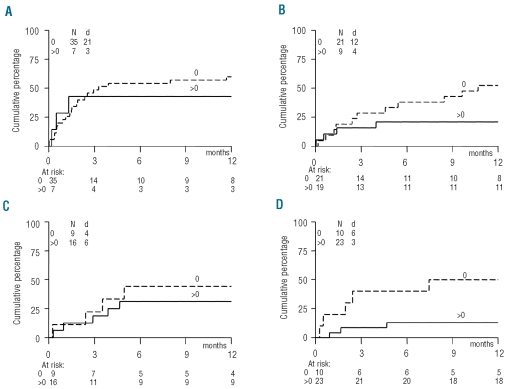
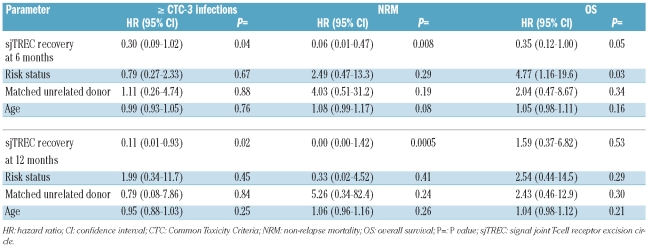
We next determined whether recovery of sjTREC+ T cells and recovery of absolute lymphocyte count and lymphocyte subsets (CD3+, CD3+CD4+, CD3+CD8+ and CD3−CD16−56+ NK cells) could predict for severe infections, non-relapse mortality and overall survival. The cumulative incidence of severe infections (CTC grade 3 or 4) occurring after a specific time-point was assessed and compared between patients with adequate or insufficient recovery, taking relapse and death as competing risks into account. The overall lymphocyte count as well as the NK-cell and T-cell subsets (CD3+, CD4+, CD8+ T cells) did not consistently predict for severe infections. However, the overall lymphocyte count at day 30 appeared moderately associated with CTC grade 4 infections (HR 0.44, 95% CI: 0.18–1.08; P=0.07). Similar results were obtained when lymphocyte recovery was analyzed as a continuous variable or when the median value was used as the cut-off to compare groups of patients (data not shown). Patients in whom sjTREC+ T-cell recovery failed (defined as absent sjTREC+ T cells in blood) were, however, at significantly higher risk of a severe infection compared to patients who did show sjTREC+ T-cell recovery, as illustrated in Figure 3. In multivariable analysis, the predictive impact of sjTREC+ cell recovery was estimated and weighed against the risk factors age, risk-status and type of donor, as these three factors were associated with overall survival in univariate analysis. The results of this analysis are presented in Table 2. A more than 3-fold lower risk of developing severe infections was observed for those patients who had effective sjTREC+ T-cell recovery at 6 months (HR 0.30; 95%CI: 0.09–1.02; P=0.04), a 4-fold lower risk at 9 months (HR 0.26, 95%CI: 0.03–2.31; P=0.2), and even a 9-fold lower risk at 12 months (HR 0.11, 95% CI: 0.01–0.93; P=0.02). The predictive impact of sjTREC+ T-cell recovery outweighed the other risk factors, including age (Table 2). While the overall lymphocyte count as well as counts of the individual subsets showed weak and inconsistent associations in univariate analysis at the 2, 6, 9, or 12-month time-points, their predictive power disappeared when weighed against the recovery of sjTREC-positive T cells in multivariable analysis (data not shown). We also tested sjTREC+ T-cell recovery as a continuous variable in both univariate and multivariate analyses. Again, the risk of severe infections was lower among patients with adequate sjTREC+ T-cell recovery as compared to patients with insufficient sjTREC+ T-cell recovery at 6 months (HR 0.05, 95% CI: 0.00–0.76; P=0.02), 9 months (HR: 0.02; 95% CI: 0.00–0.75; P=0.02) and 12 months (HR: 0.12, 95%CI: 0.03–0.56; P<0.01).
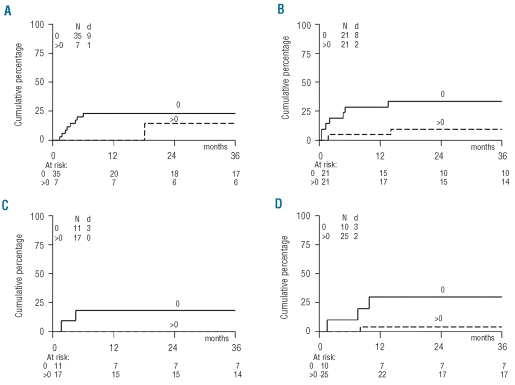
Figure 3.
CTC grade ≥ 3 infections in patients with or without recovery of thymopoiesis. Cumulative incidence of first CTC grade ≥3 infection in months beyond the time-points of measurement in patients with (>0) or without (0) sjTREC+ T-cell recovery as measured at 2 (A), 6 (B), 9 (C) and 12 (D) months after allogeneic HSCT. N: number of patients evaluated; d: number of patients reaching the endpoint. P values of likelihood ratio testing: 0.45 (2 months); 0.06 (6 months); 0.81 (9 months); and 0.01 (12 months).
Table 2.
sjTREC+ T-cell recovery predicts for infectious outcome. Results of multivariate analysis.
Accordingly, we evaluated whether the presence or absence of sjTREC+ T cells would predict for less non-relapse mortality. Again, the actual recovery of sjTREC+ T cells at both the 6 and 12 month time-points appeared to predict for subsequent reduced non-relapse mortality (Figure 4). In multivariable analysis, recovery of sjTREC+ T cells at a particular time-point outweighed the risk factors age, risk-status and donor type for subsequent non-relapse mortality (Table 2) with hazard ratios below 0.1 (P<0.01). Lastly, the predictive impact of sjTREC+ T-cell recovery on overall survival was assessed. Both risk-status and sjTREC+ T-cell recovery influenced subsequent overall survival (Table 2) taking the 6-month assessment into account, whereas sjTREC+ T-cell recovery at 12 months did not predict for subsequent overall survival. The overall lymphocyte count as well as counts of the various lymphocyte subsets did not predict for overall survival in multivariate analysis (data not shown).
Figure 4.
Non-relapse mortality in patients with or without recovery of thymopoiesis. Cumulative incidence of non-relapse mortality in months beyond the time-points of measurement in patients with (>0) or without (0) sjTREC+ T-cell recovery as measured at 2 (A), 6 (B), 9 (C) and 12 (D) months after allogeneic HSCT. N: number of patients evaluated; d: number of patients reaching the endpoint. P-values of likelihood ratio testing: 0.37 (2 months); 0.03 (6 months); 0.01 (9 months); 0.13 (12 months).
Discussion
Recovery of thymopoiesis after allogeneic HSCT may be severely protracted but plays an important role in restoration of post-transplant immune competence.1,2,20 While different parameters affecting thymic function have been identified, it was still unclear to what extent insufficient recovery of thymopoiesis as such could predict for severe opportunistic infections and non-relapse mortality in time. In the present study we show that recipients of T-cell-depleted allogeneic stem cell grafts from matched related or unrelated donors show an age-related protracted recovery of thymopoiesis, which was still significantly below the values observed in healthy stem cell donors by 24 months after allogeneic HSCT. Despite intensive follow-up and prophylactic measures, the incidence of severe CTC grade 3 and 4 post-engraftment infections was high and contributed significantly to non-relapse mortality. The predictive impact of insufficient thymopoiesis was assessed and quantified: patients with failed recovery of thymopoiesis had 3- and 9-fold higher risks for severe infections and non-relapse mortality beyond the 6- and 12-month time points, respectively. While well-known pre-transplant risk factors, such as those incorporated in the EBMT risk-score, also predicted for outcome, failed thymic recovery outweighed these risk factors, thereby highlighting the importance of adequate thymopoiesis after allogeneic HSCT. Hazard ratios referring to the risk of subsequent infections were very high in the present study, which may be explained by the predominant role of thymic function in our study population of T-cell recovery. Recipients of T-cell-replete grafts, as well as recipients of non-myeloablative grafts21 may benefit from the immune effector cells infused with the graft and depend less stringently on thymopoiesis. In addition, given the adverse effect of high-dose radiother-apy22,23 on thymic epithelium, both the myeloablative conditioning regimen and T-cell-depletion strategy may have acted in concert by impairing thymopoiesis without providing compensation by peripheral donor T-cell recovery.
Thymopoiesis is a finely-tuned selection process of thymocytes resulting in production of naïve T cells with a broad TCR repertoire which selectively recognize non-self antigens and allow the development of an adequate immune response to a broad range of infectious pathogens.24 Thymopoiesis in healthy humans gradually declines with age although the ability to generate new naïve T cells is preserved in adulthood.25 Immune reconstitution and especially restoration of the TCR repertoire in the later post-transplant period is a protracted process, which usually takes many months or years to be completed,2,4,26 as was evident in our study of older high-risk allogeneic HSCT recipients. Regeneration of a broad and functionally competent TCR repertoire after allogeneic HSCT requires intact thymic function, which is, however, compromised by pre-transplant conditioning, by an age-related involution of the thymus, and by the donor-derived allo-reactive immune response to recipient tissues.5–10,12,13,21,22 Apart from age, most pre-transplant variables did not affect post-transplant thymic function in our study. Age was also the only pre-transplant variable consistently associated with impaired post-transplantation thymopoiesis in previous studies.5–10,12,13,21
Retrospectively, we did not find an association between acute GvHD and impaired thymopoiesis, but more patients in whom thymopoiesis failed to recover at 12 months had a history of extensive chronic GVHD, which had occurred during the first year post-transplant. Several clinical and experimental studies have highlighted that GvHD severely impairs thymopoiesis, although recapitulation of thymic function after a history of acute GvHD is still possible.6–10,12,13,21,24 While it has been difficult to separate the adverse effects of immunosuppressive drugs and GvHD itself in clinical studies, experimental studies have suggested that epithelial destruction rather than the apoptotic effects of corticosteroids may account for thymic lymphoid depletion.24 Preservation of the thymic epithelium by new therapeutic approaches has, therefore, lately received more attention.27–29
We evaluated thymic function by both the frequency of sjTREC+ T cells and the sjTREC content per milliliter of peripheral blood. The latter estimate was added in order to correct for peripheral homeostatic T-cell expansion, which may result in dilution of sjTREC and subsequent underestimation of thymic function.18 However, sjTREC frequencies and contents measured in the present study were highly correlated and the predictive impact of either estimate did not differ, as observed previously.13 This observation suggests that both estimates reliably reflect recovery of thymopoiesis in the setting of allogeneic HSCT in high-risk recipients, such as those included in the present study. While allogeneic HSCT is increasingly applied as a treatment modality in patients with acute leukemia,30 opportunistic infections and non-relapse mortality remain major drawbacks of this powerful treatment. A number of risk factors predisposing to non-relapse mortality have been reported, including pre-transplant characteristics and time-dependent post-transplant risk factors. Pre-transplant risk factors include patient and donor characteristics that were derived from large retrospective studies performed by cooperative groups or large centers. Two powerful risk scores have emerged in recent years: the Seattle hematopoietic stem cell transplantation co-morbidity index (HCT-CI)31 and the EBMT-risk score.19 The HCT-CI score selectively takes the number of co-morbidities into account and has been validated prospectively in a number of centers and transplant modalities.32,33 The EBMT risk score is based on five criteria: disease stage, patient’s age, donor type, time interval from diagnosis to transplantation, and donor-recipient sex combination.19 The score was validated in several independent cohorts of patients, confirmed over time, and recently also validated in acute leukemia patients.34 The EBMT risk score also significantly predicted overall outcome in the current study, with percentages in the range of earlier studies. In addition to pre-transplant risk factors, several time-dependent post-transplant risk factors have been reported as possibly indicating failure to recover essential parts of the immune system.20 Among different parameters reported, early lymphocyte recovery and also an insufficient late recovery of (naïve) CD4+ T helper cells were correlated with outcome in a number of studies.35–45 More specifically, low numbers and/or reduced function of antigen-specific T cells were shown to predict for opportunistic viral infections in general and CMV and EBV in particular.46,47 The latter assays do, however, only cover part of the repertoire needed. Recovery of sjTREC+ T cells may be a more general parameter reflecting restoration of the adaptive immune system, needed to combat a range of opportunistic pathogens, including bacteria, fungi and viruses. Apart from the well-established role of a broad T-cell repertoire in viral complications our results suggest that failure to recover thymopoiesis also affects anti-bacterial and anti-fungal immunity. The latter observation may be supported by the recently acknowledged role of T cells in innate and mucosal immunity as well as the role of these cells in specific fungal and bacterial infections.48–53 While pre-transplant risk factors may be used for deciding whether or not to proceed to transplantation, post-transplant risk-factors may identify patients for whom supportive care measures may be intensified or who may benefit from new approaches to boost thymopoiesis. Such endeavors are nowadays of increasing importance with the broader use of alternative donors, including haploidentical donors, unrelated donors, and umbilical cord blood. The increased use of umbilical cord blood in adult patients and non-myeloablative conditioning in elderly patients, in particular, may be associated with a failure to recover thymopoiesis and late non-relapse mortality.21,54,55
In conclusion, failure to recover thymopoiesis after myeloablative T-cell-depleted allogeneic HSCT puts recipients at high risk of developing opportunistic infections of either bacterial, fungal, or viral origin. Despite effective prophylactic measures, such infections translate into increased non-related mortality and adverse outcome after allogeneic HSCT, highlighting the need to preserve or regenerate thymic function and more specifically, the restoration of the thymic epithelium that supports and directs thymopoiesis.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Hakim FT, Gress RE. Reconstitution of thymic function after stem cell transplantation in humans. Curr Opin Hematol. 2002;9(6):490–6. doi: 10.1097/00062752-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30(4):425–37. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 3.Mackall CL, Granger L, Sheard MA, Cepeda R, Gress RE. T-cell regeneration after bone marrow transplantation: differential CD45 isoform expression on thymic-derived versus thymic-independent progeny. Blood. 1993;82(8):2585–94. [PubMed] [Google Scholar]
- 4.Rufer N, Helg C, Chapuis B, Roosnek E. Human memory T cells: lessons from stem cell transplantation. Trends Immunol. 2001;22(3):136–41. doi: 10.1016/s1471-4906(00)01849-4. [DOI] [PubMed] [Google Scholar]
- 5.Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355(9218):1875–81. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg K, Blazar BR, Wagner JE, Agura E, Hill BJ, Smogorzewska M, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97(5):1458–66. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 7.Storek J, Joseph A, Dawson MA, Douek DC, Storer B, Maloney DG. Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation. 2002;73(7):1154–8. doi: 10.1097/00007890-200204150-00026. [DOI] [PubMed] [Google Scholar]
- 8.Lewin SR, Heller G, Zhang L, Rodrigues E, Skulsky E, van den Brink MR, et al. Direct evidence for new T-cell generation by patients after either T-cell-depleted or unmodified allogeneic hematopoietic stem cell transplantations. Blood. 2002;100(6):2235–42. [PubMed] [Google Scholar]
- 9.Hazenberg MD, Otto SA, de Pauw ES, Roelofs H, Fibbe WE, Hamann D, et al. T-cell receptor excision circle and T-cell dynamics after allogeneic stem cell transplantation are related to clinical events. Blood. 2002;99(9):3449–53. doi: 10.1182/blood.v99.9.3449. [DOI] [PubMed] [Google Scholar]
- 10.Poulin JF, Sylvestre M, Champagne P, Dion ML, Kettaf N, Dumont A, et al. Evidence for adequate thymic function but impaired naive T-cell survival following allogeneic hematopoietic stem cell transplantation in the absence of chronic graft-versus-host disease. Blood. 2003;102(13):4600–7. doi: 10.1182/blood-2003-05-1428. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Barfield R, Benaim E, Leung W, Knowles J, Lawrence D, et al. Prediction of T-cell reconstitution by assessment of T-cell receptor excision circle before allogeneic hematopoietic stem cell transplantation in pediatric patients. Blood. 2005;105(2):886–93. doi: 10.1182/blood-2004-04-1405. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez M, Martinez C, Ercilla G, Carreras E, Urbano-Ispizua A, Aymerich M, et al. Clinical factors influencing T-cell receptor excision circle (TRECs) counts following allogeneic stem cell transplantation in adults. Transpl Immunol. 2006;16(1):52–9. doi: 10.1016/j.trim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Clave E, Busson M, Douay C, Peffault de Latour R, Berrou J, Rabian C, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood. 2009;113(25):6477–84. doi: 10.1182/blood-2008-09-176594. [DOI] [PubMed] [Google Scholar]
- 14.Cornelissen JJ, van der Holt B, Petersen EJ, Vindelov L, Russel CA, Hoglund M, et al. A randomized multicenter comparison of CD34(+)-selected progenitor cells from blood vs from bone marrow in recipients of HLA-identical allogeneic transplants for hematological malignancies. Exp Hematol. 2003;31(10):855–64. doi: 10.1016/s0301-472x(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 15.Broers AE, van der Holt B, Haze S, Braakman E, Gratama JW, Lowenberg B, et al. A comparison of postengraftment infectious morbidity and mortality after allogeneic partially T cell-depleted peripheral blood progenitor cell transplantation versus T cell-depleted bone marrow transplantation. Exp Hematol. 2005;33(8):912–9. doi: 10.1016/j.exphem.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Broers AE, Meijerink JP, van Dongen JJ, Posthumus SJ, Lowenberg B, Braakman E, et al. Quantification of newly developed T cells in mice by real-time quantitative PCR of T-cell receptor rearrangement excision circles. Exp Hematol. 2002;30(7):745–50. doi: 10.1016/s0301-472x(02)00825-1. [DOI] [PubMed] [Google Scholar]
- 17.Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6(9):1036–42. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro RM, Perelson AS. Determining thymic output quantitatively: using models to interpret experimental T-cell receptor excision circle (TREC) data. Immunol Rev. 2007;216:21–34. doi: 10.1111/j.1600-065X.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 19.Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352(9134):1087–92. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 20.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castermans E, Hannon M, Dutrieux J, Humblet-Baron S, Seidel L, Cheynier R, et al. Thymic recovery after allogeneic hematopoietic cell transplantation with non-myeloablative conditioning is limited to patients younger than 60 years of age. Haematologica. 2011;96(2):298–306. doi: 10.3324/haematol.2010.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung B, Barbara-Burnham L, Barsky L, Weinberg K. Radiosensitivity of thymic interleukin-7 production and thymopoiesis after bone marrow transplantation. Blood. 2001;98(5):1601–6. doi: 10.1182/blood.v98.5.1601. [DOI] [PubMed] [Google Scholar]
- 23.Hauri-Hohl MM, Zuklys S, Keller MP, Jeker LT, Barthlott T, Moon AM, et al. TGF-beta signaling in thymic epithelial cells regulates thymic involution and postirradiation reconstitution. Blood. 2008;112(3):626–34. doi: 10.1182/blood-2007-10-115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krenger W, Hollander GA. The thymus in GVHD pathophysiology. Best Pract Res Clin Haematol. 2008;21(2):119–28. doi: 10.1016/j.beha.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 26.Eyrich M, Croner T, Leiler C, Lang P, Bader P, Klingebiel T, et al. Distinct contributions of CD4(+) and CD8(+) naive and memory T-cell subsets to overall T-cell-receptor repertoire complexity following transplantation of T-cell-depleted CD34-selected hematopoietic progenitor cells from unrelated donors. Blood. 2002;100(5):1915–8. doi: 10.1182/blood-2001-11-0005. [DOI] [PubMed] [Google Scholar]
- 27.Wils EJ, Cornelissen JJ. Thymopoiesis following allogeneic stem cell transplantation: new possibilities for improvement. Blood Rev. 2005;19(2):89–98. doi: 10.1016/j.blre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Chidgey AP, Seach N, Dudakov J, Hammett MV, Boyd RL. Strategies for reconstituting and boosting T cell-based immunity following haematopoietic stem cell transplantation: pre-clinical and clinical approaches. Semin Immunopathol. 2008;30(4):457–77. doi: 10.1007/s00281-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 29.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115(19):3861–8. doi: 10.1182/blood-2009-12-234096. [DOI] [PubMed] [Google Scholar]
- 30.Gratwohl A, Baldomero H, Schwendener A, Rocha V, Apperley J, Frauendorfer K, et al. The EBMT activity survey 2007 with focus on allogeneic HSCT for AML and novel cellular therapies. Bone Marrow Transplant. 2009;43(4):275–91. doi: 10.1038/bmt.2009.7. [DOI] [PubMed] [Google Scholar]
- 31.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25(27):4246–54. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 33.Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110(13):4606–13. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115(20):4715–26. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- 35.Einsele H, Ehninger G, Steidle M, Fischer I, Bihler S, Gerneth F, et al. Lymphocytopenia as an unfavorable prognostic factor in patients with cytomegalovirus infection after bone marrow transplantation. Blood. 1993;82(5):1672–8. [PubMed] [Google Scholar]
- 36.Storek J, Gooley T, Witherspoon RP, Sullivan KM, Storb R. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. Am J Hematol. 1997;54(2):131–8. doi: 10.1002/(sici)1096-8652(199702)54:2<131::aid-ajh6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 37.Small TN, Avigan D, Dupont B, Smith K, Black P, Heller G, et al. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttrans-plant graft rejection prophylaxis. Biol Blood Marrow Transplant. 1997;3(2):65–75. [PubMed] [Google Scholar]
- 38.Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467–80. [PubMed] [Google Scholar]
- 39.Storek J, Espino G, Dawson MA, Storer B, Flowers ME, Maloney DG. Low B-cell and monocyte counts on day 80 are associated with high infection rates between days 100 and 365 after allogeneic marrow transplantation. Blood. 2000;96(9):3290–3. [PubMed] [Google Scholar]
- 40.Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101(2):407–14. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 41.Kim DH, Kim JG, Sohn SK, Sung WJ, Suh JS, Lee KS, et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Br J Haematol. 2004;125(2):217–24. doi: 10.1111/j.1365-2141.2004.04891.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB. Rapid helper T-cell recovery above 200×106/L at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37(12):1119–28. doi: 10.1038/sj.bmt.1705381. [DOI] [PubMed] [Google Scholar]
- 43.Savani BN, Mielke S, Rezvani K, Montero A, Yong AS, Wish L, et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strong-ly predicts outcome after T cell-depleted allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(10):1216–23. doi: 10.1016/j.bbmt.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berger M, Figari O, Bruno B, Raiola A, Dominietto A, Fiorone M, et al. Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transplant. 2008;41(1):55–62. doi: 10.1038/sj.bmt.1705870. [DOI] [PubMed] [Google Scholar]
- 45.Le Blanc K, Barrett AJ, Schaffer M, Hagglund H, Ljungman P, Ringden O, et al. Lymphocyte recovery is a major determinant of outcome after matched unrelated myeloablative transplantation for myelogenous malignancies. Biol Blood Marrow Transplant. 2009;15(9):1108–15. doi: 10.1016/j.bbmt.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meij P, van Esser JW, Niesters HG, van Baarle D, Miedema F, Blake N, et al. Impaired recovery of Epstein-Barr virus (EBV)--specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. Blood. 2003;101(11):4290–7. doi: 10.1182/blood-2002-10-3001. [DOI] [PubMed] [Google Scholar]
- 47.Gratama JW, Brooimans RA, van der Holt B, Sintnicolaas K, van Doornum G, Niesters HG, et al. Monitoring cytomegalovirus IE-1 and pp65-specific CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation may identify patients at risk for recurrent CMV reactivations. Cytometry B Clin Cytom. 2008;74(4):211–20. doi: 10.1002/cyto.b.20420. [DOI] [PubMed] [Google Scholar]
- 48.Berg RE, Forman J. The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr Opin Immunol. 2006;18(3):338–43. doi: 10.1016/j.coi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z, O'Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008;41(2):87–102. doi: 10.1007/s12026-007-8014-9. [DOI] [PubMed] [Google Scholar]
- 50.Cook MC, Tangye SG. Primary immune deficiencies affecting lymphocyte differentiation: lessons from the spectrum of resulting infections. Int Immunol. 2009;21(9):1003–11. doi: 10.1093/intimm/dxp076. [DOI] [PubMed] [Google Scholar]
- 51.Muller I, Munder M, Kropf P, Hansch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30(11):522–30. doi: 10.1016/j.it.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Segal BH. Aspergillosis. N Engl J Med. 2009;360(18):1870–84. doi: 10.1056/NEJMra0808853. [DOI] [PubMed] [Google Scholar]
- 53.Malley R. Antibody and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J Mol Med. 2010;88(2):135–42. doi: 10.1007/s00109-009-0579-4. [DOI] [PubMed] [Google Scholar]
- 54.Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110(13):4543–51. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown JA, Stevenson K, Kim HT, Cutler C, Ballen K, McDonough S, et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood. 2010;115(20):4111–9. doi: 10.1182/blood-2009-09-244145. [DOI] [PMC free article] [PubMed] [Google Scholar]