Abstract
Overhydrated hereditary stomatocytosis, clinically characterized by hemolytic anemia, is a rare disorder of the erythrocyte membrane permeability to monovalent cations, associated with mutations in the Rh-associated glycoprotein gene. We assessed the red blood cell metabolome of 4 patients with this disorder and showed recurrent metabolic abnormalities associated with this disease but not due to the diminished half-life of their erythrocytes. Glycolysis is exhausted with accumulation of ADP, pyruvate, lactate, and malate. Ascorbate metabolic pathway is altered probably due to a limited entry of dehydroascorbate. Although no major oxydative stress has been reported in patients with overhydrated hereditary stomatocytosis, we found decreased amounts of oxydized glutathione, creatine and ergothioneine, suggesting transporter abnormalities and/or uncharacterized oxydative stress. These results pinpoint major metabolic defects of overhydrated hereditary stomatocytosis erythrocytes and emphasize the relevance of red blood cell metabolomics for a better understanding of the pathophysiological bases of hemolytic anemia associated with erythrocyte abnormalities.
Keywords: metabolome, red blood cells, hemolytic anemia
Introduction
Overhydrated hereditary stomatocytosis (OHSt), which is clinically characterized by a hemolytic anemia, is a rare disorder of red blood cells (RBCs) associated with increased membrane permeability to monovalent cations and increased activity of the Na+K+-ATPase.1 OHSt diagnosis is based on a hemolytic anemia associated with a massive right shift of the osmotic gradient ektacytometry curve and a decreased osmotic resistance, together with a major increase in a monovalent cation leak. Blood smears show stomatocytes and bowl-shaped RBCs presenting a slit-like area. OHSt is associated with mutations in the gene encoding the Rh-associated glycoprotein (RhAG), a member of the Rh complex.2 RhAG may function as an ammonium transporter and/or a gas channel.3–5 When expressed in Xenopus laevis oocytes, the human wild-type RhAG induces a monovalent cation leak considerably enhanced when mutated RhAG transporters are expressed instead.2 Among membrane abnormalities, OHSt RBCs display a sharp reduction or an absence of stomatin,6 an integral RBC membrane protein. In human RBCs, stomatin acts as a molecular switch that partly converts the glucose transporter 1 (Glut1) into a transporter for L-dehydroascorbic acid (DHA) that is metabolized producing ascorbate. In the absence of stomatin, the DHA transport by Glut1 undergoes a 2-fold decrease while glucose uptake is significantly increased,7 suggesting an enhanced glycolysis in OHSt RBCs. Altogether, these results indicate that the OHSt physiopathology affects several metabolic pathways in RBCs and may disrupt homeostasis of RBC metabolism. We have developed a simple strategy for the extraction and global mass spectrometry-based analyses of metabolites from purified human RBCs.8 Here we use this strategy to distinguish metabolic changes in RBCs from OHSt patients in order to fully characterize the metabolic bases of this disease.
Design and Methods
Blood samples were collected from 4 French patients (3 males and one female) from three different families2 carrying the Phe65Ser mutation in RhAG, and 24 healthy adults as controls, including 5 relatives of the patients. Hemoglobin levels of the patients were between 9.9 and 12.7g/dL with an average of 11.3 g/dL. Reticulocyte count (325×109/L, range 269–445×109/L; 10.8%, range 7.5–15.8%) and the mean red cell volume of the patients had increased (134 fL, range 122–144 fL). Written informed consent was obtained from all patients and controls in accordance with the Declaration of Helsinki.
Preparation of RBC lysates, metabolite extraction from RBCs, parameters for liquid chromatography coupled to electrospray-LTQ-Orbitrap mass spectrometry, and data processing were performed as previously described.8 Control RBCs were fractioned according to the age of the RBCs.9 The percentage of leukocytes in RBC purified samples was determined using an automated cell counter (Abacus Junior Hematology Analyzer, Diatron). For all samples, the percentage of leukocytes was less than 0.01%.
Results and Discussion
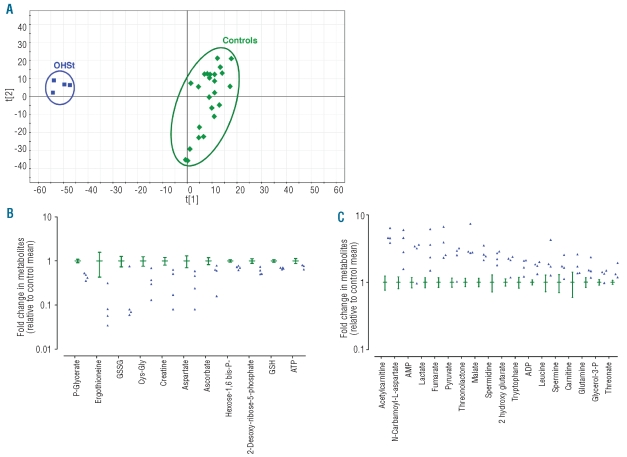
Red blood cell metabolites from OHSt patients and controls were analyzed by LC/MS with detection in both ion modes: negative and positive. Detected biomolecules are amino acids, organic acids, amines, lipids, sugars, hormones, peptides, and proteins. The selectivity of metabolite detection is obtained from the chromatographic retention time and to the accuracy of mass measurements. Processing of the metabolic fingerprints led to 1524 and 785 relevant ions in the negative and positive ion modes, respectively. The hundreds of thousands of signals contained in the data sets make statistical analysis problematic. The resulting data matrices were subjected to multivariate statistical analyses using Principal Component Analysis (PCA), an unsupervised method that can summarize the information content of the original data set and visualize it in a 2-dimensional space.10 As shown in Figure 1A, the PCA score plot discriminated the healthy subjects (green diamonds) from the OHSt patients (blue squares) on the basis of the information obtained from the metabolic fingerprints.
Figure 1.
OHSt patients and healthy subjects can be discriminated on the basis of the metabolic information contained in their red blood cells. (A) PCA score plots for RBC extracts obtained from 4 OHSt patients and 24 controls including patients’ relatives. Each point (blue squares: OHSt patients; green diamonds: healthy subjects) represents an LC/MS metabolic fingerprint. Data were acquired in negative mode of electrospray ionization. Variables included in the resulting data matrix were mean-centered and scaled to unit variance before PCA. Scores plot separates metabolic fingerprints of healthy subjects from those of OHSt patients on the first component, indicating that most of the variance of the data set correlates with OHSt. (B-C) A metabolic signature of OHSt patients is obtained from LC/MS analysis of RBC extracts. Metabolites exhibiting significant concentration variations between controls and OHSt patients are shown. Values are ratios of individual levels observed for OHSt patients (blue point) and controls (green, mean with 95% CI) to mean levels obtained for healthy subjects. (B) Metabolites whose levels are decreased in OHSt patients. (C) Metabolites whose levels are increased in OHSt patients.
Eighty-nine metabolites were identified from the metabolic fingerprints.8 Twenty-nine displayed significantly decreased (Figure 1B) or increased (Figure 1C) concentrations in OHSt patients compared with controls. No significant metabolite concentration differences were found between RBC extracts from OHSt patients with low (7.5%) and high (15.8%) reticulocyte count (data not shown) indicating that the differences observed were not directly related to the number of reticulocytes present in the analyzed samples. A similar absence of effect of reticulocyte concentration on the RBC metabolome was found in a previous study on sickle cell disease.8
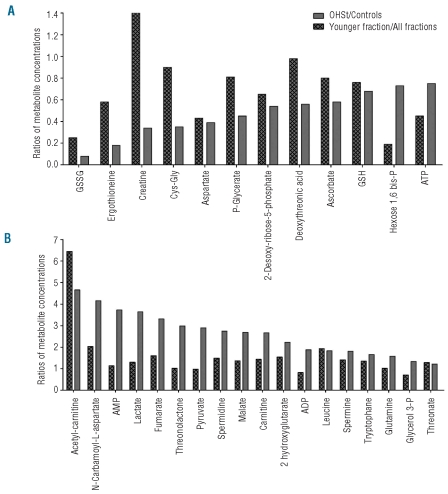
To ensure that this metabolic signature was directly related to OHSt and not to the diminished half-life of OHSt RBCs (data not shown), we compared the metabolomes of young RBCs, i.e. less than 20-day old cells, containing 1.6% reticulocytes, from healthy subjects to those of OHSt patients’ RBCs. To this end, control’s RBCs were fractioned according to their ages9 and the metabolomes of the different fractions were analyzed by LC/MS. Then, for each discriminating metabolite previously identified, we compared the metabolite concentration ratio of OHSt patients’ RBCs on controls’ RBCs with the ratio of young fraction on all fractions in normal RBCs. Metabolites exhibiting comparable concentration ratios were considered to have been impacted by the age of RBCs. This analysis showed that the concentration of metabolites, such as GSH, desoxyribose-5-phosphate, aspartate, leucine, spermine or threonate, was related to the youth of OHSt RBCs and not to intrinsic metabolic disorders of OHSt patients’ RBCs (Figure 2A and B).
Figure 2.
A metabolic signature of OHSt patients and of young normal RBCs can be obtained from LC/MS analysis of RBC extracts. Metabolites showing significant concentration variations between controls and OHSt patients are shown. (A) Metabolites whose levels had decreased in OHSt patients were analyzed in young normal RBCs. (B) Metabolites whose levels had increased in OHSt patients were analyzed in young normal RBCs. Values are ratios of mean levels observed for OHSt patients and controls (gray panel), compared to ratios of mean levels found for young RBCs and all aged RBCs (hatched panel).
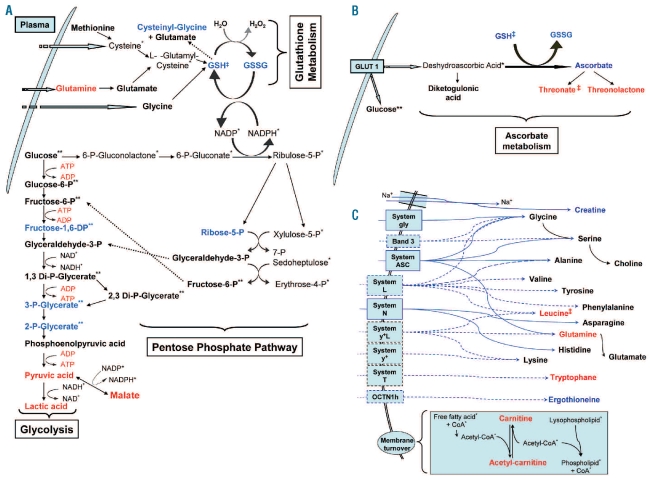
In the glycolytic and pentose phosphate pathways, we detected decreased concentrations of many metabolic intermediates, such as hexose-1,6-bisphosphate, phospho-glycerate, i.e. 2-phospho-glycerate or 3-phospho-glycerate, or desoxy-ribose-5-phosphate (Figures 1B and 3A), while others, like hexose-6-phosphate, glyceralde-hyde-3-phosphate, bisphospho-glycerate, phospho-enolpyruvic acid and disphosphoglyceric acid, did not vary (Figure 3A). All final products of glycolysis, i.e. pyruvate, lactate and malate (which can be produced from pyruvate), displayed higher concentrations in OHSt patients’ RBCs (Figures 1C, and 3A) than in controls’ RBCs. As RBCs of the OHSt patients display a very high activity of the Na+K+-ATPase, thought to compensate for the monovalent cation leakage associated with the mutated RhAG transporter, these results are consistent with the high levels of ATP required for the Na+K+-ATPase activity,1 ATP production only depending on glycolysis in RBCs. Interestingly, we did not observe any variations in concentration of glycolysis end-products, such as pyruvate or lactate, in sickle cell disease RBCs,8 strongly suggesting that the alteration in the concentrations of these glycolysis end-products was related to specific alterations of OHSt patients’ RBCs.
Figure 3.
Alterations in RBC pathways. Metabolites in red increased in OHSt patients RBCs versus control. Metabolites in blue decreased in OHSt patients RBCs versus control. Metabolites in black stayed unchanged in OHSt patients’ RBCs versus control. Metabolites with one asterisk (*) were not observed in our study. Metabolites with two asterisks (**) cannot be set apart from their isomers in our study. Metabolites with a double cross (‡) are related to RBC ages and not to OHSt. The solid black arrow indicates an enzymatic transformation and the dashed blue arrow indicates a facilitated diffusion (passive transport) through the erythrocyte membrane. The solid blue arrow indicates a secondary active transport through the erythrocyte membrane. (A) GSH metabolism, glycolytic activity and pentose phosphate pathway. (B) Ascorbate metabolism. (C) Amino-acid transport by erythrocyte membrane and role of carnitine in cell membrane turnover.
Because of the reduction or absence of stomatin in OHSt patients’ RBC membranes, the DHA transport by the Glut1 transporter decreases.7 Although DHA could not be detected by the method used, the ascorbate concentration underwent a 1.7-fold decrease in OHSt patients’ RBCs together with an increase in the concentrations of threonate and threonolactone; two metabolites originating from ascorbate degradation products (Figures 1B and C, and 3B). The same decrease in threonate concentration was found in young erythrocytes compared to whole RBC population, thus indicating the relative impacts of OHSt and age of RBCs on the increase or decrease of the metabolite concentrations detected. Finally, the variations in the concentration of metabolites that are part of the ascorbate pathway were moderate explaining why, despite the decreased uptake of DHA by OHSt patients’ RBCs, no clinical manifestation of an ascorbate deficit in OHSt patients was reported.
The moderate decrease in reduced glutathione (GSH) concentration found in OHSt patients’ RBCs is in line with decreased RBC GSH concentration in 3 cases of hemolytic anemia11 and is consistent with the decrease in GSH concentration probably being linked to the young age of OHSt patients’ RBCs (Figure 2A). We also found a 12-fold decrease of oxydized glutathione (GSSG) concentration (Figures 1B and 3A and B). This variation might be due to an increased GSSG efflux from RBCs, given that the membrane GSSG efflux transporter MRP1 is ATP-dependent and has a higher affinity for GSSG than for GSH.12 As OHSt RBCs are known to produce more ATP than normal RBCs,1 the efflux of GSSG through MRP1 might increase.
Only two amino acids (glutamine and tryptophan) showed increased concentrations in OHSt patients’ RBCs (Figures 1C and 3C), whereas the concentrations of glycine, serine, glutamate, alanine, choline, valine, tyro-sine, phenylalanine, histidine, asparagine, and lysine remained unchanged. Carnitine and acetyl-carnitine concentrations had increased in OHSt cells (Figures 1C and 3C) which might indicate increased membrane turnover. A decreased concentration of creatine (Figures 1B, 2A and 3C) was detected in OHSt patients’ RBCs. Creatine has been reported to be a youth marker in RBCs13 and in hemolytic anemia. Creatine concentration is expected to increase due to the short RBC lifespan (see also in sickle cell anemia8). This discrepancy might be the consequence of a lower intake of creatine dependent on the sodium gradient,14 itself altered in OHSt RBCs.15
Finally, we found a decreased concentration of ergothioneine in OHSt patients’ RBCs. Ergothioneine is synthesized from histidine in organisms such as actinobacteria or filamentous fungi16 but not in humans in whom its presence is due to dietary intake.17 Ergothioneine has anti-oxidant properties and is imported into human cells like erythrocytes by a specific transporter (OCTN1).18 The decreased concentrations of ergothioneine observed in OHSt patients’ RBCs may be linked to a decreased OCTN1 activity or to an increased consumption compensating for the decreased concentration of GSSG in OHSt patients’ RBCs.
In conclusion, the metabolic signature of OHSt patients’ RBCs provided new insights into their molecular alterations, including increased GSSG efflux, decreased creatine transport, accumulation of glutamine and tryptophan, and decreased concentration of ergothioneine. Some of the metabolomic alterations found in the OHSt patients’ RBCs, such as GSH or desoxyribose 5P variations, are also found both in young normal RBCs and in sickle cell disease RBCs. This strongly suggests that these alterations are related to the young age of RBCs in these 2 hemolytic anemias. Conversely other alterations, such as pyruvate or lactate variations, were found only in the metabolic signature of OHSt patients’ RBCs, and not in sickle cell disease patients’ RBCs, suggesting that these alterations are linked to OHSt patients’ RBCs phenotype. Finally, this study demonstrates that RBC metabolomics could be an appropriate and useful method to assess large cohorts of patients with frequent pathologies, like sickle cell disease,8 as well as patients with rare diseases such as OHSt, for which specific metabolomic signatures were obtained from 4 patients.
Acknowledgments
The authors thank Drs C. Armari-Alla, C. Barro, A. Robert and V. Dumas, Professors P. Sié and P. Bordigoni for their help in providing us with blood samples. DD, GM, and BK are supported by fellowships from the ‘Association pour la Recherche sur le Cancer’ (ARC), the Institut National de la Santé et de la Recherche Médicale (INSERM), the Commissariat à l’Energie Atomique (CEA), and the Académie de Médecine. JFH is supported by a grant provided by the DIANE Project (Désordres Inflammatoires dans les Affections Neurologiques) (Région Wallonne, Belgium). This work was supported by the Commissariat à l’Energie Atomique (CEA) and the Institut National de la Santé et de la Recherche Médicale (INSERM).
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Mentzer WC, Jr, Smith WB, Goldstone J, Shohet SB. Hereditary stomatocytosis: membrane and metabolism studies. Blood. 1975;46(5):659–69. [PubMed] [Google Scholar]
- 2.Bruce LJ, Guizouarn H, Burton NM, Gabillat N, Poole J, Flatt JF, et al. The monovalent cation leak in overhydrated stomatocytic red blood cells results from amino acid substitutions in the Rh-associated glycoprotein. Blood. 2009;113(6):1350–7. doi: 10.1182/blood-2008-07-171140. [DOI] [PubMed] [Google Scholar]
- 3.Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet. 2000;26(3):341–4. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- 4.Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J. 2008;22(1):64–73. doi: 10.1096/fj.07-9097com. [DOI] [PubMed] [Google Scholar]
- 5.Boron WF. Sharpey-Schafer lecture: gas channels. Exp Physiol. 2010;95(12):1107–30. doi: 10.1113/expphysiol.2010.055244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fricke B, Parsons SF, Knopfle G, von Düring M, Stewart GW. Stomatin is mis-trafficked in the erythrocytes of overhydrated hereditary stomatocytosis, and is absent from normal primitive yolk sac-derived erythrocytes. Br J Haematol. 2005;131(2):265–77. doi: 10.1111/j.1365-2141.2005.05742.x. [DOI] [PubMed] [Google Scholar]
- 7.Montel-Hagen A, Kinet S, Manel N, Mongellaz C, Prohaska R, Battini JL, et al. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132(6):1039–48. doi: 10.1016/j.cell.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Darghouth D, Koehl B, Madalinski G, Heilier JF, Bovee P, Xu Y, et al. Patho-physiology of sickle cell disease is mirrored by the red blood cell metabolome. Blood. 2011;117(6):e57–66. doi: 10.1182/blood-2010-07-299636. [DOI] [PubMed] [Google Scholar]
- 9.Bosch FH, Werre JM, Roerdinkholder-Stoelwinder B, Huls TH, Willekens FL, Halie MR. Characteristics of red blood cell populations fractionated with a combination of counterflow centrifugation and Percoll separation. Blood. 1992;79(1):254–60. [PubMed] [Google Scholar]
- 10.Trygg J, Holmes E, Lundstedt T. Chemo-metrics in metabonomics. J Proteome Res. 2007;6(2):469–79. doi: 10.1021/pr060594q. [DOI] [PubMed] [Google Scholar]
- 11.Lo SS, Marti HR, Hitzig WH. Hemolytic anemia associated with decreased concentration of reduced glutathione in red cells. Acta Haematol. 1971;46(1):14–23. doi: 10.1159/000208555. [DOI] [PubMed] [Google Scholar]
- 12.Cole SP, Deeley RG. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol Sci. 2006;27(8):438–46. doi: 10.1016/j.tips.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Jiao Y, Okumiya T, Saibara T, Tsubosaki E, Matsumura H, Park K, et al. An enzymatic assay for erythrocyte creatine as an index of the erythrocyte life time. Clin Biochem. 1998;31(2):59–65. doi: 10.1016/s0009-9120(97)00164-1. [DOI] [PubMed] [Google Scholar]
- 14.Bennett SE, Bevington A, Walls J. Regulation of intracellular creatine in erythrocytes and myoblasts: influence of uraemia and inhibition of Na,K-ATPase. Cell Biochem Funct. 1994;12(2):99–106. doi: 10.1002/cbf.290120204. [DOI] [PubMed] [Google Scholar]
- 15.Delaunay J. The hereditary stomatocytoses: genetic disorders of the red cell membrane permeability to monovalent cations. Semin Hematol. 2004;41(2):165–72. doi: 10.1053/j.seminhematol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Genghof DS. Biosynthesis of ergothioneine and hercynine by fungi and Actinomycetales. J Bacteriol. 1970;103(2):475–8. doi: 10.1128/jb.103.2.475-478.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melville DB, Horner WH, Otken CC, Ludwig ML. Studies on the origin of ergothioneine in animals. J BiolChem. 1955;213(1):61–8. [PubMed] [Google Scholar]
- 18.Grundemann D, Harlfinger S, Golz S, Geerts A, Lazar A, Berkels R, et al. Discovery of the ergothioneine transporter. Proc Natl Acad Sci USA. 2005;102(14):5256–61. doi: 10.1073/pnas.0408624102. [DOI] [PMC free article] [PubMed] [Google Scholar]