Abstract
Platelet glycoprotein GPIbα mutations are the basic defect behind Bernard-Soulier syndrome, a rare inherited macrothrombocytopenia characterized by anomalies of the GPIbα, GPIbβ and GPIX subunits of von Willebrand factor receptor. A 32-year old man was investigated for suspected Bernard-Soulier syndrome. Ristocetin induced agglutination was absent. Flow cytometry and Western blot analysis showed a severe reduction in GPIbα, but sequencing revealed only a biallelic c.386A>G substitution, theoretically leading to a p.Asn110Glu variation. To further clarify the data, megakaryocyte cultures were set. Though the maturation of megakaryocytes was normal, proplatelet formation was defective and GPIbα mRNA was not detectable. GPIX protein was slightly reduced and GPIbβ polypeptide almost absent. Computational analysis showed that the c.386A>G mutation disrupted an exon splicing enhancer motif involved in the proper maturation of the GPIbα transcript. The c.386A>G mutation suggests a unique mutational mechanism causing the virtual absence of GPIbα without creating a stop codon.
Keywords: Bernard-Soulier, congenital thrombocytopenia, glycoprotein Ibα, platelets
Introduction
Bernard-Soulier syndrome (BSS) is a rare inherited form of macrothrombocytopenia characterized by a defective platelet von Willebrand Factor (vWF) receptor.1 This complex comprises 4 different subunits, the glycoproteins (GPs) Ibα, Ibβ, IX, and V, belonging to the family of leucine-rich repeat (LRR) proteins. The major component, GPIbα (CD42b), contains eight LRR,2 all but the second of which are characterized by a consensus sequence with a conserved asparagine (N) in the sixth position.3 Mutations of these GPs have been recognized as being responsible for different forms of classical (recessive) or autosomal dominant BSS. Many different GPIbα gene mutations have been described so far, leading to amino acid substitutions or stop codons, often occurring after frameshifts producing aberrant proteins.1, 4–10
Biallelic mutations causing a stop codon are generally responsible for the absence of the protein from the platelet surface. In some cases, the truncated protein lacks transmembrane anchoring and is retrievable as a soluble protein fraction.7,8 Other BSS mutations are biallelic or monoallelic single base variations responsible for various amino acid substitutions. BSS patients expressing a mutated protein usually have an impaired capacity to bind natural receptor ligands and/or anti-CD42b monoclonal antibodies (mAbs).3
GPIbα interactions with its ligand play an important part in proplatelet formation. The megakaryocytes (MK) of BSS patients with the monoallelic Bolzano mutation reveal quantitative and qualitative tubulin distribution abnormalities that affect proplatelet formation in vitro, contributing to macrothrombocytopenia.11
Design and Methods
The study followed the guidelines approved by our local ethical committee (Prot. n. 1192P) and patient consent was obtained in accordance with the Declaration of Helsinki. The patient also gave his approval to the publication of the present paper.
The patient is a 32-year old man with Klinefelter syndrome (XXY genotype) born of blood-related parents (2nd generation). He has had a life-long history of thrombocytopenia (39×109/L by automatic count, 51×109/L by manual count; 50% giant platelets), diagnosed as idiopathic thrombocytopenic purpura (ITP) at the age of one year, when he underwent surgery for ventriculo-peritoneal shunting for endocranial hypertension secondary to Klinefelter-related hydrocephalus. No complications were recorded during or after the procedure, but the patient was given prophylactic platelet concentrate transfusions. In adulthood, the patient refused steroid treatments or splenectomy. The patient shares a thalassemic trait with his mother and sister. None of his family members consented to the study. As for his medical history, the patient only reported a mild hemorrhagic diathesis with occasional epistaxis, and no hematologic abnormalities other than those relating to the thalassemic trait.
Platelet-rich plasma (PRP) was prepared and processed from patient and healthy controls as previously described.6
Ristocetin-induced platelet agglutination (RIPA) at 1.2, 1.5 and 2 mg/mL was conducted on PRP according to the Born method, as already described 6 Platelet agglutination values were expressed as percentage differences between the light transmission of PRP (0%) and PPP (100%) in patients and controls.
For the flow cytometric study, 22.5 μL of PRP were incubated with monoclonal antibodies (mAbs) in a 25 μL final reaction volume for 15 min at room temperature. Appropriate normal and isotopic controls were set for each experiment, carried out as described previously.7 DNA extraction, amplification and sequencing of the GPIbα gene were performed as explained elsewhere,6 using the primer sets published by Savoia et al.12
Platelet lysate analysis by Western blot (WB) was carried out as previously described.7 The features of the mAbs used for flow cytometry and WB13 are available in the Online Supplementary Appendix, along with details of cDNA analysis.
Differentiation of MKs from patient’s peripheral blood and proplatelet formation (PPF) evaluation was carried out as previously described.11,14 The MK yield and PPF were evaluated at the end of the culture as reported in detail elsewhere.11 Briefly, MKs were identified on the basis of CD41 expression and assigned to distinct stages of maturity according to standard morphological criteria.15 MKs forming proplatelets were identified as large CD41+ cells extending α-tubulin-positive, filament-like structures. Details are available in the Online Supplementary Appendix.
Specimens mounted in Mowiol 4–88 were observed with an Axioscope 2 Plus microscope (Carl Zeiss, Gottingen, Germany) using a 63/1.25 or 100/1.30 Plan Neofluar oil-immersion objective.
For computational analysis data, we submitted both the wild-type and the mutated sequences to the web-based program ESE-finder 3.0 (http://rulai.cshl.edu/tools/ESE).16
Matrices and thresholds used are available in the Online Supplementary Table S1.
Results and Discussion
Platelet agglutination was absent in response to ristocetin (RIPA) at 1.2, 1.5 and 2 mg/mL.
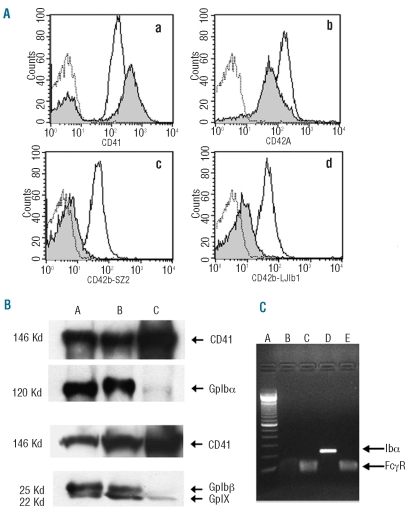
Compared to normal controls, flow cytometry analysis showed increased membrane expression of CD41, consistent with their larger size. Conversely, the anti-GPIbα mAbs signal was nearly absent for both the SZ2 and the LJIb1 clones, directed against different N-term epitopes. Finally, GPIX expression was slightly reduced (Figure 1A). CD49b expression, PAC-1 or anti-CD62P binding before and after ADP stimulation were normal (data not shown).
Figure 1.
GPIbα expression. (A) Flow cytometry tests (empty curves: healthy control histograms; gray filled curves: patient’s histograms; dotted curves: non-specific binding). Panel a: CD41 expression. The increased anti-CD41 mAb binding rate is consistent with the larger average size of the patient’s platelets. Panel b: CD42a (GPIX) expression is only slightly reduced on platelet surface. Panels c and d: weak GPIbα expression of CD42b (GPIbα) on patient’s platelets compared to control. This test was conducted with two different anti-CD42b mAbs clones: SZ2 (Panel c) and LJIb1 (Panel d). (B) WB analysis on total platelet lysates. Upper panel: reduced GPIbα expression in the patient with c.386A>G substitution (C) compared to a subject with the N41H heterozygous substitution (A) and with healthy control (B). Lower panel: the GPIbβ band (25 kDa) is absent and the GPIX band at 22 KDa is slightly reduced in the patient with c.386A>G substitution (C) compared to control subjects (AB), in agreement with flow cytometry data. (C) RT-PCR. c.386A>G patient’s cDNA retrotranscription is undetectable (B) compared to healthy control (D, 250 bp). No differences between patient and healthy control are evident for the housekeeping gene FCγRIIA (Lanes C and E, respectively). Lane A: Invitrogen 100 bp molecular weights.
Genetic analysis on GPIbα gene (annotations refer to GeneBank nucleotide sequence gi:3668108; Accession number: AC004771) revealed a single base mutation, c.386A>G substitution (Online Supplementary Figure S1), supposed to cause a p.N110D replacement in the fourth LLR of GPIbα. The biallelic condition of the mutation is hypothetic, due to the unavailability of the patient’s relatives’ DNA samples, but it is strongly suggested by the strict consanguinity of his parents.
Analysis of the entire coding region in our patient revealed the following polymorphic set: −5 T/T; HPA2 a/a and VNTR c/c allele with a synonymous C>T substitution at T419 codon in the second VNTR repeat, seldom found in the normal population. GPIbβ and GPIX coding regions were not mutated (data not shown).
WB analysis was performed on total platelet lysates using both samples from healthy and monoallelic Bernard-Soulier N41H patients as controls. Regular bands of GPIbα were present at about 120 kilodalton (kDa) in the healthy controls and in the BSS N41H, as expected from a single amino acid substitution N41H.7 On the contrary, GpIbα expression was markedly decreased in platelet lysates from our patient. Compared to the healthy control, GPIbβ was absent whereas a band correspondent to GPIX (22Kd) was visible, albeit significantly reduced (Figure 1B).
Absence of free GpIbβ in our blot analysis does not necessarily rule out the possibility that the glycoprotein may still be present in a complex with GpIX. To test this hypothesis, we studied the reactivity of the monoclonal antibody SZ1 in our patient. This antibody recognizes a conformational epitope on GPIX generated as a consequence of the interaction between GpIbβ and GpIX.17 As shown in Online Supplementary Figure S2, the antibody was able to detect a band corresponding to the GpIX-GpIbβ complex (47 KDa) in the healthy control and in the BSS N41H control, whereas the same band could not be visualized in our patient. However, using the same conformational antibody, a faint band corresponding to the full GpIb/GpIX (161 kDa) complex was visible instead. Therefore, residual GpIbβ appears to be able to make complexes and generate conformational changes in GpIX only in the presence of residual GpIbα (Online Supplementary Figure S2). These data suggest that severe reduction of GpIbα, as in our case, is associated to degradation of GpIbβ.
To establish whether the dramatic reduction of GPIbα protein was due to a transcriptional or a translational impairment, we studied the mRNA from MKs. The retrotranscription demonstrated the absence of the GPIbα mRNA in our patient, while the expression of the house-keeping gene was normal both in patient and control (Figure 1C). Overall these data demonstrate that the c.386A>G substitution leads, surprisingly, to the disappearance of the mature transcript in MKs, thus explaining the virtual absence of GPIbα protein as seen from flow cytometry and WB analysis.
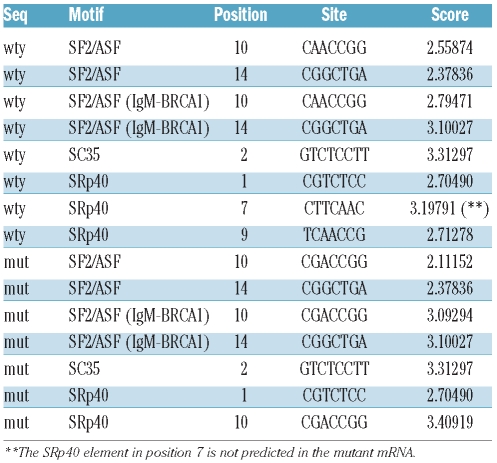
In order to further characterize this mutation, the sequence surrounding the nucleotide substitution was investigated for the presence of any element involved in the recruitment of the splicing machinery (other than the canonical splice sites), and exon splicing enhancer (ESE) motifs in particular.16,8 ESE motifs are short sequences of coding exons required for efficient splicing and splice site recognition. To establish whether the mutation might involve putative ESE, we submitted both the wild-type and the mutated sequences to the web-based program ESE finder 3.0 (http://rulai.cshl.edu/tools/ESE).16,17 The c.386A>G mutation dramatically affects an ESE motif “CTTCAAC” predicted to be a SRp40 binding site. This motif is predicted in the wild-type exon with a score of 3.19791, while the prediction disappears completely in the mutant exon (Table 1).
Table 1.
ESEfinder results. The table shows the ESE sequences predicted for the wild-type transcript.
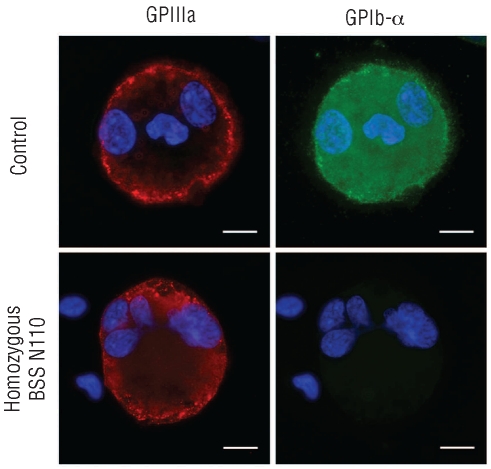
Additional studies were carried out on MK cultures. As shown in Figure 2, and in agreement with our data in platelets, control-derived MKs expressed both GPIIIa (CD61) and GPIbα, whereas patient-derived MKs expressed GPIIIa, but not GPIbα, on the surface. We also demonstrated that the c.386A>G mutation does not affect MK differentiation or maturation as the immunomorphological evaluation of cultured peripheral CD45+ blood progenitors revealed no difference in the percentage of CD41+ cells between patient and controls (Online Supplementary Table S2). As expected, mature MKs from patient’s peripheral blood reseeded in fresh medium did not extend any proplatelets as compared to controls that regularly did (Online Supplementary Figure S3). The same results emerged when patient-derived MKs were allowed to adhere to fibrinogen or VWF (data not shown). Finally, as seen for the controls, the patient-derived MKs did not extend any proplatelets adhering to type I collagen, while around 20% of them underwent cytoplasm reorganization and spreading (data not shown).
Figure 2.
Immunofluorescence analysis on megakaryocytes. Absence of GPIbα on the cell surface of patient-derived MKs. Megakaryocytes were derived from the control and the patient’s peripheral blood and stained with anti-GPIIIa (red) and anti-GPIbα (green) antibodies. Nuclei were counterstained with Hoechst 33288 (blue). Representative immunofluorescence images are shown (scale bars=10 μm).
A mutated GPIbα is a very common defect in the classical forms of BSS and its variants. The GPIbα gene mutations described to date are small deletions, single nucleotide insertions, and missense or nonsense substitutions, all resulting in sequence variations or premature stop codons.
The patient described in this paper had a severe macrothrombocytopenia with mild hemorrhagic diathesis, identified during routine analyses and classified as ITP in childhood. When the patient came under our observation, his parents’ consanguinity, the early onset of the disease, and the high number of macrothrombocytes prompted us to search for an inherited macrothrombocytopenia. The absence of RIPA pointed to a diagnosis of BSS which was confirmed by flow cytometry, immunofluorescence and WB analysis, all showing the severe reduction of GPIbα protein on the platelet surface and lysates. The WB analysis showed that also GPIbβ expression was virtually absent in the patient, whereas GPIX band resulted only slightly reduced. Based on these findings, we hypothize that the defect of GPIbα is indirectly responsible for the GPIbβ altered maturation process and early degradation. This impairment does not seem to affect the GPIX to the same extent, as flow cytometry and WB analysis showed only a slight reduction in this subunit.
Therefore, in our patient the GPIb/V/IX complex assembly is severely defective, a finding that is in agreement with the absence of a severe bleeding diathesis, as we have already reported.6
GPIbα gene sequencing showed a homozygous c.386A>G substitution in the first base of the codon encoding the asparagine (N) 110. The effect of this point mutation would theoretically be to substitute the amino acid 110 from N to aspartic acid (D), rather than affect the protein expression level. To better understand the mechanisms responsible for the protein’s disappearance, mRNA expression was analyzed. The low number of platelets from the patient and the contamination of the RNA preparation by lymphocytes prevented us from studying the platelet RNA directly, so we performed the analysis on cultured MK RNA. The RT-PCR showed that in the patient the GPIbα transcript was undetectable, implying that GPIbα mRNA is either not produced in the MK cytoplasm or that it is unstable. Recently, Savoia et al. used in silico analysis to explore whether a p.N110D substitution heavily destabilizes the protein folding.19 Our data on mRNA, together with WB on total lysates, are more consistent with an instability and/or anomalous maturation of the GPIbα mRNA as being responsible for the quantitative defect of the protein.
MK cultures also confirmed the virtual absence of the GPIbα protein on the MK surface. The c.386A>G mutation does not affect the MK maturation process, but only their capacity to extend proplatelets, confirming that a normal GPIbα is required for platelet formation.11 A thorough understanding of the mRNA instability mechanisms responsible for the behavior of the protein is beyond the scope of this report and deserves more accurate expression studies, but we tried to design a hypothetical scenario using computational analysis to compare the mutated and normal RNAs. GPIbα is a gene comprising 2 exons, the first being a non-coding exon. ESEfinder web tool analysis showed that the described mutation can dramatically affect a “CTTCAAC” ESE sequence motif, thought to be a SRp40 binding site, predicted in the wild-type exon with a score of 3.19791. The prediction disappears completely in the mutant exon. SR proteins form a family of splicing factors with RNA recognition motifs.20 ESE disruption has been linked to several genetic disorders and the disruption of this ESE motif might be responsible for exon skipping and the production of an aberrant or unstable transcript. Although there are examples in the literature supporting this interpretation, we believe this hypothesis needs to be investigated experimentally.21–23
GPIbα mRNA degradation as a consequence of the protein unfolding may also explain our finding.24 However, the lack of protein accumulation in platelet total lysates and MK, demonstrated with different mAbs clones of anti-GPIbα, makes this mechanism unlikely in our case.
Moreover, the GPIbα impaired expression is in part accountable also for the anomalous expression and/or maturation of GPIX and GPIbβ. Our data on GPIbβ apparently do not agree with previous reports suggesting that absence of GpIbα does not affect the generation of GpIbβ/GpIX complexes. However, these data deriving from transfected cellular systems may not entirely mimic physiological conditions. On the contrary, our results are more consistent with previous experimental data on platelets, showing almost complete absence of GpIbβ in a case of mutation generating a GpIbα truncated form,9 again suggesting a protective role of GpIbα toward GPIbβ stability. The fact that in the cited report the defective GpIbα was still produced in considerable amounts in platelet cytoplasm may explain the presence of residual GPIbβ compared to our patient in whom GpIbα, present only in traces, could not exert its protective role adequately.
In conclusion, we suggest that the c.386A>G homozygous mutation of GPIbα gene could be responsible for an altered mRNA maturation leading to a nearly undetectable level of the protein in patient platelets and to a clinical BSS phenotype even without introducing a stop codon in the protein sequence.
Acknowledgments
The authors are indebted to FA Coburn for reviewing English Language use in this manuscript.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding: this work was supported by grants from the Italian Ministry for University and Research (MIUR), Rome, Italy and from the University of Padua, Italy to FF and from the Cariplo Foundation 2006.0596/10.8485 to AB.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Lopez JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard-Soulier syndrome. Blood. 1998;91(12):4397–418. [PubMed] [Google Scholar]
- 2.Peng Y, Shrimpton CN, Dong FJ, Lopez JA. Gain of von Willebrand factor binding function by mutagenesis of a species-conserved residue within the leucine-rich repeat region of platelet glycoprotein Ibα. Blood. 2005;106(6):1982–7. doi: 10.1182/blood-2005-02-0514. [DOI] [PubMed] [Google Scholar]
- 3.Afshar-Kharghan V, Gineys G, Schade AJ, Sun L, Li CQ, McIntire LV, et al. Necessity of conserved asparagine residues in the leucine-rich repeats of platelet glycoprotein Ibalpha for the proper conformation and function of the ligand-binding region. Biochemistry. 2000;39(12):3384–91. doi: 10.1021/bi992061j. [DOI] [PubMed] [Google Scholar]
- 4.Kenny D, Jonsson OG, Morateck PA, Montgomery RR. Naturally occurring mutations in glycoprotein Ib alpha that result in defective ligand binding and synthesis of a truncated protein. Blood. 1998;92(1):175–83. [PubMed] [Google Scholar]
- 5.Kunishima S, Imai T, Hamaguchi M, Saito H. Novel heterozygous missense mutation in the second leucine-rich repeat of GPIbα affects GPIb/IX/V expression and results in macrothrombocytopenia in a patient initially misdiagnosed with idiopathic thrombocytopenic purpura. Eur J Haematol. 2006;74(4):348–55. doi: 10.1111/j.1600-0609.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 6.Vettore S, Scandellari R, Scapin M, Lombardi AM, Duner E, Randi ML, et al. A case of Bernard-Soulier Syndrome due to a biallelic four bases deletion TGAG of GPIbα gene: lack of GPIbα but absence of bleeding. Platelets. 2008;19(5):388–91. doi: 10.1080/09537100801949976. [DOI] [PubMed] [Google Scholar]
- 7.Vettore S, Scandellari R, Moro S, Lombardi AM, Scapin M, Randi ML, et al. Novel point mutation in a leucine-rich repeat of the GPIbα chain of the platelet von Willebrand factor receptor, GPIb/IX/V, resulting in an inherited dominant form of Bernard-Soulier syndrome affecting two unrelated families: the N41H variant. Haematologica. 2008;93(11):1743–7. doi: 10.3324/haematol.12830. [DOI] [PubMed] [Google Scholar]
- 8.Afshar-Kharghan V, Craig FE, Lopez JA. Bernard-Soulier syndrome in a patient doubly heterozygous for two frameshift mutations in the glycoprotein Ibα gene. Br. J Haemat. 2000;110(4):919–24. doi: 10.1046/j.1365-2141.2000.02261.x. [DOI] [PubMed] [Google Scholar]
- 9.Kunishima S, Miura H, Fukutani H, Yoshida H, Osumi K, Kobayashi S, et al. Bernard-Soulier Syndrome Kagoshima: Ser444>Stop mutation of glycoprotein GP Ib alpha resulting in circulating truncated GPIbα and surface expression of GPIbβ and GPIX. Blood. 1994;84(10):3356–60. [PubMed] [Google Scholar]
- 10.Kenny D, Newman PJ, Mopratek PA, Montgomery RR. A dinucleotide deletion results in defective membrane anchoring and circulating soluble glycoprotein Ib alpha in a novel form of Bernard-Soulier Syndrome. Blood. 1997;90(7):2626–33. [PubMed] [Google Scholar]
- 11.Balduini A, Malara A, Pecci A, Badalucco S, Bozzi V, Pallotta I, et al. Proplatelet formation in heterozygous Bernard-Soulier syndrome type Bolzano. J Thromb Haemost. 2009;7(3):478–84. doi: 10.1111/j.1538-7836.2008.03255.x. [DOI] [PubMed] [Google Scholar]
- 12.Savoia A, Balduini CL, Savino M, Noris P, Del Vecchio M, Perrotta S, et al. Autosomal dominant macrothrombocytopenia in Italy is most frequently a type of heterozygous Bernard-Soulier syndrome. Blood. 2001;97(5):1330–5. doi: 10.1182/blood.v97.5.1330. [DOI] [PubMed] [Google Scholar]
- 13.De Marco L, Mazzucato M, Fabris F, De Roia D, Coser P, Girolami A, et al. Variant Bernard-Soulier syndrome type Bolzano. A congenital bleeding disorder due to a structural and functional abnormality of the platelet glycoprotein Ib-IX complex. J Clin Invest. 1990;86(1):25–31. doi: 10.1172/JCI114692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi A, Zuccarello LV, Zanaboni G, Monzani E, Dyne KM, Cetta G, et al. Type I collagen CNBr peptides: species and behavior in solution. Biochemistry. 1996;35(19):6048–57. doi: 10.1021/bi9518151. [DOI] [PubMed] [Google Scholar]
- 15.Williams N, Levine RF. The origin, development and regulation of megakaryocytes. Br J Haematol. 1982;52(2):173–80. doi: 10.1111/j.1365-2141.1982.tb03878.x. [DOI] [PubMed] [Google Scholar]
- 16.Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR. ESEfinder: a web resource to identify exonic splicing enhancers. Nucleic Acid Research. 2003;31(13):3568–71. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez JA, Li CQ, Weisman S, Chambers M. The glycoprotein Ib-IX complex-specific monoclonal antibody SZ1 binds to a conformation-sensitive epitope on glycoprotein IX: implications for the target antigen of quinine/quinidine-dependent autoantibodies. Blood. 1995;85(5):1254–58. [PubMed] [Google Scholar]
- 18.Blencowe BJ. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem Sci. 2000;25(3):106–10. doi: 10.1016/s0968-0004(00)01549-8. [DOI] [PubMed] [Google Scholar]
- 19.Savoia A, Pastore A, De Rocco D, Civaschi E, Di Stazio M, Bottega R, et al. Clinical and genetic aspects of Bernard-Soulier syndrome: searching for genotype/phenotype correlations. Haematologica. 2011;96(3):417–23. doi: 10.3324/haematol.2010.032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PJ, Zhang C, Wang J, Chew SL, Zhang MQ, Krainer AR. An increased specificity score matrix for the prediction of SF2/ASF-specific exonic splicing enhancers. Hum Mol Genet. 2006;15(16):2490–508. doi: 10.1093/hmg/ddl171. [DOI] [PubMed] [Google Scholar]
- 21.Ridout CK, Keighley P, Krywawych S, Brown RM, Brown GK. A putative exonic splicing enhancer in exon 7 of the PDHA1 gene affects splicing of adjacent exons. Hum Mutat. 2008;29(3):451. doi: 10.1002/humu.9525. [DOI] [PubMed] [Google Scholar]
- 22.Liu HX, Cartegni L, Zhang MQ, Krainer AR. A mechanism for exon skipping caused by nonsense or missense mutations in BRCA1 and other genes. Nat Genet. 2001;27(1):55–8. doi: 10.1038/83762. [DOI] [PubMed] [Google Scholar]
- 23.Shotelersuk V, Desudchit T, Tongkobpetch S. ASA E382K disrupts a potential exonic splicing enhancer and causes exon skipping, but missense mutations in ASA are not associated with ESEs. Int J Mol Med. 2004;14(4):683–9. [PubMed] [Google Scholar]
- 24.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–7. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]