The prognosis for elderly patients with diffuse large B-cell lymphomas (DLBCL) remains particularly poor. The most common explanation involves co-morbidities related to advanced age, which strongly impact chemotherapy feasibility and tolerance.1 Despite a generally poor prognosis, a recent clinical trial dedicated to patients over 80 years of age demonstrated that a significant proportion of DLBCL patients could be cured using rituximab (R) and reduced-intensity chemotherapy (R-miniCHOP).2 In addition to well-known clinical conditions related to aging, the poor prognosis of DLBCL in elderly patients may also be related to intrinsic biological features of the tumor. The germinal center B-cell like (GCB)/activated B-cell like (ABC) signature is considered a major biological determinant of prognosis, independent of the international prognostic index (IPI), remaining predictive of outcome in patients treated by immunochemotherapy.3 However, the relationship between aging and the distribution of these two main gene expression profiles (GEP) has not been specifically studied, even though a trend for a higher proportion of ABC patients was reported by Rosenwald and colleagues in patients over 60 years of age.4 To address this question, we retrospectively determined the GEP of a series of 131 primary de novo DLBCL patients over 50 years of age (median age 68 years, range 50–91 years), selected on the basis of histopathological diagnosis and available tumor RNA. The GCB/ABC signature was determined by DASL technology with RNA extracted from fresh frozen material as previously reported.5,6 By this reproducible and robust method, we observed a concordance rate of 70–95% with immunohistochemistry.5,6 A direct comparison with microarray-based technology using RNA extracted from formalin-fixed paraffin-embedded tissues is ongoing.
Using this approach, 51 cases (39%) were classified in the GCB group, 57 cases (44%) in the ABC group and 23 (17%) in an unclassified (intermediate) group (Figure 1A). To determine the distribution of the GCB/ABC phenotype according to age, the overall population was divided into four age-related categories, i.e. Group 1: 50–60 years (n=36); Group 2: 60–70 years (n=41); Group 3: 70–80 years (n=36), and Group 4: 80 years and over (n=18). Figure 1B shows the rates of ABC/GCB/unclassified DLBCL according to these categories with a significant increase in the ABC subtype in proportion to increasing age. There was a significant difference in ABC/non-ABC (including GCB and unclassified) distribution between Group 1 and Group 4 (28% ABC vs. 67%, P=0.01, Fisher’s exact test). To consolidate our results, we next determined whether the same aging effect was also observed in an independent published series of DLBCL (data accessible from the GEO data-bank, NCBI, GSE10846).3 Despite technological differences in the determination of GEP (Affymetrix array vs. DASL), a similar increase in the ABC DLBCL proportion with age was observed (Figure 1C). In this series of patients over 50 years of age (median 68 years, range 50–92 years), the percentage of ABC DLBCL increases with age, with a differential distribution between patients aged between 50–60 years and those over 80 years of age (33% ABC vs. 54%, P=0.04, Fisher’s exact test, Figure 1C).
Figure 1.
GCB/ABC distribution with aging in DLBCL patients older than 50 years. (A) Hierarchical clustering. DLBCL were clustered according to gene expression as assessed by DASL. Genes fitting the ABC signature and genes fitting the GCB signature are indicated on the right. Patients are identified by their unique patient numbers. A color code related to age category is indicated (upper part of the HeatMap). Unsupervised hierarchical clustering was performed using ‘COR’ distances (the opposite of Pearson’s r) after Cubic Spline normalization (GenomeStudio V2009.1 software, Illumina). DLBCL diagnosis was performed according to WHO criteria. Primary mediastinal B-cell lymphomas and T-cell rich B-cell lymphomas were excluded. (B) ABC and non-ABC proportions according to age categories in a monocentric series of DLBCL. Number of patients by age category are indicated in brackets. (C) ABC and non-ABC proportions according to age in an independent series of DLBCL. Number of patients by age category are indicated in brackets.
We observed an average increase of 13.7% in ABC DLBCL for each ten years of aging after the age of 50 years in our series and of 7.5% in the Lenz series. A relationship between GCB/ABC distribution and age has been suggested in the context of pediatric lymphoma. Immunohistochemistry analysis indicated that, in this setting, DLBCL is associated most strongly with the GCB subgroup, which reflects the opposite pattern to that observed in the elderly.7 Whether a continuous increase in the ABC DLBCL in proportion to age can be observed remains to be determined.
Attempts to explain such a skewed ABC distribution during aging remain speculative. Diversity of normal repertoire of B/plasma cells is reduced with age, and this loss of diversity is characterized by clonal expansions of B cells in vivo.8 For instance, an increase in the number of B cells expressing the VH4-34 IgH gene has been noted during aging.9 We have previously shown that VH4-34 + DLBCL frequently expressed IgM, frequently displayed the t(3;14)(q27;q32) translocation, and are typically classified in the ABC subtype.5,10 This suggests that the increase in the proportion of ABC DLBLC with age may reflect a change in the B-cell population during aging. Another hypothesis relates to the putative pathological specificity of DLBCL occurring in elderly patients. EBV-related DLBCL are almost exclusively reported in elderly or very old patients, but this provisional WHO entity seems mostly limited to Asia and is rare in Western countries.11
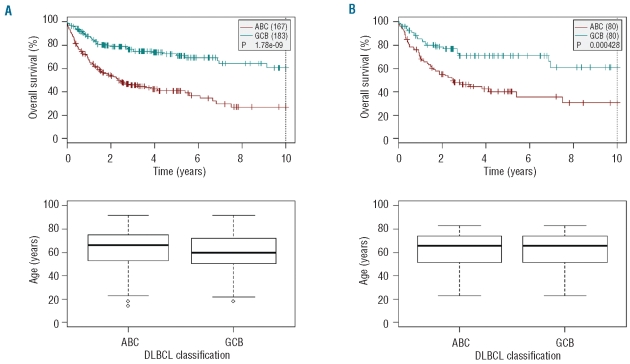
Age is an obvious and major prognostic factor which impacts directly on treatment strategies, chemotherapy feasibility and tolerance. This precludes, therefore, any comparison of survival between young and elderly patients with ABC DLBCL. To confirm the prognostic value of the ABC/GCB signature regardless of age, we compared overall survival according to molecular classification by producing random pairs of age-matched patients from the publicly available data published by Lenz et al.12 For each patient randomly selected in the GCB subtype, an age-matched patient was randomly selected from the ABC subtype, in order to form 80 GCB-ABC pairs of patients. In the 50 tested random paired combinations, the ABC subtype remains constantly correlated to an unfavorable outcome indicating that the unfavorable prognostic value of the molecular signature is not related to the skewed ABC distribution during aging (Figure 2).
Figure 2.
Impact of the GCB/ABC molecular classification in age-matched patients. Random combinations of patients were produced from the series published by Lenzet et al. (n=350). For each patient randomly selected in the GCB group, a patient with the same age (in years) was randomly selected in the ABC group, in order to form 80GCB/ABC pairs of age-matched patients. In the 50 subseries produced, 91% of the 350 samples were selected at least once, and all Log rank P values were significative (max=0.002). (A) Overall survival in the overall population. (B) Example of survival curves obtained in a series of 80 age-matched patients.
In conclusion, our results indicate that in addition to constitutive factors related to advanced age, the prognosis of DLBCL is also conditioned by intrinsic biological features of the tumor cells. Despite promising results obtained using conventional immunochemotherapy, such as R-miniCHOP, new therapeutic strategies in geriatric populations should include molecules able to target oncogenic pathways related to the ABC phenotype, such as the NFKB pathway.
Footnotes
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Kobayashi Y, Miura K, Hojo A, Hatta Y, Tanaka T, Kurita D, et al. Charlson Comorbidity Index is an independent prognostic factor among elderly patients with diffuse large B-cell lymphoma. J Cancer Res Clin Oncol. 2011;137(7):1079–84. doi: 10.1007/s00432-010-0973-x. [DOI] [PubMed] [Google Scholar]
- 2.Peyrade F, Jardin F, Thieblemont C, Thyss A, Emile JF, Castaigne S, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12(5):460–8. doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 3.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359(22):2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–47. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 5.Ruminy P, Etancelin P, Couronné L, Parmentier F, Rainville V, Mareschal S, et al. The isotype of the BCR as a surrogate for the GCB and ABC molecular subtypes in diffuse large B-cell lymphoma. Leukemia. 2011;25(4):681–8. doi: 10.1038/leu.2010.302. [DOI] [PubMed] [Google Scholar]
- 6.Lanic H, Mareschal S, Mechken F, Picquenot JM, Cornic M, Maingonnat C, et al. Interim positron emission tomography scan associated with international prognostic index and germinal center B cell-like signature as prognostic index in diffuse large B-cell lymphoma. Leuk Lymphoma. 2011 Aug 1; doi: 10.3109/10428194.2011.600482. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Oschlies I, Klapper W, Zimmermann M, Krams M, Wacker HH, Burkhardt B, et al. Diffuse large B-cell lymphoma in pediatric patients belongs predominantly to the germinal-center type B-cell lymphomas: a clinicopathologic analysis of cases included in the German BFM (Berlin-Frankfurt-Munster) Multicenter Trial. Blood. 2006;107(10):4047–52. doi: 10.1182/blood-2005-10-4213. [DOI] [PubMed] [Google Scholar]
- 8.Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8(1):18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Stollar BD. Immunoglobulin VH gene expression in human aging. Clin Immunol. 1999;93(2):132–42. doi: 10.1006/clim.1999.4781. [DOI] [PubMed] [Google Scholar]
- 10.Jardin F, Sahota SS, Ruminy P, Parmentier F, Picquenot JM, Rainville V, et al. Novel Ig V gene features of t(14;18) and t(3;14) de novo diffuse large B-cell lymphoma displaying germinal center-B cell like and non-germinal center-B cell like markers. Leukemia. 2006;20(11):2070–4. doi: 10.1038/sj.leu.2404370. [DOI] [PubMed] [Google Scholar]
- 11.Hofscheier A, Ponciano A, Bonzheim I, Adam P, Lome-Maldonado C, Vela T, et al. Geographic variation in the prevalence of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly: a comparative analysis of a Mexican and a German population. Mod Pathol. 2011;24(8):1046–54. doi: 10.1038/modpathol.2011.62. [DOI] [PubMed] [Google Scholar]
- 12.Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA. 2008;105(36):13520–5. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]