With great interest we read the recent review on familial myelodysplastic syndromes (MDS) published in this journal by Liew and Owen.1 Besides telomere disorders and familial monosomy 7, the review focused on familial platelet disorder with propensity to myeloid malignancies (FPDMM) and also surveyed syndromic cases of heterozygous loss of chromosome 21q22. To further highlight the clinical diversity of FPDMM and to discuss the challenges posed by the clinical management of patients with germline RUNX1 deficiency, we report here on a patient with a constitutional loss of RUNX1 due to a de novo deletion of 21q22.
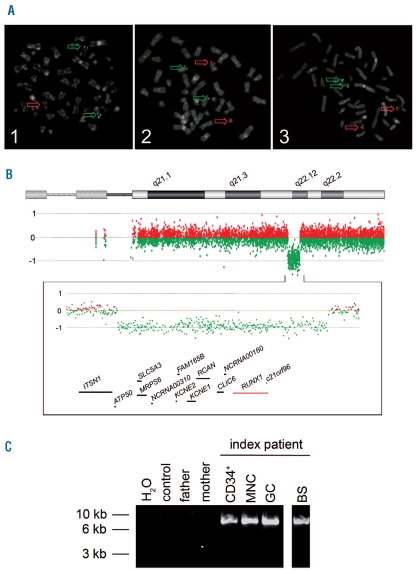
Due to chronic, idiopathic thrombocytopenia, retrospectively found to have been already present in childhood, mild anemia and neutropenia, cytogenetic investigations were performed in a 19-year old patient and displayed a loss of one RUNX1 allele in bone marrow cells. There was no evidence of MDS or acute myeloid leukemia (AML). Following genetic counseling, karyotyping and fluorescence in situ hybridization of phytohemagglutinin-stimulated peripheral blood cells confirmed a heterozygous deletion in 21q22 (Figure 1A, Online Supplementary Appendix). High-resolution array comparative genomic hybridization (aCGH) displayed a 1.6 Mb deletion in the long arm of a chromosome 21 involving among others RUNX1 (Figure 1B). Breakpoint spanning long distance PCR reconfirmed the deletion in DNA isolated from peripheral blood and a buccal swab (Figure 1C). Mutations of the remaining RUNX1 allele were excluded by DNA sequencing.
Figure 1.
(A) ETV6/RUNX1 metaphase fluorescence in situ hybridization (FISH). Metaphase plates of PHA-stimulated peripheral blood cells of the index patient (1) and his parents (2, 3) were investigated using specific probes for RUNX1 (LSI AML1, 21q22, red) and ETV6 (LSI TEL, 12p13, green) (Vysis, Abott, LSI ETV6(TEL)/RUNX1(AML1) ES dual color translocation probe set, Wiesbaden, Germany). While metaphases of the index patient (1) displayed one red and two green signals indicative of a loss of one RUNX1 allele, the metaphases of the parents (2, 3) showed two red and two green signals. Interphase FISH analysis of 100 nuclei displayed the submicroscopic loss of one RUNX1 allele in 93% of cell nuclei investigated. (B) High resolution oligo array comparative genomic hybridization (aCGH) of the index patient. DNA of peripheral blood cells was analyzed using a 400k oligo array following the manufacturer’s instructions (Agilent Technologies, Boeblingen, Germany). In comparison to a healthy control sample, aCGH displayed a 1.6 Mb deletion in the long arm of a chromosome 21 of the index patient which is probably due to a de novo rearrangement: arr 21q22.11q22.12(35,304,856–36,864,010)x1 dn. Below the ideogram of chromosome 21 from 21pter to 21qter, the aberration states based on normalized log2 transformed fluorescent intensity ratios are shown indicating the heterozygous interstitial deletion. Further on, an enlarged view of the aberrant region is given (chromosome 21: 34,912,522–37,106,477; 2.19Mb) displaying genes located within the region lost as well as some of the neighboring genes. The RUNX1 locus is highlighted in red. (C) Breakpoint spanning long-distance PCR. Using primers located within the last aCGH probes before and after the deletion detected, a breakpoint spanning PCR product of approximately 9 kb was generated in the index patient (lanes 5–8) while no product was seen in peripheral blood DNA samples of his parents (lane 3, 4) and a healthy control (lane 2). Analyses of DNA of several peripheral blood cell populations of the index patient displayed the deleted allele in CD34+ cells (CD34+, lane 5), cells of a mononuclear (MNC, lane 6), and a granulocytic cell fraction (GC, lane 7). Finally, in DNA of a buccal swab of the patient, the PCR product could also be amplified indicating the germline origin of the submicroscopic deletion in 21q (BS, lane 8). The identified de novo deletion is probably the result of a recombination between two L1PA2 long interspersed nuclear elements (LINE) that lie in the breakpoint region and display a sequence identity of 97%.
In contrast to the reviewed syndromic cases with deletions in 21q22 that, with the exception of one case,2 displayed a complex phenotype,1 our patient did not show any growth or developmental delay, dysmorphic features or other abnormalities. Most of the previously reported patients had been described in early childhood when a complex phenotype probably prompted cytogenetic analyses. However, as demonstrated by our patient, deletions of 21q22 including RUNX1 do not necessarily lead to a complex phenotype, highlighting again the clinical variability of FPDMM.1
In view of the early onset of leukemias in 3 out of 12 patients, Liew and Owen hypothesized that the age of leukemic transformation seems to be earlier in patients with deletions in 21q22 than in classical FPDMM.1 Despite detailed morphological and cytogenetic investigations, there was no evidence of MDS or AML in our 19-year old patient, although he carries a deletion similar to that seen in the case2 of non-syndromic thrombocytopenia with myelodysplasia. Moreover, we recently reported on a patient with a RUNX1 mutation showing a malignant transformation by the age of 13 years, indicating that in RUNX1 mutation carriers early onset of leukemia is also possible.3 It is known that secondary genetic alterations are required for the development of leukemia in FPDMM.3,4 Possibly, the age of onset depends more on the time and nature of additionally acquired alterations than on the presence of a deletion or mutation of RUNX1.
As pointed out by Liew and Owen,1 there is fortunately an increased awareness of FPDMM. In addition, the report on the association of RUNX1 mutation with thrombocytopenia, bone marrow blasts, and poor overall survival in patients with MDS5 will further increase the number of patients with germline RUNX1 deficiency. While there is an estimated risk of up to 60% of developing MDS or AML in patients with FPDMM, no surveillance guidelines are available for these patients and their families. In cases presenting with overt leukemia, the clinical management is dictated by the disease and it is broadly accepted that bone marrow transplantations by donors carrying the familial mutation have to be avoided.6 But what can we offer otherwise healthy individuals, e.g. the patient reported herein or individuals identified during screening as potential donors for a bone marrow transplant? We provided genetic counseling for the patient and his parents before we proceeded with further analyses and would recommend that this be the standard procedure whenever possible. In addition, we recommended peripheral blood cell counts and clinical evaluation of the patient every six months. So far, regular investigations of bone marrow aspirates have not been scheduled. In view of the association of KCNE2, KCNE1, RCAN1 and CLIC6, also affected by the deletion (Figure 1B), with cardiac malformations and arrhythmias, the index patient was referred to a cardiologist.7,8 However, is that enough? As far as the increased awareness of individuals with germline RUNX1 deficiency is concerned, efforts for the development of broadly accepted surveillance programs and clinical management guidelines for FPDMM are the next critical step. These are urgently needed to ensure that the increased aware-ness of this disease is translated into a clinically useful management approach towards the affected individuals and their families.
Acknowledgments
The authors would like to thank the participating patient and his family, and Gillian Teicke for her excellent assistance in editing the manuscript.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding: TR was funded by a grant from Hannover Biomedical Research School, Graduate School of Excellence, PhD Program Molecular Medicine, Hannover Medical School, Hannover, Germany.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Liew E, Owen CJ. Familial myelodysplastic syndromes - a review of the literature. Haematologica. 2011;96(10):1536–42. doi: 10.3324/haematol.2011.043422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Crabben S, van Binsbergen E, Ausems M, Poot M, Bierings M, Buijs A. Constitutional RUNX1 deletion presenting as non-syndromic thrombocytopenia with myelodysplasia: 21q22 ITSN1 as a candidate gene in mental retardation. Leuk Res. 2010;34:e8–12. doi: 10.1016/j.leukres.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Ripperger T, Steinemann D, Gohring G, Finke J, Niemeyer CM, Strahm B, et al. A novel pedigree with heterozygous germline RUNX1 mutation causing familial MDS-related AML: can these families serve as a multistep model for leukemic transformation? Leukemia. 2009;23(7):1364–6. doi: 10.1038/leu.2009.87. [DOI] [PubMed] [Google Scholar]
- 4.Preudhomme C, Renneville A, Bourdon V, Philippe N, Roche-Lestienne C, Boissel N, et al. High frequency of RUNX1 biallelic alteration in acute myeloid leukemia secondary to familial platelet disorder. Blood. 2009;113(22):5583–7. doi: 10.1182/blood-2008-07-168260. [DOI] [PubMed] [Google Scholar]
- 5.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodys-plastic syndromes. N Engl J Med. 2011;364(26):2496–506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen CJ, Toze CL, Koochin A, Forrest DL, Smith CA, Stevens JM, et al. Five new pedigrees with inherited RUNX1 mutations causing familial platelet disorder with propensity to myeloid malignancy. Blood. 2008;112(12):4639–45. doi: 10.1182/blood-2008-05-156745. [DOI] [PubMed] [Google Scholar]
- 7.Shinawi M, Erez A, Shardy DL, Lee B, Naeem R, Weissenberger G, et al. Syndromic thrombocytopenia and predisposition to acute myelogenous leukemia caused by constitutional microdeletions on chromosome 21q. Blood. 2008;112(4):1042–7. doi: 10.1182/blood-2008-01-135970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzaki E, Morin G, Pollazzon M, Papa FT, Buoni S, Hayek J, et al. Syndromic mental retardation with thrombocytopenia due to 21q22.11q22.12 deletion: Report of three patients. Am J Med Genet A. 2010;152A(7):1711–7. doi: 10.1002/ajmg.a.33478. [DOI] [PubMed] [Google Scholar]