Abstract
Cryptotanshinone (CPT), isolated from the plant Salvia miltiorrhiza Bunge, is a potential anticancer agent. However, the underlying mechanism remains to be defined. Here we show that CPT inhibited lymphangiogenesis in an in vitro model (tube formation). This effect was partly attributed to inhibiting expression of vascular endothelial growth factor receptor 3 (VEGFR-3) in murine lymphatic endothelial cells (LECs), as overexpression of VEGFR-3 conferred resistance to CPT inhibition of the tube formation, whereas downregulation of VEGFR-3 mimicked the effect of CPT, blocking the tube formation. Furthermore, CPT inhibited phosphorylation of the extracellular signal-related kinase 1/2 (ERK1/2). Overexpression of VEGFR-3 attenuated CPT inhibition of ERK1/2 phosphorylation, whereas downregulation of VEGFR-3 inhibited ERK1/2 phosphorylation in LECs. Expression of constitutively active MKK1 resulted in activation of ERK1/2, and partially prevented CPT inhibition of LEC tube formation. In addition, CPT also inhibited protein expression and activities of Rac1 and Cdc42, but not RhoA. Expression of constitutively active Rac1 and Cdc42 concurrently, but not Rac1 or Cdc42 alone, conferred resistance to CPT inhibition of LEC tube formation. Taken together, the results suggest that CPT inhibits LEC tube formation in part by inhibiting VEGFR-3-mediated ERK1/2 phosphorylation, and in part by inhibiting expression of the small GTPases.
Keywords: Cryptotanshinone, lymphatic endothelial cells, tube formation, VEGFR-3, ERK, small GTPases
Introduction
Cryptotanshinone (CPT), a natural compound isolated from Salvia miltiorrhiza Bunge (Danshen), has been used in traditional oriental medicine for the treatment of a variety of diseases, such as coronary artery disease (1), hyperlipidemia, acute ischemic stroke (2), chronic renal failure (3), chronic hepatitis (4), and Alzheimer's disease (5). In addition, CPT has been recently shown to possess anticancer activity in a spectrum of human cancer cells (6–10). For instance, CPT inhibits growth of prostate cancer cells (DU145) by inactivating the signal transducer and activator of transcription-3 (Stat3) activity (6); induces apoptosis in DU145 cells by augmenting Fas sensitivity (7); inhibits growth of hepatocarcinoma and gastric cancer cells by arresting cell cycle at S phase (8); and inhibits proliferation of skin cancer cells (B16 and B16BL6) (9). Most recently we have further demonstrated that CPT displayed anticancer activity by inhibiting proliferation of human rhabdomyosarcoma (Rh30), prostate cancer (DU145) and breast cancer cells (MCF-7), by arresting cells in G1/G0 phase of the cell cycle (10). CPT inhibition of cell proliferation was associated with downregulation of cyclin D1 expression and phosphorylation of retinoblastoma (Rb) protein, due to inhibition of the mammalian target of rapamycin (mTOR) signaling pathway (10). These findings suggest that CPT is a potential novel anticancer agent.
Lymphangiogenesis, like angiogenesis, plays an important role in promoting tumor growth and metastasis (11–13). For many types of solid tumors, the lymphatic system acts as the primary conduit for initial metastasis, which is an indication of disease progression and prognosis for reduced survival (14–17). Therefore, inhibition of lymphangiogenesis is a promising strategy for treatment or prevention of tumor metastasis (18,19). Previous studies have shown that CPT inhibits angiogenesis in bovine aortic endothelial cells (BAECs) (20) and human umbilical vein endothelial cells (HUVEC) (21). However, the effect of CPT on lymphangiogenesis is not known.
Vascular endothelial growth factors (VEGFs) and their receptors are central controllers of vasculogenesis, angiogenesis and lymphangiogenesis (22). Five VEGFs (VEGF or VEGF-A, placenta growth factor, VEGF-B, VEGF-C and VEGF-D) and three VEGF receptors (VEGFR-1, -2 and -3) have been identified in mammals (22). VEGFR-1/2 and VFGFR-3 are primarily expressed on the surface of vascular and lymphatic endothelial cells (LECs), respectively (22). It is known that VEGF-A binds to VEGFR-1/2, regulating vasculogenesis and angiogenesis, while VEGF-C/D binds to VEGFR-3, mediating lymphangiogenesis. In particular, VEGF-C/D binds to and activates VEGFR-3, leading to activation of the downstream signaling molecules, such as phosphatidylinositol 3′-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK) pathways, which are crucial for LEC survival and lymphangiogenesis (22, 23), as well as metastasis (11–13). Thus, VEGFR-3 pathway has become an attractive target for cancer prevention and treatment.
The small GTPases (Rac1, Cdc42 and RhoA) regulate migration, survival, and vacuole and capillary lumen formation in LECs, which are critical for lymphangiogenesis (24–27). Recent studies have demonstrated a requirement for Rac1 and Cdc42 in capillary lumen formation of LECs, indicating that the small GTPases play key roles in the lymphangiogenic process (28). Therefore, targeting small GTPases is an alternative approach for cancer prevention and treatment.
Using murine LEC tube formation, an in vitro lymphangiogenesis model (29), we studied the effect of CPT on lymphangiogenesis. The results indicate that CPT inhibited the LEC tube formation, which was in part by inhibition of VEGFR-3-mediated phosphorylation of extracellular signal-related kinase 1/2 (ERK1/2), and in part by inhibition of expression and activities of Rac1 and Cdc42.
Materials and Methods
Chemicals
CPT was extracted from the roots of Salvia miltiorrhiza Bunge (Danshen), as described previously (10), and dissolved in 100% ethanol to prepare stock solutions (20 mM), which was aliquoted and stored at −20°C. U0126, a selective inhibitor of MKK1/2, was obtained from LC Laboratories (Woburn, MA, USA).
Cell lines and culture
Murine LECs (30) were grown in antibiotic-free Dulbecco’s modified Eagle medium (DMEM)/F12 (Mediatech, Herndon, VA, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) at 37°C and 5% CO2. Human embryonic kidney 293 (American Type Culture Collection, Manassas, VA, USA), 293TD and 293A cells (Invitrogen, Carlsbad, CA, USA) were grown in antibiotic-free DMEM (Mediatech) supplemented with 10% heat-inactivated FBS and non-essential amino acids (Mediatech) at 37°C and 5% CO2.
Plasmids and transfection
LEC clones stably overexpressing p3×Flag-VEGFR-3-TV1 and p3×Flag-TV1 plasmid (empty vector, as a control) were generated and used as described (29).
Lentiviral shRNA cloning, production and infection
To generate lentiviral shRNA to Rac1 or Cdc42, oligonucleotides containing the target sequences were synthesized, annealed and inserted into FSIPPW lentiviral vector (31) through the EcoR1/BamH1 restriction enzyme site. The oligonucleiotides used were: Rac1 sense: 5’-AATTCCCATACCGGAGTGCTCAGCTTGCAAGAGAAGCTGAGCACTCCAG GTATTTTTTG -3’, antisense: 5’-GATCCAAAAAATACCTGGAGTGCTCAGCTT CTCTTGCAAGCTGAGCACTCC AGGTATGGG-3’; Cdc42 sense: 5’-AATTCCCCATGTCTCCTGATATCCTATGCAAGAGATAGGATATCAGGAGACATGTTTTTG-3’, antisense: 5’-GATCCAAAAACATGTCTCCTGATATCCTA TCTCTTGCATAGGATATCAGGAGACATGGGG-3’. Lentiviral shRNAs to Rac1 and Cdc42 were made as described previously (32), and lentiviral shRNAs against VEGFR-3 and green fluorescence protein (GFP, as a control) were shown (29). Subsequently, LEC cells, when grown to about 70% confluence, were infected with the above lentiviral shRNAs in the presence of 8 µg/ml polybrene and exposed to 2 mg/ml puromycin after 24 h of infection. In 5 days, cells were used for experiments.
Recombinant adenoviral constructs and infection
The recombinant adenoviral vectors expressing GFP (Ad-GFP), Flag-tagged constitutively active MKK1 (Ad-MKK1-R4F), RhoA (RhoA-L63) (Ad-RhoA-L63), Cdc42 (Ad-Cdc42-L28), and Rac1 (Ad-Rac1-L61) were described previously (29,33). All adenoviruses were amplified, titrated and used as described (33).
Western blot analysis
Western blot analysis was performed as described previously (32). The primary antibodies used included antibodies to VEGFR-3, Akt, Cdc42, RhoA, ERK2, JNK1, phospho-JNK (Thr183/Tyr185), p38, phospho-p38 (Thr180/Tyr182), MKK1, Flag (Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-ERK1/2 (Thr202/Tyr204), phospho-Akt (Ser473) (Cell Signaling, Beverly, MA, USA), Rac1 (Cytoskeleton, Denver, CO, USA), and β-tubulin (Sigma, St. Louis, MO, USA).
Cell morphological analysis
LECs were seeded at a density of 5 × 105 cells/well in 6-well plates. Next day, the cells were treated with CPT (0–10 µM) for 24 h, or with 10 µM CPT for 0–24 h, followed by taking images under an Olympus inverted phase-contrast microscope (Olympus Optical, Melviller, NY, USA) (200×) equipped with the Quick Imaging system.
Tube formation assay
Tube formation assay was performed, as described previously (29).
Small GTPase activity assay
The activity of Rac1, Cdc42, or RhoA was determined using Rac/Cdc42 assay kit and Rho assay kit (Millipore, Billerica, MA, USA), respectively, as described previously (33).
Statistical analysis
Results were expressed as mean values ± standard deviation (mean ± SD). The data were analyzed by one-way analysis of variance (ANOVA) followed by post-hoc Dunnett’s t-test for multiple comparisons. A level of P < 0.05 was considered to be statistically significant.
Results
CPT inhibits LEC tube formation
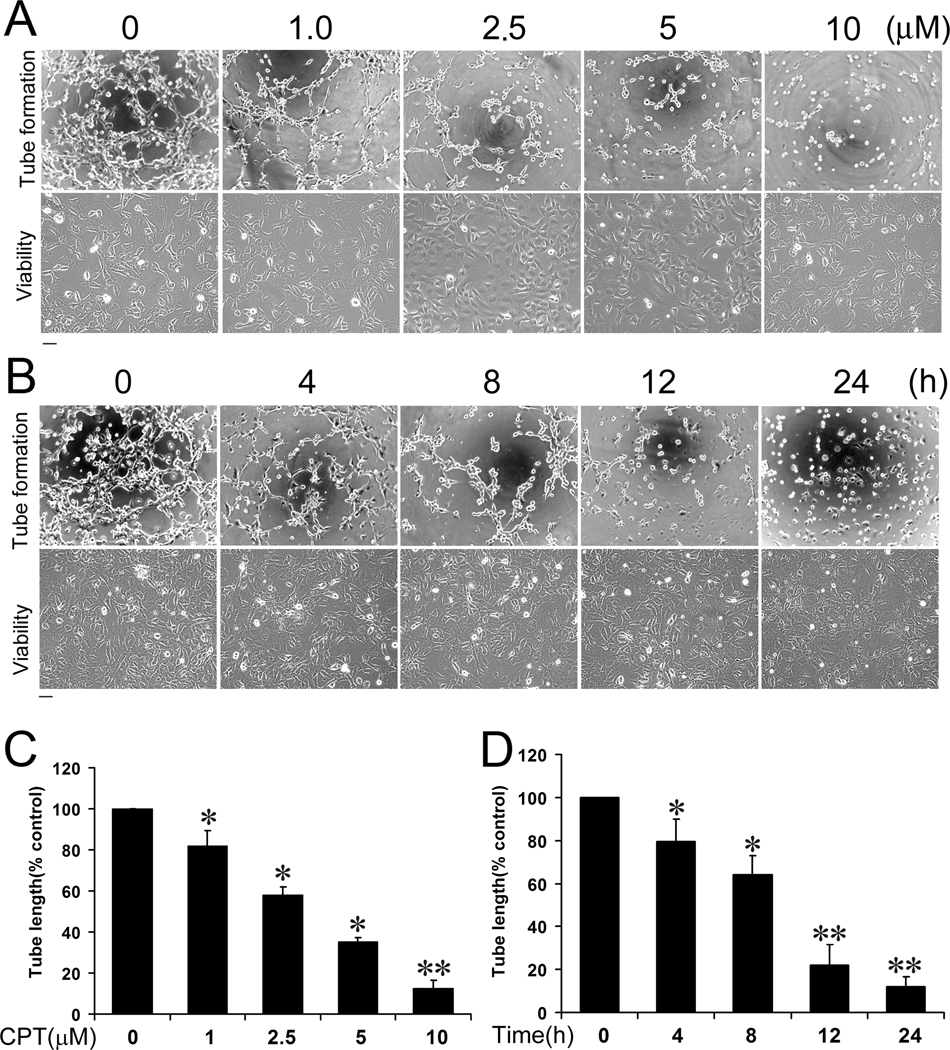
Studies have shown that CPT inhibits angiogenesis (20, 21), but the effect of CPT on lymphangiogenesis is not known. To find out whether CPT inhibits lymphangiogenesis, we chose murine LEC tube formation as an in vitro model for lymphangiogenesis. Treatment with CPT (0–10 µM) for 24 h did not apparently affect LEC cell viability according to cell morphology (Fig.1A, bottom panel). However, pretreatment with CPT (0–10 µM) for 24 h inhibited LEC tube formation in a concentration-dependent manner (Fig.1A, upper panel). At 5 and 10 µM, CPT inhibited the tube formation by approximately 65% and 90%, respectively, comparing with the control group (Fig.1C). Furthermore, CPT (10 µM) also inhibited LEC tube formation in a time-dependent manner (Fig. 1B, upper panel), in spite of no obvious effect on cell viability (Fig.1B, bottom panel). After treatment for 4 h, CPT (10µM) was able to significantly inhibit the tube formation (by ~20%). When LECs were treated with CPT (10µM) for 24 h, the tube formation was suppressed by ~90%, comparing with the control group (Fig.1D).
Figure 1.
CPT inhibits LEC tube formation in a concentration- and time-dependent manner. LECs were treated with CPT (0–10 µM) for 24 h, or CPT (10 µM) for 0–24 h, followed by tube formation assay and morphological analysis, as described in “Materials and Methods”. Representative images are shown in (A) and (B), respectively. Bar = 100 µm. The length of tube-like formation was evaluated by NIH Image J software. Quantitative data are presented as mean ± SD (n = 3) in (C) and (D), respectively. *P < 0.05, **P < 0.01, difference vs. control group.
CPT inhibition of LEC tube formation is associated with suppressing VEGFR-3 protein expression
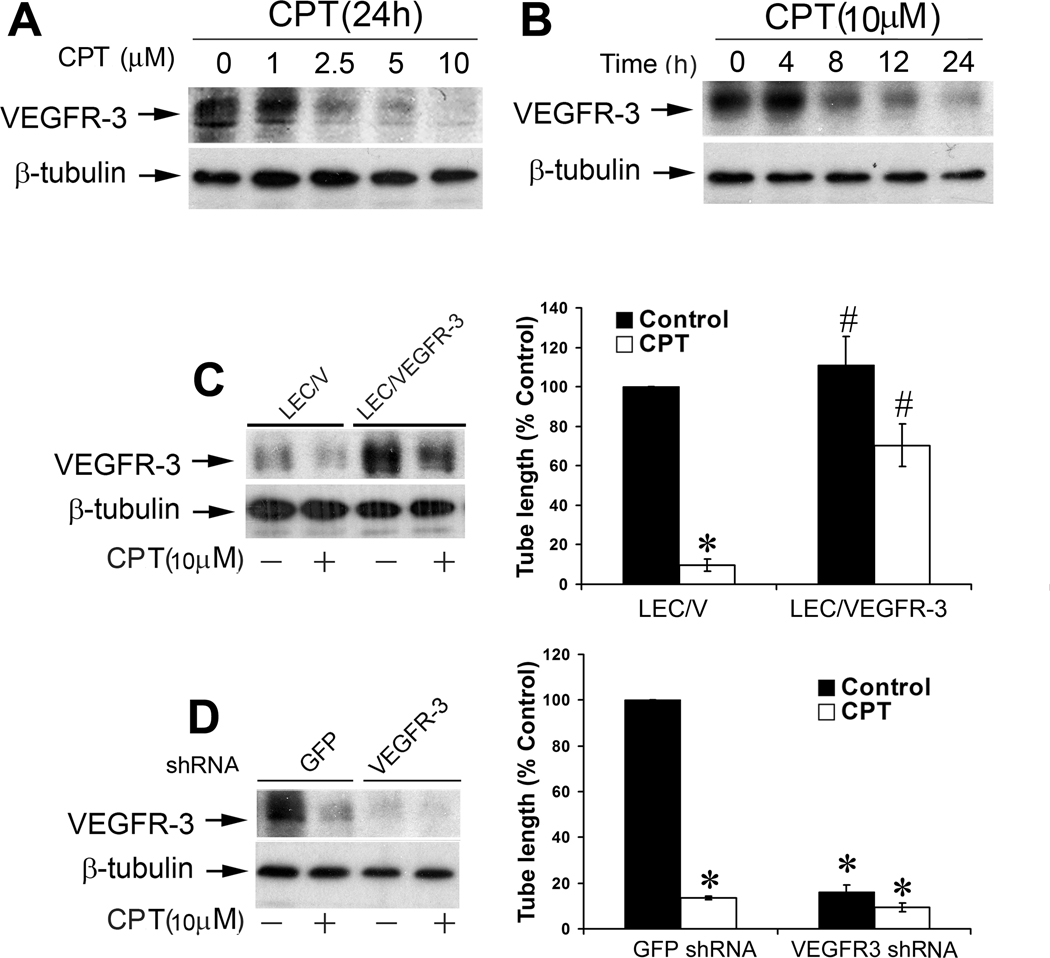
Since VEGFR-3 is primarily expressed in LECs (34), and essential for lymphangiogenesis (11–13,22), we studied whether CPT inhibits LEC tube formation by targeting VEGFR-3. When LECs were treated with CPT (0–10 µM) for 24 h, a concentration-dependent reduction of VEGFR-3 protein expression was detected by Western blotting (Fig.2A). When the cells were exposed to CPT at 10 µM, a time-dependent inhibition of VEGFR-3 expression was also observed (Fig.2B). Treatment with CPT for 8 h was able to remarkably downregulate VEGFR-3 protein level. Prolonged treatment with CPT resulted in more reduction of VEGFR-3 (Fig.2B).
Figure 2.
CPT inhibition of LEC tube formation is associated with suppressing VEGFR-3 protein expression. A, B, CPT inhibited protein expression of VEGFR-3 in a concentration- and time-dependent manner. LECs, treated with CPT (0–10 µM) for 24 h (A) or CPT (10 µM) for 0–24 h (B), were harvested and subjected to Western blot analysis with antibodies to VEGFR-3. β-tubulin was used as a loading control. C, Overexpression of VEGFR-3 partially prevented CPT inhibition of LEC tube formation. LEC/V (control) and LEC/VEGFR-3 cells were treated with CPT (10 µM) for 24 h, followed by Western blot analysis with indicated antibodies (Left panel), or tube formation assay (Right panel), as described in “Materials and Methods”. Quantitative results of tube formation are shown as mean ± SD (n = 3). *P < 0.05, difference vs. control group; #P < 0.05, difference vs. LEC/V group. D, Lentiviral shRNA to VEGFR-3, but not GFP, downregulated VEGFR-3 protein expression in LECs, as detected by Western blotting (Left panel). LECs, infected with lentiviral shRNAs to VEGFR-3 and GFP (control), respectively, were treated with CPT (10 µM) for 24 h, followed by tube formation assay (Right panel), as described in “Materials and Methods”. Quantitative results of tube formation are shown as mean ± SD (n = 3). * P < 0.05, difference vs. GFP shRNA control group.
To define the role of VEGFR-3 in CPT inhibition of LEC tube formation, LEC cells (LEC/VEGFR-3) stably overexpressing VEGFR-3 were generated by transfection with p3×Flag-VEGFR-3-TV1 plasmid, as described (29). About 3-fold increase of VEGFR-3 protein expression was detected in LEC/VEGFR-3 cells, comparing with the control cells (LEC/V) transfected with the empty vector (Fig.2C, left panel). Overexpression of VEGFR-3 did not alter the cell viability and growth rate in LECs. Treatment with CPT (10 µM) for 24 h inhibited VEGFR-3 protein expression by ~80% (Fig. 2C, left panel), and suppressed the tube formation by ~90% in LEC/V cells (Fig.2C, right panel). When LEC/VEGFR-3 cells were exposed to CPT (10 µM) for 24 h, VEGFR-3 protein expression was reduced by ~50%, but the VEGFR-3 protein level was still slightly higher than the basal level in the control (LEC/V) cells (Fig.2C, left panel). Interestingly, overexpression of VEGFR-3 rendered high resistance to CPT inhibition of the tube formation (Fig.2C, right panel), suggesting that CPT suppresses LEC tube formation in part by reducing VEGFR-3 protein expression.
To further substantiate the role of VEGFR-3 in CPT inhibition of LEC tube formation, RNA interference was utilized. Infection with lentiviral shRNA to VEGFR-3 downregulated the protein expression of VEGFR-3 by ~90%, in comparison with the controls infected with lentiviral shRNA to GFP (Fig.2D, left panel). Silencing VEGFR-3 mimicked the effect of CPT, inhibiting LEC tube formation by ~90% (Fig.2D, right panel), which supports that VEGRR-3 is essential for LEC tube formation. Addition of CPT (10 µM) did not further enhance VEGFR-3 shRNA inhibition of LEC tube formation, suggesting that downregulation of VEGFR-3 by 90% might have maximally inhibited the tube formation.
CPT inhibits LEC tube formation by targeting VEGFR-3-mediated ERK1/2 pathway
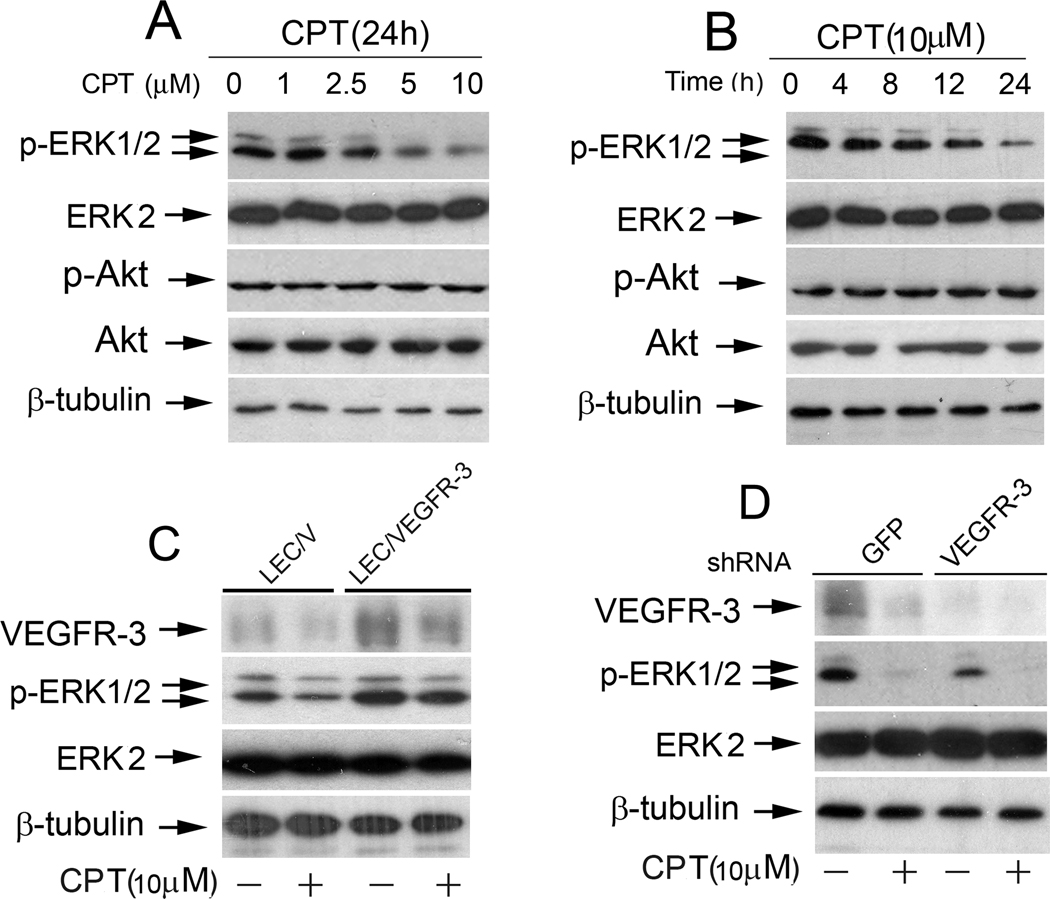
As PI3K/Akt and MAPK pathways are the two major downstreams of VEGFR-3 (22), we next wondered whether CPT inhibits LEC tube formation through targeting these pathways. Treatment with CPT failed to alter protein expression or phosphorylation of Akt (Fig.3A and B), and JNK and p38 MAPK pathways obviously (data not shown), but resulted in a concentration- and time-dependent inhibition of phosphorylation of ERK1/2, despite no effect on total protein level of ERK2 (Fig.3A and B), suggesting a selective inhibition of the ERK pathway in the LECs.
Figure 3.
CPT inhibits VEGFR-3-mediated ERK1/2 pathway. A, B, CPT inhibited phosphorylation of ERK1/2, but not AKT, in LECs in a concentration- and time-dependent manner. LECs treated with CPT (0–10 mM) for 24 h (A) or CPT (10 µM) for 0–24 h (B) were harvested and subjected to Western blot analysis with indicated antibodies. b-tubulin was used as a loading control. C, Overexpression of VEGFR-3 conferred resistance to CPT (10 µM) for 24 h, followed by Western blotting with indicated antibodies. D, Downregulation of VEGFR-3 mimicked the effect of CPT, inhibiting phosphorylation of ERK1/2 in LECs. LECs, infected with lentiviral shRNAs to VEGFR-3 and GFP (control), respectively, were treated with CPT (10 µM) for 24 h, followed by Western blotting with indicated antibodies.
To determine whether CPT inhibition of phosphorylation of ERK1/2 is through regulation of VEGFR-3 pathway, LEC/VEGFR-3 and LEC/V cells were treated with CPT (10 µM) for 24 h, respectively. As expected, overexpression of VEGFR-3 enhanced ERK1/2 phosphorylation and rendered high resistance to CPT inhibition of ERK1/2 phosphorylation (Fig.3C). In contrast, silencing VEGFR-3 by lentiviral shRNA mimicked the effect of CPT, decreasing ERK1/2 phosphorylation (Fig.3D). The results reveal that CPT inhibition of ERK1/2 phosphorylation is a consequence of downregulation of VEGFR-3 protein expression in the LECs.
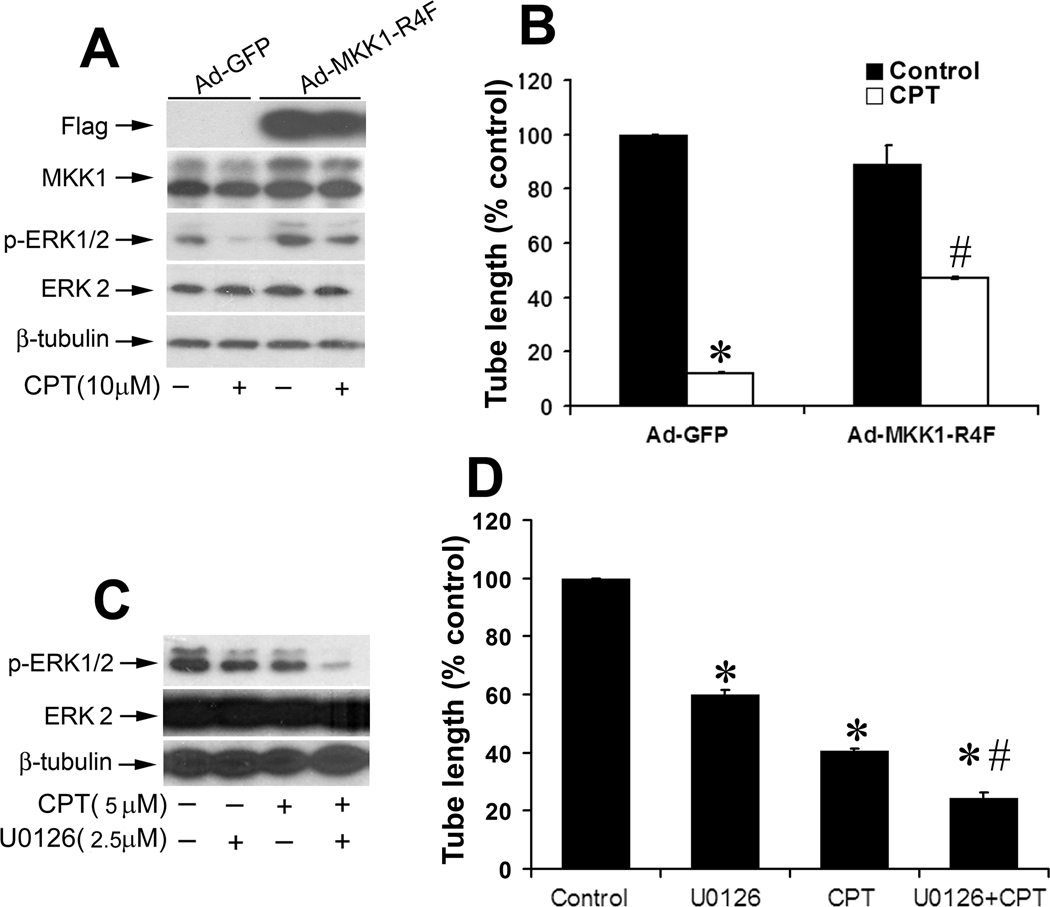
To further verify whether CPT inhibition of LEC tube formation is truly attributed to inhibition of ERK1/2 pathway, we generated recombinant adenoviral vector (Ad-MKK1-R4F) expressing Flag-tagged constitutively active MKK1, which activates ERK1/2 (35). As shown in Fig.4A, Flag-MKK1 was expressing in the LECs infected with Ad-MKK1-R4F, but not Ad-GFP (control). Expression of constitutively active MKK1 elevated phosphorylation of ERK1/2 in LECs. Treatment with CPT (10 µM) for 24 h suppressed phosphorylation of ERK1/2 in both Ad-GFP-infected (control) and Ad-MKK1-R4F-infected cells. However, the ERK1/2 phosphorylation level in Ad-MKK1-R4F-infected cells exposed to CPT was comparable to the basal level in the control cells (Fig.4A). Of notice, expression of constitutively active MKK1, but not GFP, conferred high resistance to CPT inhibition of LEC tube formation (Fig.4B). As a control, U0126 (a selective inhibitor of MKK1/2, upstream of ERK1/2) was employed to treat the LECs. We observed that 5 µM U0126 inhibited ERK1/2 phosphorylation almost completely and suppressed the tube formation by 90% in LECs. Addition of 10 µM CPT failed to enhance U0126 inhibition of the tube formation (data not shown). However, treatment with either 2.5 µM U0126 or 5 µM CPT alone inhibited ERK1/2 phosphorylation by ~50% (Fig.4C), and inhibited LEC tube formation by approximately 60% and 40%, respectively (Fig.4D). Combined treatment with 2.5 µM U0126 and 5 µM CPT displayed an additive or synergistic inhibitory effect on ERK1/2 phosphorylation and the tube formation (Fig.4C and D). The results suggest that CPT inhibits LEC tube formation partly through targeting VEGFR-3-mediated ERK pathway.
Figure 4.
CPT inhibition of LEC tube formation is through targeting VEGFR-3-mediated ERK1/2 pathway. A, B, Expression of constitutively active MKK1 attenuated CPT inhibition of ERK1/2 phosphorylation and the tube formation in LECs. LECs, infected with Ad-MKK1-R4F and Ad-GFP (control), respectively, were treated with or without CPT (10 µM) for 24 h, followed by Western blotting with indicated antibodies (A), or by tube formation assay (B) as described in “Materials and Methods”. Quantitative results are shown as mean ± SD (n = 3). *P < 0.05, difference vs. control group; #P < 0.05, difference vs. Ad-GFP group. C, D, LECs were treated with U0126 (2.5 µM) or CPT (5 µM) alone, or both for 24 h, followed by Western blotting using the indicated antibodies (C), or by tube formation assay (D) as described in “Materials and Methods”. Quantitative results of tube formation are shown as mean ± SD (n = 3). *P < 0.05, difference vs. control group. #P < 0.05, difference vs. U0126 or CPT treatment group.
Rac1 and Cdc42, but not RhoA, are involved in CPT inhibition of LEC tube formation
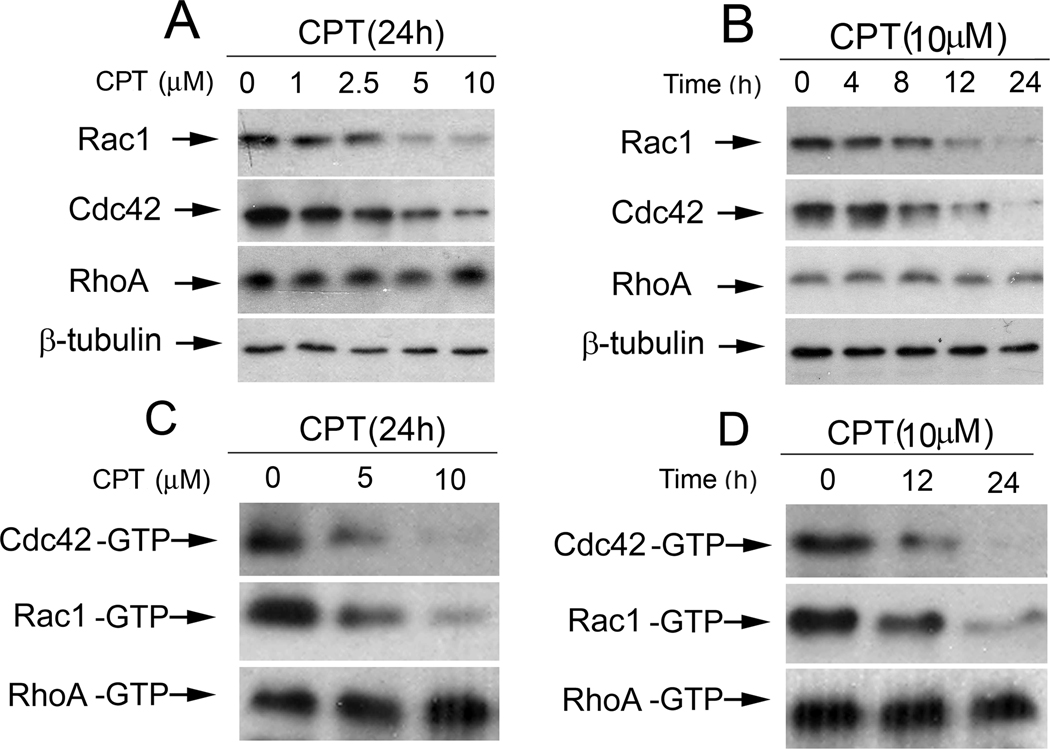
As the small GTPases play critical roles in lymphangiogenesis (24–28), we were also curious to ask whether CPT inhibits LEC tube formation by targeting the small GTPases. When LECs were treated with CPT (0–10 µM) for 24 h or 10µM CPT for 0–24 h, a concentration- and time-dependent inhibition of protein expression of Rac1 and Cdc42, but not RhoA, was observed (Fig.5A and B). Similarly, CPT treatment also markedly decreased the active (GTP-bound) protein levels of Rac1 and Cdc42, but not RhoA (Fig.5C and D), indicating inhibition of the activities of Rac1 and Cdc42.
Figure 5.
CPT inhibits protein expression and activities of Rac1 and Cdc42, but not RhoA, in a concentration- and time-dependent manner. A, B, LECs, treated with CPT (0–10 µM) for 24 h (A) or CPT (10 µM) for 0–24 h (B), were harvested and subjected to Western blotting with indicated antibodies. b-tubulin was used as a loading control. C, D, CPT inhibited activity of Rac1 and Cdc42, but not RhoA, in LECs in a concentration- and time-dependent manner. LECs treated with CPT (0–10 µM) for 24 h (C) or CPT (10 µM) for 0–24 h (D), were harvested for the small GTPase activity assay as described in “Materials and Methods”.
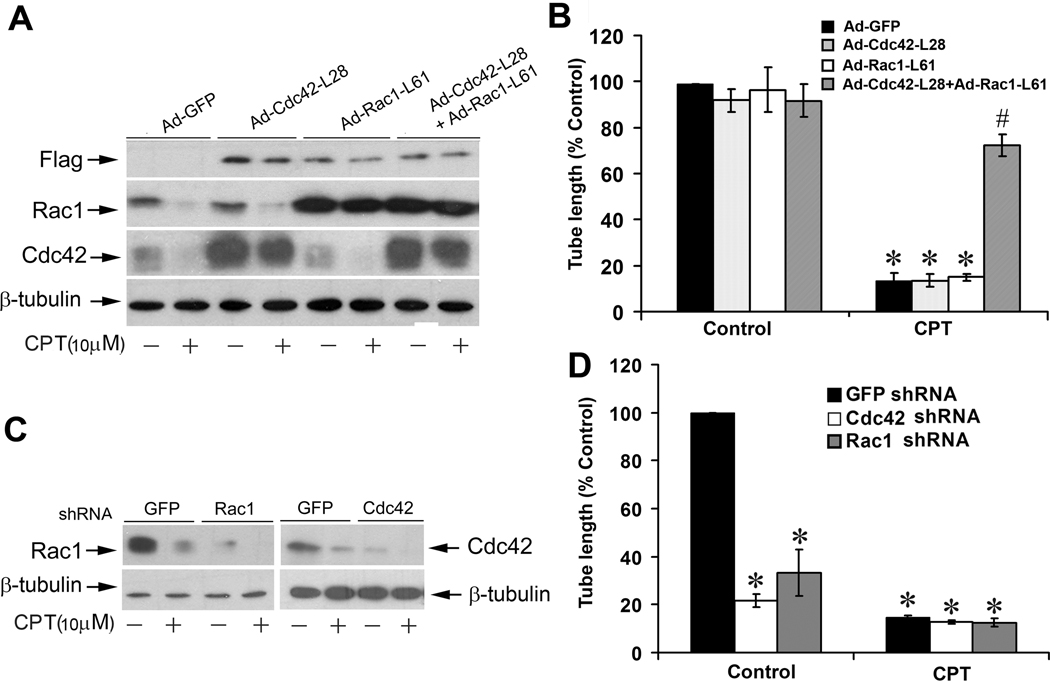
To demonstrate the roles of the small GTPases in the CPT inhibition of LEC tube formation, we constructed recombinant adenoviruses encoding constitutively active Rac1 and Cdc42. LECs infected with these adenoviruses were treated with or without CPT, followed by tube formation assay. As shown in Fig.6A, considerable levels of constitutively active Rac1 (Rac1-L61) and Cdc42 (Cdc42- L28) were expressing in LECs, respectively, as detected by Western blotting using antibodies to Flag and individual small GTPases. Expression of Rac1-L61 or Cdc42-L28 alone neither significantly altered the basal tube formation, nor rendered apparent resistance to CPT inhibition of LEC tube formation (Fig.6B). However, interestingly, concurrent expression of Rac1-L61 and Cdc42-L28 conferred high resistance to CPT inhibition of LEC tube formation (Fig.6B).
Figure 6.
Rac1 and Cdc42 pathways are involved in CPT inhibition of LEC tube formation. A, B, Expression of constitutively active Rac1 and Cdc42 concurrently, but not Rac1 or Cdc42 alone, rendered resistance to CPT inhibition of LEC tube formation. LECs, infected with Ad-Cdc42-L28, Ad-Rac1-L61, Ad-Cdc42-L28+Ad-Rac1-L61, or Ad-GFP (control), respectively, were treated with or without CPT (10 µM) for 24 h, followed by Western blotting using the indicated antibodies (A), or by tube formation assay (B) as described in “Materials and Methods”. Quantitative results of tube formation are shown as mean ± SD (n = 3). *P < 0.05, difference vs. Ad-GFP control group; #P < 0.05, difference vs. Ad-GFP/CPT, Ad-Cdc42-L28/CPT, or Ad-Rac1-L61/CPT treatment group. C, Lentiviral shRNA to Rac1 or Cdc42, but not GFP, downregulated Rac1 or Cdc42 in LECs, respectively, as detected by Western blotting. D, Downregulation of Rac1 or Cdc42 inhibited tube formation in LECs. Quantitative results are shown with mean ± SD (n = 3). *P < 0.05, difference vs. GFP shRNA control group.
To further corroborate the roles of the small GTPases in CPT inhibition of LEC tube formation, we generated lentiviral shRNAs to silence Rac1 (Fig.6C, left panel) and Cdc42 (Fig.6C, right panel), respectively. Of interest, downregulation of Rac1 or Cdc42 by ~90% alone was able to inhibit LEC tube formation by ~70% and ~80%, respectively (Fig.6D). The data suggest that both Rac1 and Cdc42 are essential for LEC tube formation, and CPT inhibits LEC tube formation partly by suppressing expression/activities of the two small GTPases.
Discussion
Recent studies have shown that CPT exhibits anticancer activity by inhibiting proliferation and inducing apoptosis in cancer cells, as well as inhibiting angiogenesis (6–10, 20–21). Here, for the first time, we present evidence that CPT inhibited LEC tube formation, an in vitro lymphangiogenesis model, suggesting that CPT inhibits lymphangiogenesis. It is well accepted that lymphangiogenesis, like angiogenesis, is pivotal for tumor growth and metastasis, and anti-lymphangiogenesis has become a new avenue for prevention and treatment of cancer (11–13). Therefore, our findings strongly support the notion that CPT is a potential anticancer agent.
VEGFR-3 has been characterized as a key player for lymphangiogenesis, and disruption of this pathway has been explored for the development of anticancer drugs (36–41). In the present study, we found that the anti-lymphangiogenic effect of CPT is associated with inhibition of VEGFR-3 protein expression in the LECs. This is supported by the findings that (1) CPT inhibited VEGFR-3 protein expression; (2) overexpression of VEGFR-3 conferred high resistance to CPT inhibition of LEC tube formation; and (3) downregulation of VEGFR-3 mimicked the effect of CPT, blocking LEC tube formation. The data are consistent with previous reports that blockade of the VEGFR-3 pathway by soluble receptor form (38, 40), small molecule inhibitors (23, 39, 42), or specific antibodies (43) effectively inhibited lymphangiogenesis.
It has been described that activation of VEGFR-3 pathway promotes LEC proliferation, migration and survival through PI3K/AKT and MAPK pathways (22, 23). Here we found that CPT affected neither cellular protein expression nor phosphorylation of Akt, JNK and p38 MAPK pathways, but inhibited phosphorylation of ERK1/2 in murine LECs. Further, we identified that CPT inhibition of ERK1/2 pathway was a consequence of downregulation of VEGFR-3 protein expression, as overexpression of VEGFR-3 attenuated CPT inhibition of ERK1/2 phosphorylation, whereas downregulation of VEGFR-3 mimicked the effect of CPT, reducing ERK1/2 phosphorylation in the LECs. In addition, we also observed that expression of constitutively active MKK1 increased ERK1/2 phosphorylation and rendered high resistance to CPT inhibition of LEC tube formation. Selective suppression of ERK1/2 by U0126 (5 µM) directly blocked LEC tube formation by 90%, whereas no additive or synergistic inhibitory effect on the tube formation was observed by treatment with CPT (10 µM) (data not shown). However, when LECs were treated with lower concentrations of these two compounds, CPT (5 µM) either additively or synergistically enhance the inhibition of U0126 (2.5 µM) on the tube formation, implying that ERK pathway is a critical controller for the LEC tube formation, and CPT inhibited LEC tube formation, at least partly through inhibition of VEGFR-3-mediated ERK1/2 pathway.
In our study, we also noticed that overexpression of VEGFR-3 (Fig.2C) or constitutively active MKK1 (Fig.4B) failed to fully rescue the tube formation inhibited by CPT, implying that there may be other signaling molecules involved in LEC tube formation. Since the small GTPases play important roles in cytoskeletal reorganization, cell motility and protrusion formation, which are essential for tube formation (44), we then investigated the effect of CPT on the small GTPases. Our results demonstrated that CPT inhibited protein expression and activities of Rac1 and Cdc42, but not RhoA. Furthermore, only concurrent expression of constitutively active Rac1 and Cdc42 conferred significant resistance to CPT inhibition of LEC tube formation. This is in agreement with other findings that both Racl and Cdc42 are necessary for the tube formation (25, 28, 45, 46). It is worthy mentioning that in the studies, we observed that silencing RhoA by shRNA alone also inhibited LEC tube formation (data not shown), suggesting that RhoA is essential for LEC tube formation as well, although RhoA is not a target of CPT. However, expression of constitutively active RhoA (RhoA-L63) alone, or even concurrent expression of RhoA-L63 + Cdc42-L28 or RhoA-L63 + Rac1-L61, failed to prevent CPT inhibition of LEC tube formation (data not shown).
Studies have shown that Rac1 is regulated by VEGFR-3 pathway in normal mouse endothelial cells (47). Following observation that CPT inhibits VEGFR-3 and Rac1/Cdc42, we originally hypothesized that CPT inhibition of Rac1 and Cdc42 is probably by inhibiting VEGFR-3. However, to our surprise, neither overexpression nor downregulation of VEGFR-3 had any influence on activities/expression of Rac1 and Cdc42, regardless of presence or absence of CPT (data not shown), suggesting that Rac1 and Cdc42 are not regulated by VEGFR-3 pathway in our murine LECs. This is probably due to the different cell lines or experimental conditions employed. Further studies are needed to address whether CPT inhibits lymphangiogenesis in vivo and uncover the underlying molecular mechanisms. Also, more studies should be helpful to unveil how CPT inhibits expression of VEGFR-3, Rac1 and Cdc42.
In summary, we have shown that CPT inhibited LEC tube formation in a concentration- and time-dependent manner. CPT inhibition of the LEC tube formation was related to suppression of VEGFR-3-mediated ERK pathway (Fig.1S). Furthermore, our data indicate that CPT inhibition of LEC tube formation was also in part by targeting Rac1and Cdc42 (Fig.1S). CPT may act as a novel anti-lymphangiogenic agent.
Supplementary Material
Figure 1S. A model showing that CPT inhibits LEC tube formation in part by inhibiting VEGFR-3-mediated ERK1/2 phosphorylation, and in part by inhibiting expression of Rac1/Cdc42. Arrows represent activation, whereas bars represent inhibition.
Acknowledgments
This work was supported in part by NIH (CA115414; S. Huang), American Cancer Society (RSG-08-135-01-CNE; S. Huang), and National Natural Science Foundation of China (30371727 and 30772766, Y. Lu).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 3.Wojcikowski K, Johnson DW, Gobe G. Herbs or natural substances as complementary therapies for chronic kidney disease: ideas for future studies. J Lab Clin Med. 2006;147:160–166. doi: 10.1016/j.lab.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Wang BE. Treatment of chronic liver diseases with traditional Chinese medicine. J Gastroenterol Hepatol. 2000;15 Suppl:E67–E70. doi: 10.1046/j.1440-1746.2000.02100.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu XY, Lin SG, Chen X, Zhou ZW, Liang J, Duan W, et al. Transport of cryptotanshinone, a major active triterpenoid in Salvia miltiorrhiza Bunge widely used in the treatment of stroke and Alzheimer's disease, across the blood-brain barrier. Curr Drug Metab. 2007;8:365–378. doi: 10.2174/138920007780655441. [DOI] [PubMed] [Google Scholar]
- 6.Shin DS, Kim HN, Shin KD, Yoon YJ, Kim SJ, Han DC, et al. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 2009;69:193–202. doi: 10.1158/0008-5472.CAN-08-2575. [DOI] [PubMed] [Google Scholar]
- 7.Park IJ, Kim MJ, Park OJ, Park MG, Choe W, Kang I, et al. Cryptotanshinone sensitizes DU145 prostate cancer cells to Fas (APO1/CD95)-mediated apoptosis through Bcl-2 and MAPK regulation. Cancer Lett. 2010;298:88–98. doi: 10.1016/j.canlet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Lee WYW, Chiu LCM, Yeung JHK. Cytotoxicity of major tanshinones isolated from Danshen (Salvia miltiorrhiza) on HepG2 cells in relation to glutathione perturbation. Food Chem Toxicol. 2008;46:328–338. doi: 10.1016/j.fct.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Zheng SZ, Sun ZG, Wang AY, Huang CH, Punchard NA, et al. Cryptotanshinone has diverse effects on cell cycle events in melanoma cell lines with different metastatic capacity. Cancer Chemother Pharmacol. 2011;68:17–27. doi: 10.1007/s00280-010-1440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Luo Y, Liu L, Zhou H, Xu B, Han X, et al. Cryptotanshinone inhibits cancer cell proliferation by suppressing mammalian target of rapamycin-mediated cyclin D1 expression and Rb phosphorylation. Cancer Prev Res. 2010;3:1015–1025. doi: 10.1158/1940-6207.CAPR-10-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 12.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nature Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 13.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onogawa S, Kitadai Y, Tanaka S, Kuwai T, Kimura S, Chayama K. Expression of VEGF-C and VEGF-D at the invasive edge correlates with lymph node metastasis and prognosis of patients with colorectal carcinoma. Cancer Sci. 2004;95:32–39. doi: 10.1111/j.1349-7006.2004.tb03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nature Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 16.Achen MG, Mann GB, Stacker SA. Targeting lymphangiogenesis to prevent tumour metastasis. Br J Cancer. 2006;94:1355–1360. doi: 10.1038/sj.bjc.6603120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roma AA, Magi-Galluzzi C, Kral MA, Jin TT, Klein EA, Zhou M. Peritumoral lymphatic invasion is associated with regional lymph node metastases in prostate adenocarcinoma. Mod Pathol. 2006;19:392–398. doi: 10.1038/modpathol.3800546. [DOI] [PubMed] [Google Scholar]
- 18.Pepper MS. Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res. 2001;7:462–468. [PubMed] [Google Scholar]
- 19.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 20.Hur JM, Shim JS, Jung HJ, Kwon HJ. Cryptotanshinone but not tanshinone IIA inhibits angiogenesis in vitro. Exp Mol Med. 2005;37:133–137. doi: 10.1038/emm.2005.18. [DOI] [PubMed] [Google Scholar]
- 21.Gong Y, Li Y, Lu Y, Li L, Abdolmaleky H, Blackburn GL, et al. Bioactive tanshinones in Salvia Miltiorrhiza inhibits the growth of prostate cancer cells in vitro and in mice. Int J Cancer. 2011;129:1042–1052. doi: 10.1002/ijc.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Wissmann C, Detmar M. Pathways targeting tumor lymphangiogenesis. Clin Cancer Res. 2006;12:6865–6868. doi: 10.1158/1078-0432.CCR-06-1800. [DOI] [PubMed] [Google Scholar]
- 24.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Zhang C, He Y, Wu H, Wang Z, Song W, et al. Lymphatic endothelial cell-secreted CXCL1 stimulates lymphangiogenesis and metastasis of gastric cancer. Int J Cancer. 2011 doi: 10.1002/ijc.26035. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci. 2002;115:1123–1136. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- 27.Navarro A, Rezaiekhaligh M, Keightley JA, Mabry SM, Perez RE, Ekekezie II. T1a/podoplanin is essential for capillary morphogenesis in lymphaticendothelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:543–551. doi: 10.1152/ajplung.90262.2008. [DOI] [PubMed] [Google Scholar]
- 28.Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Zhou H, Liu L, Shen T, Chen W, Xu B, et al. The fungicide ciclopirox inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3-mediated ERK signaling pathway. Oncogene. 2011;30:2098–2107. doi: 10.1038/onc.2010.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ando T, Jordan P, Joh T, Wang Y, Jennings MH, Houghton J, et al. Isolation and characterization of a novel mouse lymphatic endothelial cell line: SV-LEC. Lymphat Res Biol. 2005;3:105–115. doi: 10.1089/lrb.2005.3.105. [DOI] [PubMed] [Google Scholar]
- 31.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Li F, Cardelli JA, Martin KA, Blenis J, Huang S. Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene. 2006;25:7029–7040. doi: 10.1038/sj.onc.1209691. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Luo Y, Chen L, Shen T, Xu B, Chen W, et al. Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J Biol Chem. 2010;285:38362–38373. doi: 10.1074/jbc.M110.141168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 36.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 37.Karpanen T, Wirzenius M, Mäkinen T, Veikkola T, Haisma HJ, Achen MG, et al. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am J Pathol. 2006;169:708–718. doi: 10.2353/ajpath.2006.051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–6909. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- 39.Ruggeri B, Singh J, Gingrich D, Angeles T, Albom M, Yang S, et al. CEP-7055: a novel, orally active pan inhibitor of vascular endothelial growth factor receptor tyrosine kinases with potent antiangiogenic activity and antitumor efficacy in preclinical models. Cancer Res. 2003;63:5978–5991. [PubMed] [Google Scholar]
- 40.Mäkinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nature Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 41.Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, et al. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–4746. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 43.Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 44.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 45.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 46.Hoang MV, Nagy JA, Senger DR. Cdc42-mediated inhibition of GSK-3β improves angio-architecture and lumen formation during VEGF-driven pathological angiogenesis. Microvasc Res. 2011;81:34–43. doi: 10.1016/j.mvr.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1S. A model showing that CPT inhibits LEC tube formation in part by inhibiting VEGFR-3-mediated ERK1/2 phosphorylation, and in part by inhibiting expression of Rac1/Cdc42. Arrows represent activation, whereas bars represent inhibition.