Abstract
We previously demonstrated that allopregnanolone (APα) increased proliferation of neural progenitor cells and reversed neurogenic and cognitive deficits prior to AD pathology (Wang et al., 2005; 2010). Herein, we determined efficacy of APα to restore neural progenitor cell survival and associative learning and memory subsequent to AD pathology in male 3xTgAD mice and their non-transgenic (nonTg) counterparts. APα significantly increased survival of BrdU+ cells and hippocampal-dependent associative learning and memory in 3xTgAD mice in the presence of intraneuronal Aβ whereas APα was ineffective subsequent to development of extraneuronal Aβ plaques. Restoration of hippocampal-dependent associative learning was maximal by the first day and sustained throughout behavioral training. Learning and memory function in APα-treated 3xTgAD mice was 100% greater than vehicle-treated and comparable to maximal normal nonTg performance. In aged 15-month-old nonTg mice, APα significantly increased survival of BrdU+ cells and hippocampal-dependent associative learning and memory. Results provide preclinical evidence that APα promoted survival of newly generated cells and restored cognitive performance in the pre-plaque phase of AD pathology and in late-stage normal aging.
Keywords: Allopregnanolone, Alzheimer's disease therapeutics, adult neurogenesis, trace eyeblink conditioning, mild cognitive impairment therapeutics, translational neuroscience
1. Introduction
Generation of new neurons is well documented to occur throughout adulthood across various species, including rodents (Ma, et al., 2009, Zhao, et al., 2008) and humans (Eriksson, et al., 1998). In most mammals, adult neurogenesis is restricted to the subgranular zone (SGZ) of the dentate gyrus in the hippocampus (Aimone, et al., 2010) and subventricular zone (SVZ) of the lateral ventricle (Alvarez-Buylla, et al., 2008). Both SGZ and SVZ neurogenesis have been shown to play a significant role in various forms of learning and memory (Bath, et al., 2008, Kim, et al., 2007, Lazarini, et al., 2009, Saxe, et al., 2006, Waddell and Shors, 2008, Wang, et al., 2010). While the regenerative potential of the mammalian brain is sustained throughout the life span, the magnitude of the proliferative efficacy of neural progenitors declines with age and diseases, such as Alzheimer’s disease (AD) (Hattiangady and Shetty, 2008, Hattiangady, et al., 2007, Kuhn, et al., 1996, Lazarov, et al., 2010, Rao, et al., 2005). Age-and AD-associated declines in hippocampal neurogenesis have been observed in multiple mouse models of AD along with a concomitant decline in cognitive function (Ermini, et al., 2008, Niidome, et al., 2008, Rodriguez, et al., 2008, Rodriguez, et al., 2009, Taniuchi, et al., 2007, Verret, et al., 2007, Zhang, et al., 2007). Recent studies have shown that impaired neurogenesis is an early event in the etiology of mouse models of familial AD (Demars, et al., 2010, Wang, et al., 2010). Malfunctioning presenilin-1, misprocessing of amyloid precursor protein and toxic effects of hyperphosphorylated tau and Aβ could be contributing to impaired neurogenesis in AD models.
Neural stem cells have been proposed to be a therapeutic strategy to restore brain function in neurodegenerative disease. Several studies have shown restoration of cognitive function following neural stem cell transplants (Blurton-Jones, et al., 2009, Ebert, et al., 2008, Lu, et al., 2003, Park, et al., 2006). Ablated regions can be repopulated by neural stem cells under optimal conditions. Collectively, these data provide preclinical evidence for the potential of neural progenitor cells as a therapeutic avenue to regenerate neuronal circuits and to restore neurological function. While the transplant strategy shows efficacy, the clinical utility of this approach is limited. An alternative approach is to promote the intrinsic regenerative capacity of the brain. The challenge in this approach is the neurochemical and neuropathological milieu of the diseased brain. Changes in local biochemical milieu, including growth factors, cytokines, neurotransmitters, and neurosteroids can contribute to impaired neurogenesis (Brinton, 2009, Brinton, et al., 2008, Demars, et al., 2010, Wang, et al., 2010). Reversing the decline in adult neurogenesis using regenerative factors could be a therapeutic strategy to reverse disease- and age-associated cognitive decline.
Neurosteroids are a class of therapeutic agents with blood-brain barrier penetrating physicochemical properties (Brinton and Wang, 2006). One such neurosteroid is allopregnanolone (APα), a metabolite of progesterone, which is synthesized de novo in both embryonic and adult CNS (Baulieu, et al., 2001, Mellon and Griffin, 2002a, Mellon and Griffin, 2002b) as well as in pluripotent progenitor cells (Gago, et al., 2004, Lauber and Lichtensteiger, 1996) and which exhibits an age- and AD-associated decline (Bernardi, et al., 1998,Brinton, et al., 2008,Marx, et al., 2006,Weill-Engerer, et al., 2002). We have recently shown that the triple-transgenic mouse model of Alzheimer’s disease (3xTgAD), over-expressing APPSWE, PS1M146V, and tauP301L (Oddo, et al., 2003b), has both neurogenic and cognitive deficits beginning at 3-months of age (Wang, et al., 2010). Despite the lack of evident AD pathology, basal level of newborn cells in the SGZ of the hippocampus was significantly lower in these mice as compared to the age-matched non-transgenic (nonTg) mice. The concentration of APα was found to be significantly reduced within the brain and serum of 3xTgAD mice as compared to nonTg mice, similar to that observed in human AD patients (Weill-Engerer, et al., 2002). Furthermore, severe cognitive deficits were observed in 3-month-old 3xTgAD male mice as compared to nonTg mice in a hippocampal-dependent associative task. Treatment with APα reversed this cognitive deficit and restored learning and memory performance to the level of normal nonTg mice. APα also induced a significant increase in survival of neural progenitor cells, significantly correlated with APα-induced enhanced memory performance.
3xTgAD mice manifest age-dependent neuropathology of AD in the form of both Aβ plaques and neurofibrillary tangles along with an age-dependent learning and memory deficits (Billings, et al., 2005, Oddo, et al., 2003a, Oddo, et al., 2003b). The current study was directed at determining the efficacy of APα to restore associative learning and memory function and to promote neural progenitor generation and survival in the aged 3xTgAD mice at varying degrees of Aβ burden, from intraneuronal accumulation to extraneuronal plaque formation. Parallel analyses were conducted in the age-matched nonTg male mice to investigate the neurogenic and cognitive deficits that accompany aging in normal mice and probe the efficacy of APα to reverse those deficits. Results of our current analyses are further indicative of the potential of APα as a regenerative therapeutic to prevent or delay neurogenic and cognitive deficits associated with mild cognitive impairment, Alzheimer’s disease and late stage normal aging.
2. Materials and methods
2.1 Chemicals
Allopregnanolone (APα: 3α-hydroxy-5α-pregnan-20-one) (aka AP, Allo or THP) used for this study was purchased from Steraloids Inc. (Newport, Rhode Island, USA). Chemicals were from MP Biomed (Irvine, CA) unless otherwise noted.
2.2 Animals and treatment
Breeding pairs of the triple transgenic Alzheimer’s disease mouse (3xTgAD, homozygous mutant of human APPSwe, PS1M146v and tauP301L) and its background strain (129/Sv × C57BL/6) were obtained from Dr. Frank LaFerla (University of California, Irvine) and the colonies were established at University of Southern California. The characterization of Aβ and tau pathologies, as well as synaptic dysfunction in this line of mice has been described previously (Billings, et al., 2005, Oddo, et al., 2003b) and confirmed in our laboratory. Mice were genotyped regularly to confirm the purity of the colony. Experiments were performed using 6, 9, 12 and 15-month-old male 3xTgAD and nonTg mice. The number of mice per condition is indicated within the results section. Mice were maintained under a 12 hr light/dark cycle with continuous access to food and water. APα stock solution was prepared in pure ethanol and diluted in PBS before injection (with a final ethanol concentration of 0.002% of the body weight). The experimental paradigm used was similar to our previous study (Wang, et al., 2010).
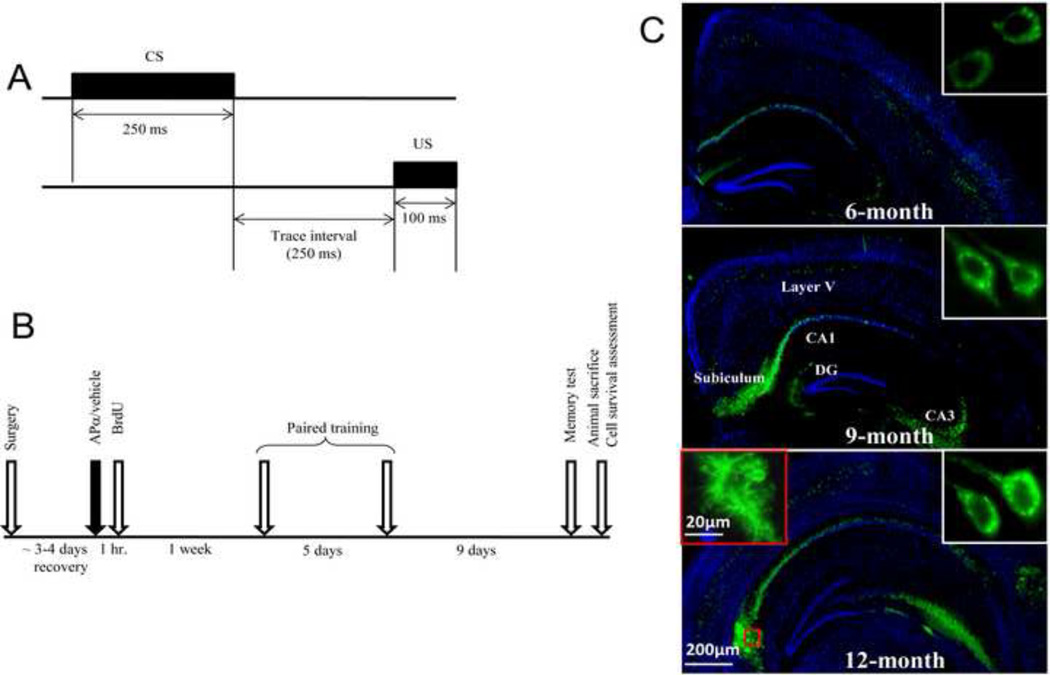
Mice of each genotype and age received a subcutaneous (s.c.) injection of APα at a concentration of 10 mg/kg body weight (BW), an optimal dose of APα based on our previous study (Wang, et al., 2010). One hour after APα injection, mice were intraperitoneally (i.p.) injected with 100 mg/kg BW bromodeoxyuridine (BrdU). This was followed by a 7-day neurogenesis and migration phase, then followed by 5-day trace eyeblink conditioning and subsequently followed 9 days later by a 1-day memory test. Mice were sacrificed after the memory test and FACS analysis of BrdU positive cells was performed to assess the number of cells that survived. Timings of APα and BrdU injections, training, and perfusion were based on our previous analysis indicating that APα-induced neurogenesis significantly increased learning of trace eyeblink conditioning in 3-month old 3xTgAD male mice following an injection of APα one week prior to behavioral testing (Wang, et al., 2010).
All experiments strictly conformed to the Animal Welfare Act, Guide to Use and Care of Laboratory Animals, and the U.S. Government Principles of the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training guidelines on the ethical use of animals. In addition, the minimal number of required animals was used for these experiments and pain was minimized.
2.3 Trace eyeblink conditioning
A 4-pin head stage (Digi-Key, MN) was cemented to the skull with dental acrylic, under deep anesthesia by intraperitoneal (i.p.) injection with ketamine (100 mg/kg) and xylazine (25 mg/kg). The connector had four Teflon-coated stainless steel wires and one bare stainless steel wire (0.003” bare and 0.0055” coated, A-M Systems, Inc., WA). The bare wire was attached via a gold pin (Time Electronics, England) to the head stage. Coated wires were implanted subcutaneously (s.c) in the orbicularis oculi dorsal to the left upper eyelid to record the electromyographic (EMG) activity and s.c. periorbitally to deliver the shock (unconditioned stimulus; US). All animals were placed on a warm isothermal pad after surgery to recover for 30 min. After surgery, mice were individually housed, provided with ad libitum access to food and water, and maintained on a 12 hr light/dark cycle.
After one week of acclimation to the colony room, mice with no obvious adverse responses to surgery were randomly assigned to an experimental condition. Mice were injected subcutaneously with 10 mg/kg APα or vehicle followed one hour later with an i.p. injection of BrdU (100 mg/kg). After these injections, mice were returned to their home cages for 7 days until the onset of behavioral testing.
During the first day of training, mice were placed within Plexiglas cylinders in a sound-attenuated chamber and were habituated to the test environment for one session consisting of 30 stimulus-free trials at 30–60 sec inter-trial intervals while spontaneous eyeblink rate was recorded using electromyographic (EMG) activity recorded from the obicularis oculi dorsal to the orbit during each trial. EMG activity was rectified and integrated using custom designed computational Labview routines (Ohno, et al., 2005,Tseng, et al., 2004).
Following habituation to the test environment, mice underwent paired eyeblink conditioning for five days. Mice were trained by pairing delivery of a tone (CS, 250 ms, 2 kHz, 85 dB) as the conditioned stimulus followed by a 250 ms period of no stimuli, followed by the periobital shock as the unconditioned stimulus (US, 100 ms). Mice received two blocks of 30 trials per day (30–60 sec inter-trial intervals, 3–4 h inter-block intervals). This trace eyeblink conditioning paradigm is subthreshold for inducing neurogenesis, shown in our previous study (Wang, et al., 2010). Shock intensity (US) was adjusted daily for each mouse to elicit a head-movement response. Following the paired conditioning, mice were returned to their home cages for nine days followed by a single session to assess memory of the learned association on the tenth day. The percentage of CR was computed as the ratio of the number of CRs to the total number of valid trials. Trials were defined as CR, UR, unstable, etc. (Lee and Kim, 2004). Animals were perfused at the end of the memory test for further analysis.
2.4 Animal dissection and tissue collection
Mice were sacrificed at the end of memory test for cell survival assessment (Fig.1A). On the day of sacrifice, mice were deeply anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg), and perfused with PBS. Brains were dissected into two hemispheres, and one hemisphere was fixed immediately in cold 4% paraformaldehyde and was used for FACS analysis to determine the number of BrdU+ cells that survived.
Figure 1. Experimental design.
(A) Hippocampal-dependent trace eyeblink conditioning was used as a behavioral assay to study the effect of APα on cognitive function in 3xTgAD and age-matched nonTg mice. During each trial a tone (conditioned stimulus; CS) was paired with periorbital shock (unconditioned stimulus; US) on the eyelid eliciting an eyeblink response. The trace period between the CS offset and US onset was 250 ms. (B) Experimental plan for behavior test and cell survival determination. Mice were treated with either APα (10 mg/kg) or vehicle and 1 h later with BrdU (100 mg/kg). One week later, mice were subjected to trace eyeblink conditioning, with one day of habituation and five days of paired training. Following paired training, mice were left undisturbed in their home cages for 9 days and subsequently were tested for memory. (C) Age-associated intraneuronal and extraneuronal Aβ plaque development in coronal sections derived from male 3xTgAD mouse hippocampus. In 6-month-old 3xTgAD mouse brain, a low level of intraneuronal 6E10 immunoreactivity (green immunofluorescence; see inset) was detectable in subicular and CA1 regions of the hippocampus and layer V of the cerebral cortex. At 9-months-of-age, a moderate level of intraneuronal 6E10 immunoreactivity was expressed. At 12-months-of-age, intra- and extraneuronal 6E10 staining is apparent. At this age, plaque structures developed around the area of subiculum (see inset). Immunofluorescent staining with 6E10 is shown in green and nuclear stain DAPI shown in blue.
2.5 Immunohistochemistry
Free-floating multibrain sections were rinsed extensively in PBS containing 0.1% Triton X-100 (PBST). They were blocked in blocking buffer (PBST with 5% normal goat serum, NGS, and 0.3% Triton X-100) for 60 min, followed by incubation with primary antibody in blocking buffer overnight at 4°C. To detect total Aβ, the monoclonal antibody 6E10 (1:1000, Signet, Cat No. 9320) was used. The next day, sections were rinsed 3 times with PBST and incubated in FITC-conjugated goat anti-mouse (1:500, Vector Laboratories) secondary antibody in PBST for 60 min at room temperature. After intense washing with PBST the sections were mounted with DAPI-containing mounting medium on coverslips. The immunoreactivity was observed and images were captured with the Axiovert 200 M Marianas Digital Microscopy Workstation (Intelligent Imaging Innovations, Denver, CO).
2.6 Nuclei extraction and flow cytometry counting
In order to reduce the labor intensive and time consuming demands of using immunohistochemistry combined with unbiased stereology to analyze the APα-induced formation of new cells in hippocampus of 6, 9, 12, and 15 month old mice, we chose to assess the BrdU immunopositive nuclei using flow cytometry assay, which we have demonstrated to more objectively screen a large amount of samples in a high-throughput manner to evaluate the efficacy of a neurogenic agent versus immunohistochemistry combined with unbiased stereology (Henry, et al., 2009, Wang, et al., 2010). Hippocampi were dissected from the fixed hemispheres from the cell survival assessment experiment using consistent anatomical landmarks as criteria for dissection as described before (Bilsland, et al., 2006). The fornix adjacent to the hippocampal lobe was removed to avoid the subventricular zone and rostral migratory stream proliferative pools. The extracted hippocampi were homogenized using Next Advance 24-sample homogenizer (Next Advance Inc., NY) for 3 min on speed 7. This procedure lyses the plasma lemma while preserving the integrity of the nuclear envelope. The nuclei were collected into a 1.5 mL microcentrifuge tube by washing the beads and tube 4 times using 200 µL of PBS, and then centrifuged for 10 minutes at 10,000 rpm. Once all of the nuclei were collected in a pellet, the supernatant was discarded. The pellet was then re-suspended in 600µL of PBS plus 0.5% Triton x-100. The number of nuclei was estimated by staining with propidium iodide (PI), a fluorescent molecule which stoichiometrically binds to DNA by intercalating between the bases with no sequence preference. Aliquots of 25 µl were re-suspended in 200 µL of a 0.2 M solution of boric acid, pH 9.0, and heated for 1 h at 75°C for epitope retrieval. After washing in PBS, the nuclei were incubated for 24 h at 4°C with primary mouse monoclonal anti-BrdU antibody (1:100, Abcam, Ab12219) and subsequently incubated with FITC-conjugated goat anti-mouse IgG secondary antibody (1:100 in PBS; Vector Labs, FI-2001). The remainder of the cell suspension was diluted to 500µL and sent for flow cytometry assay using Beckman FC 500 System with CXP Software. Propidium iodide (PI) cells were first gated on a histogram; the expressing cells were visualized on a forward/side scatter plot. PI cells were ‘back-gated’ on the forward/side scatter plot to eliminate debris prior to analysis; this also eliminated auto fluorescence of the sample. Gates were always set using dissociates with cell aliquots which lack of the first antibody, but which were incubated with secondary antibody and processed alongside the experimental procedure. PI-labeled cells in a fixed volume were gated, and the number of cells showing BrdU+ signal was analyzed. Data were expressed as total BrdU+ cells per hippocampus.
2.7 Statistical analysis
Data were analyzed using a one-way ANOVA followed by Neuman–Keuls post hoc analysis. Data displayed in graphs were reported as mean ± SEM.
3. Results
3.1 Rationale for and design of APα treatment experiments
Our previous analyses demonstrated that APα reversed cognitive and neurogenic deficits of male 3xTgAD mice (3-month-old) prior to the onset of AD pathology (Wang, et al., 2010). The current study was designed to determine the efficacy of APα to promote cognitive and neurogenic function in 3xTgAD male mice with mild (6-month-old), moderate (9-month-old) and severe (12-month-old) Aβ accumulation. Cognitive performance was assessed using the trace eyeblink conditioning paradigm (Fig. 1A) which provides a measure of associative learning and memory that is dependent upon the generation of new neurons in the dentate gyrus (Shors, et al., 2001, Shors, et al., 2002) and is sensitive to aging and Alzheimer’s disease burden (Ewers, et al., 2006, Kishimoto, et al., 2001, Woodruff-Pak, et al., 1990).
As indicated in the experimental design (Fig. 1B), animals received a single s.c. injection of APα (10 mg/kg) or vehicle 7 days prior to the start of the learning trials. Subsequent to that week, during which newly generated cells can migrate into the dentate gyrus, is the start of the five day learning trial. Surprising to us was that learning performance in the APα treated group was already improved above vehicle at day 1 of the learning trial indicating that some process transpired between the time that the animals were treated with APα and assessment of learning performance. Because neurogenesis and migration into the dentate gyrus are well documented to occur within this time frame and because of published findings (Gage, 2002, Shors, et al., 2001, Shors, et al., 2002, Wang, et al., 2010) demonstrate that induction of neurogenesis promotes cognitive function whereas blockade of neurogenesis impairs cognitive function, the most likely mechanism to explain APα enhancement of cognitive function at the first day of learning trial is the generation and migration of newly generated cells into the dentate gyrus (Gage, 2002, Wang, et al., 2010). Our assessment of BrdU+ cell survival was conducted 21 days following APα injection. Because many newly generated cells die during the first two weeks and because only those cells that integrate into existing neural networks in the granular cell layer within 2–3 weeks survive (Lie, et al., 2004). Thus the increased number of BrdU+ cells induced by APα treatment indicated increased survival of cells generated coincident to exposure to APα.
3xTgAD mice develop age-dependent AD neuropathology, including both Aβ plaques and neurofibrillary tangles (Billings, et al., 2005, Oddo, et al., 2003a, Oddo, et al., 2003b). In our study, 3xTgAD mice developed intraneuronal and extraneuronal Aβ in coronal sections derived from the hippocampus (Fig. 1C). In 6-month-old 3xTgAD mouse brain, a low level of intraneuronal Aβ immunoreactivity was detectable in subicular and CA1 regions of the hippocampus and layer V of the cerebral cortex. At 9-months-of-age, a moderate level of intraneuronal Aβ immunoreactivity was expressed. At 12-months-of-age, intraneuronal and extraneuronal Aβ immunoreactivity was apparent and plaque structures developed around the area of the subiculum.
3.2 APα reversed cognitive and neurogenic deficits in 6-month-old 3xTgAD mice
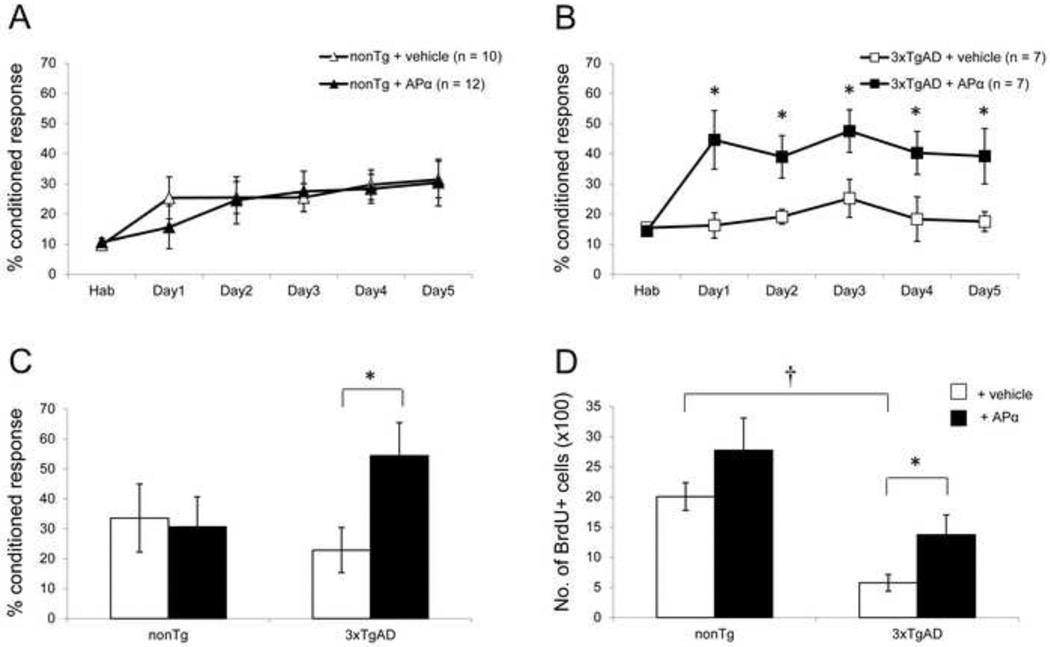
At the age of 6 months, intraneuronal Aβ accumulation is apparent in the hippocampus of 3xTgAD mice, whereas Aβ plaques have not yet formed (see Fig. 1C). In 6-month-old 3xTgAD mice, APα reversed the learning and neurogenic deficits observed while exerting no effect in nonTg mice (Fig. 2). Normal 6-month-old nonTg APα-treated mice achieved a maximal conditioned response of 30.4 ± 6.1%, which was not different from the vehicle-treated group (Fig. 2A). There was no significant difference in learning during the course of training between the vehicle-treated and APα-treated groups. Learning performance was mirrored in the memory performance test conducted 9 days after thelearning phase and resulted in no statistically significant difference between the vehicle- and APα-treated groups (Fig. 2C). In contrast, APα significantly increased the learning of 6-month-old 3xTgAD mice from a basal maximal learning of 17.4 ± 3.3% to 39.2 ± 9.2% (F(1, 12) = 4.943; P < 0.05) (Fig. 2B). During the entire learning phase, APα-treated 3xTgAD mice performed significantly better than the vehicle-treated group who failed to learn the task (F(1, 68) = 31.072; P < 0.001). Further, at day 5, learning performance of APα-treated 3xTgAD mice was comparable to the vehicle-treated nonTg mice. Analysis across the entire learning phase indicated that the performance of APα-treated 3xTgAD mice was maximal from day 1 of the training (Fig. 2B). On day 1, learning of APα-treated 3xTgAD mice increased to 44.6 ± 9.7%, as compared to 16.2 ± 4.2% in vehicle-treated mice, representing a doubling or more than 100% increase in learning performance, which persisted until the last day of the learning trial. These data indicate that APα restored maximal normal learning capacity of these mice and that the effect of APα was not simply due to increased learning performance. Whereas nonTg mice required five days of learning trials to reach maximum performance, APα-treated 3xTgAD mice performed and maintained maximal performance from day 1 of the learning trials. APα also significantly increased the retention of the conditioned responses in the memory test in 3xTgAD mice as compared to the vehicle-treated group (F(1, 10) = 5.560; P < 0.05) (Fig. 2C). Memory performance of APα-treated 3xTgAD mice was also comparable to vehicle-treated normal nonTg mice. Similar to learning performance, memory of APα-treated 3xTgAD mice increased to 54.4 ± 11.1%, as compared to 22.8 ± 7.5% in vehicle-treated 3xTgAD mice, demonstrating an increase of more than 100%.
Figure 2. APα reversed the learning and neurogenic deficits in 6-month-old 3xTgAD mice.
(A) Learning curve of 6-month-old nonTg mice: NonTg mice displayed a modest level of learning, which was not augmented by APα. (B) Learning curve of 6-month-old 3xTgAD mice: Vehicle-treated 3xTgAD group showed a reduced basal level of learning compared to vehicle-treated nonTg mice. 3xTgAD treated with APα exhibited a significant increase in associative learning at day 1, which was sustained at asymptote throughout the learning trial period compared to vehicle-treated 3xTgAD mice and restored learning to a level comparable to vehicle-treated nonTg performance at day 5 (P > 0.05). (C) Memory test in 6-month-old mice: APα significantly enhanced the memory in 3xTgAD mice and showed no effect on the age-matched nonTg mice. (D) Cell survival analyses: 3xTgAD mice had a significantly lower basal level of cell survival in SGZ as compared to the nonTg mice. Treatment with APα significantly increased the number of BrdU+ cells to the level of nonTg mice. No effect was evident in nonTg mice after APα treatment. * P < 0.05 as compared to 3xTgAD + vehicle group; † P < 0.05 as compared to nonTg + vehicle group. Data are presented as average ± SEM (n ≥ 7).
Following the memory test, survival of newly generated hippocampal cells was determined by FACS analysis to assess the total number of surviving BrdU+ cells. As BrdU was administered one hour after APα injection at the start of the behavioral experiment (Fig. 1B), FACS analysis detected the total number of surviving BrdU+ cells to indicate the number of neural progenitor cells that integrated into the hippocampal neuronal network (Lie, et al., 2004). In 6-month-old vehicle-treated nonTg mice, BrdU+ cell survival was 2004 ± 230 (Fig. 2D). In APα-treated nonTg mice, 2772 ± 535 BrdU+ cells survived, which was not significantly different from the vehicle-treated group. BrdU+ cell survival was significantly lower in 6-month-old 3xTgAD mice (574 ± 137) relative to nonTg mice (F(1, 15) = 27.94; P < 0.001). APα significantly increased BrdU+ cell survival (1372 ± 326) as compared to the vehicle-treated group (F(1, 19) = 4.07; P < 0.05), promoting cell survival by greater than 2-fold in 3xTgAD mice (Fig. 2D).
3.3 APα reversed the cognitive and neurogenic deficits in 9-month-old 3xTgAD mice
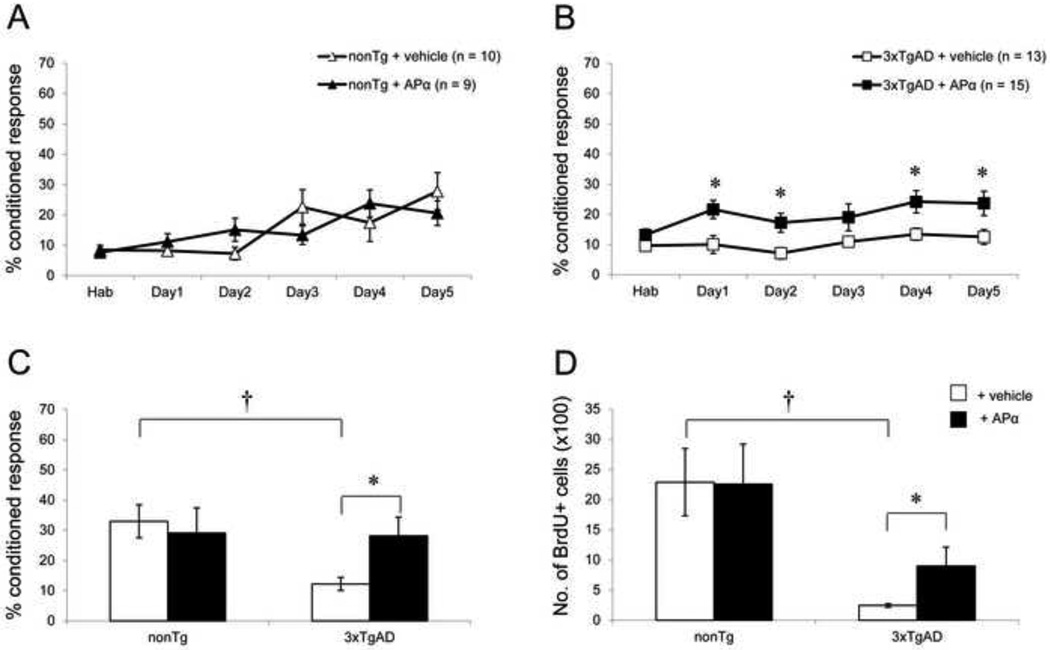
At the age of 9 months, intra-neuronal Aβ is abundant in the hippocampus of 3xTgAD mice and, in very rare instances, Aβ plaques have developed (see Fig. 1C). Similar to its effect in 6-month-old animals, APα reversed the learning and neurogenic deficits of 3xTgAD mice and showed no effect in nonTg mice. Normal 9-month-old nonTg mice achieved a maximal conditioned response of 27.8 ± 6.1% following behavioral training (Fig. 3A). In 9-month-old nonTg mice, APα had no significant effect on learning. The maximal level of learning for the APα-treated group was 20.6 ± 4.1%, which was not different from the vehicle-treated group (Fig. 3A). Similarly, there was no significant difference between vehicle- and APα-treated 9-month-old nonTg mice on the memory test (Fig. 3C). Vehicle-treated 3xTgAD mice exhibited a deficit in their maximal level of learning relative to the vehicle-treated nonTg mice (F(1, 20) = 6.03; P < 0.05). Vehicle-treated 3xTgAD mice showed no learning and their maximal response rate was 12.5 ± 2.4% (Fig. 3B). Compared to the vehicle-treated group, the final learning performance of APα-treated 3xTgAD was significantly increased to a level of 23.6 ± 4.0% (F(1, 25) = 4.82; P < 0.05) (Fig. 3B). Similar to the effects observed in 6-month-old 3xTgAD mice, APα significantly increased the learning of 9-month-old 3xTgAD mice during the course of training as compared to the vehicle-treated group (F(1, 129) = 24.84; P < 0.001). Analysis across the entire learning phase indicated that the performance of APα-treated 3xTgAD mice was maximal from day 1 of the training (Fig. 3B), similar to what was observed in the 6-month-old group. On day 1, learning of APα-treated 3xTgAD mice increased to 21.6 ± 3.0% as compared to 10.0 ± 2.9% in vehicle-treated mice, representing a doubling or more than 100% increase in learning performance which persisted until the last day of the learning trial.
Figure 3. APα reversed the learning and neurogenic deficits in 9-month-old 3xTgAD mice.
(A) Learning curve of 9-month-old nonTg mice: NonTg mice showed low level of learning similar to 6-month-old nonTg mice. Treatment with APα did not affect their learning. (B) Learning curve of 9-month-old 3xTgAD mice: Compared to the nonTg group, 3xTgAD mice showed a significant deficit in their maximum level of learning (P < 0.05). APα significantly increased learning compared to the vehicle-treated group (P < 0.05). 3xTgAD treated with APα exhibited a significant increase in associative learning at day 1, which was sustained at asymptote throughout the learning trial period compared to vehicle-treated 3xTgAD mice and restored learning to a level comparable to vehicle-treated nonTg performance at day 5 (P > 0.05). (C) Memory test in 9-month-old mice: 3xTgAD mice exhibited memory deficit as compared to nonTg mice (P < 0.001). APα significantly enhanced the memory performance of 3xTgAD mice to a level comparable to that of age-matched nonTg group. No effect of APα was observed in nonTg mice. (D) Cell survival analyses: 3xTgAD mice had a significantly reduced cell survival as compared to nonTg mice (P < 0.001). APα treatment increased the number of BrdU+ cells in 3xTgAD mice significantly, but showed no effect on cell survival in the nonTg group. * P < 0.05, as compared to 3xTgAD + vehicle group; † P < 0.001 as compared to nonTg + vehicle group. Data are presented as average ± SEM (n ≥ 9).
These data indicate that APα restored maximal normal learning capacity of these mice and that the effect of APα was not simply to increase learning performance. Whereas nonTg mice required five days of learning trials to reach maximum performance, APα-treated 3xTgAD mice performed and maintained maximal performance from day 1 of the learning trials. APα-treated 3xTgAD mice displayed improved memory relative to the vehicle-treated group (F(1, 24) = 5.14; P < 0.05) (Fig. 3C). Vehicle-treated 3xTgAD mice showed a significant deficit in the memory test as compared to the vehicle-treated nonTg mice (F(1, 20) = 15.24; P < 0.001). The memory deficit of 3xTgAD mice was reversed after treatment with APα to the level of vehicle-treated nonTg mice. Memory performance of APα-treated 3xTgAD mice was also comparable to vehicle-treated normal nonTg. Similar to learning performance, memory of APα-treated 3xTgAD mice increased to 28.2 ± 6.2% as compared to 12.2 ± 2.1% in vehicle-treated 3xTgAD mice, an increase of more than 100%.
Additionally, APα induced a significant increase in the survival of BrdU+ cells in 9-month-old 3xTgAD mice. In 9-month-old vehicle-treated nonTg mice, BrdU+ cell survival was 2289 ± 558 (Fig. 3D) which was not different from vehicle-treated 6-month-old nonTg mice. In APα-treated nonTg mice, 2256 ± 663 BrdU+ cells survived which was not different from the vehicle-treated group. BrdU+ cell survival was significantly lower in 9-month-old vehicle-treated 3xTgAD mice (244 ± 31), relative to vehicle-treated nonTg mice (F(1, 15) = 17.29; P < 0.001) (Fig. 3D). APα significantly increased the survival of BrdU+ cells (900 ± 314) as compared to the vehicle-treated group (F(1, 19) = 7.23; P < 0.05) in 3xTgAD mice. However, APα did not restore survival of BrdU+ cells in 9-month-old APα-treated 3xTgAD mice to the level of nonTg mice (F(1, 17) = 7.29; P < 0.05).
3.4 Loss of APα cognitive and neurogenic efficacy in 12-month-old 3xTgAD mice parallels development of Aβ plaques
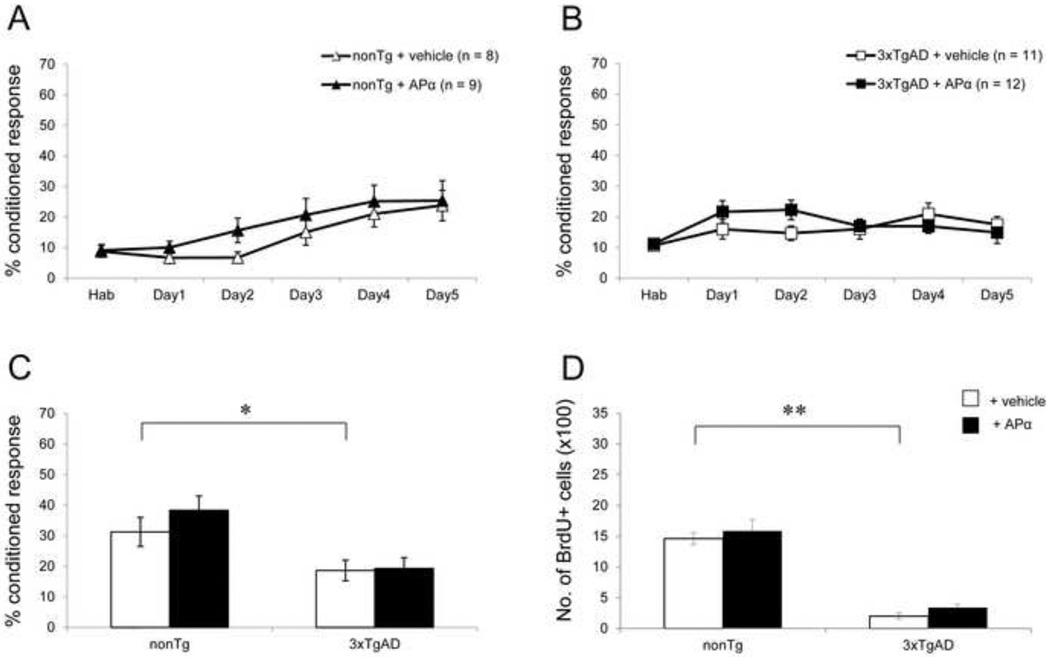
At the age of 12 months, maximal level of intraneuronal Aβ accumulation is apparent in the hippocampus and Aβ plaques are widespread in 3xTgAD mice (see Fig. 1C). In 12-month-old 3xTgAD mice, APα failed to reverse the learning and neurogenic deficits. In parallel to the loss of efficacy in 3xTgAD mice, emergence of trend towards an APα effect in 12-month-old nonTg mice appeared. Twelve-month-old vehicle-treated nonTg mice showed a profound decline in associative learning. The maximal conditioned response of vehicle-treated nonTg mice was 23.8 ± 5% which was not different from the APα-treated mice (Fig. 4A). There was no difference in learning during the course of training between the vehicle-treated and APα-treated groups. However, APα -treated nonTg mice showed a trend towards increased learning on day 2 as compared to the vehicle-treated group. APα induced a positive but not significant effect on memory performance of 12-month-old nonTg mice (Fig. 4C).
Figure 4. APα showed no significant effect in 12-month-old nonTg and 3xTgAD mice.
(A) Learning curve of 12-month-old nonTg mice: NonTg mice showed low level of learning similar to 6 and 9-month-old nonTg mice. Treatment with APα did not alter the learning in nonTg mice significantly, although a positive trend was observed in the initial phase of training. (B) Learning curve of 12-month-old 3xTgAD mice: Vehicle-treated 3xTgAD mice showed almost no learning. APα did not affect the learning in these mice. (C) Memory test in 12-month-old mice: 3xTgAD mice exhibited a significant deficit in their memory compared to age-matched nonTg mice (P < 0.05). APα did not restore memory in both 3xTgAD and nonTg mice. (D) Cell survival analyses: 3xTgAD mice had a significantly reduced cell survival compared to nonTg mice (P < 0.0001). APα treatment showed no significant effect on cell survival in both 3xTgAD and nonTg mice. * P < 0.05; ** P < 0.0001 as compared to nonTg + vehicle group. Data are presented as average ± SEM (n ≥ 8).
Vehicle-treated 12-month-old 3xTgAD mice exhibited a deficit in the final level of learning relative to the vehicle-treated nonTg mice. Vehicle-treated 12-month-old 3xTgAD mice showed impaired learning (17.5 ± 2.6%) (Fig. 4B). Compared to the vehicle-treated group, the learning of APα-treated group was not increased (14.9 ± 3.6%). Twelve-month-old 3xTgAD mice exhibited no improvement in learning over the 5 days of training (Fig. 4B) which was also evident in the memory performance (Fig. 4C). Vehicle-treated 12-month-old 3xTgAD mice showed a significant deficit in the memory test as compared to the vehicle-treated nonTg mice (F(1, 17) = 4.96; P < 0.05), which was not affected following treatment with APα. APα-treated 3xTgAD mice also showed a significant deficit relative to vehicle-treated nonTg mice (F(1, 19) = 4.29; P < 0.05). In 12-month-old nonTg mice, APα induced a modest but not significant trend towards an increase in BrdU+ cell survival (Fig. 4D). Basal level of BrdU+ cell survival was significantly decreased in 12-month-old 3xTgAD mice (202 ± 52) as compared to age-matched nonTg mice (1461 ± 100) (F(1, 16) = 135.58; P < 0.001). Cell survival in 12-month-old nonTg mice was not significantly different from that of 6- and 9-month-old nonTg mice. APα exerted no significant effect on BrdU+ cell survival in 12-month-old nonTg and 3xTgAD mice.
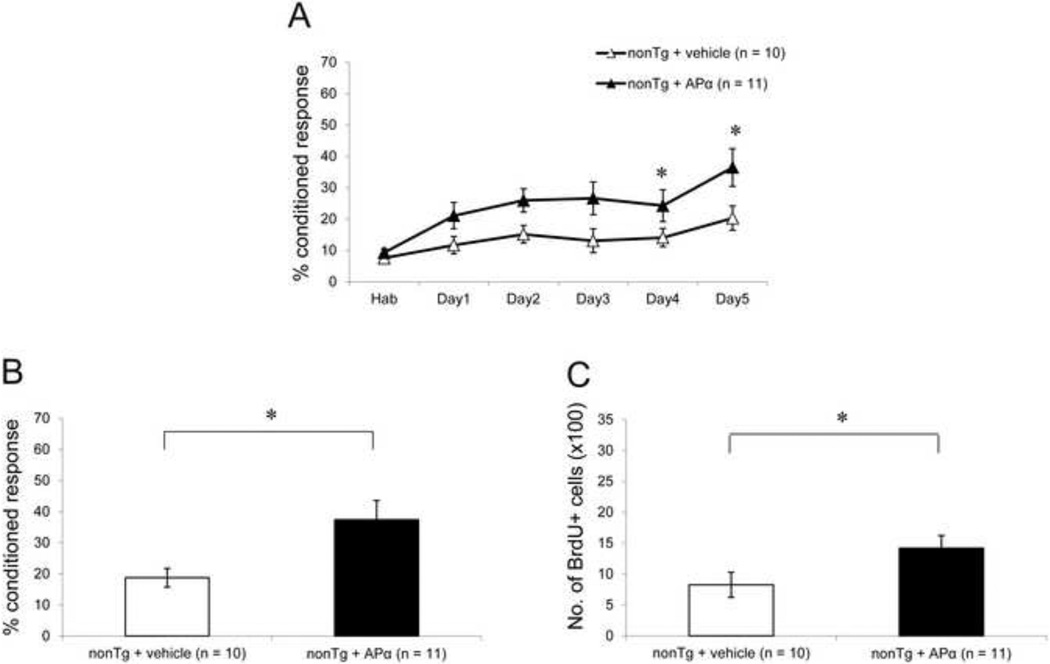
3.5 APα reversed the cognitive and neurogenic deficits in 15-month-old nonTg mice
At an age of 15 months, extracellular Aβ plaques and tau hyperphosphorylation are observed in the hippocampus of 3xTgAD mice. Since no significant effect of APα was seen in 12-month-old 3xTgAD mice, analyses of 15-month-old 3xTgAD mice were not conducted. To determine whether the trend towards a positive effect of APα in the 12-month-old nonTg mice extended to 15-month-old nonTg mice, we studied this age group. Vehicle-treated 15-month-old nonTg mice exhibited a basal level of learning performance (maximum of 20.3 ± 3.7%) comparable to that of 6-month-old 3xTgAD mice. Treatment with APα led to a significant increase in their learning (maximal of 36.5 ± 5.5%), as compared to the vehicle-treated group (F(1, 19) = 4.94; P < 0.05) (Fig. 5A). On day 1 of learning, APα-treated 15-month-old nonTg mice performed better than vehicle-treated 12-month-old nonTg mice. Compared to vehicle-treated 15-month-old nonTg mice, learning on day 1 increased from 11.7 ± 2.4% to 21.1 ± 3.8% in APα-treated nonTg mice, representing an 80% increase in learning performance which persisted until the last day of the learning trial. APα also induced a comparable improvement on memory function with APα-treated mice (37.4 ± 5.7%) performing significantly better than vehicle-treated mice (18.8 ± 2.6%) (F(1, 19) = 6.15; P < 0.05) (Fig. 5B), an increase of more than 100%. Vehicle-treated 15-month-old nonTg mice had a significant reduction in BrdU+ cell survival as compared to vehicle-treated 12-month-old nonTg mice (F(1, 19) = 5.49; P < 0.05). APα treatment induced a significant increase in the survival of BrdU+ cells in 15-month-old nonTg mice (1422 ± 204) as compared to the age-matched vehicle-treated mice (827 ± 202) (F(1, 23) = 4.13; P < 0.05). BrdU+ cell survival in APα-treated 15-month-old mice increased to the level of vehicle-treated 12-month-old nonTg mice (F(1, 18) = 0.02; P < 0.05).
Figure 5. APα reversed the learning and neurogenic deficits in 15-month-old nonTg mice.
(A) Learning curve of 15-month-old nonTg mice: Basal level of learning in 15-month-old nonTg mice was significantly low as compared to 6, 9, and 12-month-old nonTg mice. Treatment with APα significantly increased 15-month-old nonTg mouse learning performance compared to the vehicle-treated mice. Further, learning performance of APα-treated 15-month-old nonTg mice was comparable to maximal learning performance of 12-month-old mice. (B) Memory test in 12-month-old nonTg mice: Vehicle-treated 15-month-old nonTg mice showed reduced memory as compared to 6, 9 and 12-month-old nonTg mice. Treatment with APα significantly increased their memory to level of 12-month-old nonTg mice. (C) Cell survival analyses: 15-month-old nonTg mice showed a significantly reduced basal level of BrdU+ cells as compared to 6, 9 and 12-month-old nonTg mice. Treatment with APα significantly increased the cell survival, comparable to that of 12-month-old nonTg mice. * P < 0.05 as compared to nonTg + vehicle group. Data are presented as average ± SEM (n ≥ 10).
4. Discussion
Using a triple transgenic mouse model of Alzheimer’s disease (3xTgAD) and its background strain control (nonTg), the data indicate that aging and Alzheimer’s disease burden diminishes hippocampal neurogenesis and associated cognitive performance. These effects on hippocampal neurogenesis were differentially regulated by aging and AD pathology, with AD pathology affecting cell survival and hippocampal-dependent associative learning and memory more severely than normal aging. APα reversed the neurogenic and cognitive deficits in 3xTgAD mice with mild and moderate levels of Aβ. Further, efficacy of APα to reverse neurogenic and cognitive deficits in the normal nonTg aging mouse hippocampus was evident at 15-months of age. Further, at day 5, learning and memory performance of APα-treated 3xTgAD mice was comparable to the vehicle-treated nonTg mice. In 6- and 9-month-old APα-treated 3xTgAD mice, learning performance was maximal from day 1 of training and was sustained at maximum level throughout the five days of training. These data indicate that APα restored maximal learning capacity of these mice and that the effect of APα was not simply to increase learning performance. APα-treated 3xTgAD mice reached asymptotic learning performance within 60 trials (on day 1) relative to 300 trials (accumulated over 5 days) required by nonTg vehicle-treated mice. Whereas nonTg mice required five days of learning trials to reach maximum performance, APα-treated 3xTgAD mice performed and maintained maximal performance from day 1 of the learning trial. These results are supportive of our initial hypothesis that APα is an effective neurogenic agent when there is an intrinsic deficiency in neurogenesis.
4.1 APα regulation of neurogenesis and cognitive performance in aging and AD brain
We have previously shown that APα reverses both the neurogenic and cognitive deficits of 3-month-old 3xTgAD mice (Wang, et al., 2010). Herein, we extended our analyses to investigate the neurogenic and cognitive efficacy of APα in 3xTgAD mice expressing low (6-month-old) to moderate intraneuronal Aβ (9-month-old) and extraneuronal Aβ plaques (12-month-old). In parallel, neurogenic and cognitive analyses were conducted in age-matched nonTg background mice. In 3xTgAD mice, APα sustained survival of neural progenitor cells and reversed learning and memory deficits in 6- and 9-month-old 3xTgAD mice when intraneuronal Aβ burden was mild to moderate. The magnitude of neural progenitor survival and cognitive performance of APα treated 6- and 9-month-old 3xTgAD mice were comparable to that of age-matched normal nonTg mice. When extraneuronal Aβ plaques were present in the 12-month-old 3xTgAD mice, APα was ineffective in reversing the profound neurogenic and cognitive deficits of these mice.
The efficacy of APα in 3xTgAD mice expressing intraneuronal Aβ is consistent with its efficacy in pre-Aβ expressing 3-month-old 3xTgAD mice. Collectively, these data indicate that APα is efficacious at stages when Aβ has not yet transitioned to extraneuronal Aβ plaque. The data indicate that presence of intraneuronal Aβ in the hippocampus does not interfere with survival of newly generated cells and restoration of cognitive function. Further, these data suggest that the mechanism by which APα promotes neurogenesis (Wang, et al., 2005) remains functional in this population of adult neural progenitor cells. The data also indicate that when plaques are present, newly generated neurons do not survive and integrate into the neural circuitry.
In 12-month-old 3xTgAD mice, Aβ plaques are abundant in the hippocampus as is tau hyperphosphorylation (Chen, 2008,Oddo, et al., 2003b). Tau phosphorylation plays a key role in adult hippocampal neurogenesis (Hong, et al., 2009) and as would be expected, the functional integrity of tau protein is critical for mitosis and migration. As such, hyperphosphorylated tau could be a major player in impaired neurogenesis and hence the loss of APα efficacy. Aβ and hyperphosphorylated tau in the perforant pathway could alter the activity patterns that are required for neuronal survival. Consistent with this postulate, Aβ requires tau to impair axonal transport of mitochondria and the neurotrophic receptor Trk A (Vossel, et al., 2010), critical for neuronal function. Although APα was ineffective when administered once to 12-month-old 3xTgAD mice, early intervention and an optimal treatment regimen could delay extraneuronal Aβ and hyperphosphorylated tau (Chen, 2008) thereby extending the therapeutic window for APα neurogenic efficacy.
In nonTg 6- and 9-month-old mice, APα was without significant effect on both neural progenitor survival and cognitive performance. However, when mice aged to 12 months, APα induced a trend towards increased neural progenitor survival and improved learning and memory. This trend became significant in the 15-month-old nonTg mice. Efficacy of APα emerged in the 15-month-old group, when the basal level of neurogenesis was significantly reduced compared to 9- and 12-month old nonTg mice. Treatment with APα increased neural progenitor survival and restored learning and memory to that of 12-month old nonTg mice.
Age- and AD-related decline in neurogenesis has been reported in various studies and may contribute to cognitive decline (Chevallier, et al., 2005, Ewers, et al., 2006, Kishimoto, et al., 2001, Kuhn, et al., 1996, Rodriguez, et al., 2008). Efficacy of APα to the improved behavioral performance and survival of newly generated cells could be impacted by a neuroprotective effect of APα. Stein and colleagues have shown that APα is neuroprotective when given within several hours of traumatic brain injury (Djebaili, et al., 2005, Sayeed, et al., 2006). Mechanistically, Stein and colleagues have shown that APα directly inhibits the mitochondrial permeability transition pore which could be associated with improved neuroprotective efficacy over progesterone (Sayeed, et al., 2009). While the age-associated mechanism of APα-induced efficacy remains to be fully determined, substantial evidence indicates the decline in neurogenic growth and survival factors in the aged brain contributes to a decline in the neurogenic potential of the subgranular zone (Brinton, et al., 2008, Brinton and Wang, 2006, Hattiangady, et al., 2005, Kuhn, et al., 1997, Rao, et al., 2006a, Rao, et al., 2006b, Rao, et al., 2006c, Shetty, et al., 2005). Moreover, neural progenitor cells are capable of synthesizing and secreting their own growth factors, including APα, which when in decline is associated with a deterioration of neuronal networks (Gago, et al., 2004, Taupin, et al., 2000). Consistent with this postulate, APα concentration was significantly lower in both brain and plasma of the 3xTgAD male mouse (Wang, et al., 2010). Likewise, AD patients have significantly lower APα levels compared to age-matched controls (Naylor, et al., 2010, Weill-Engerer, et al., 2002). Collectively, the data indicate that the efficacy of APα to increase neuroprogenitor survival and associated cognitive functions is consistent with APα functioning as a neuroregenerative agent in the aged brain.
Notably, although both nonTg and 3xTgAD mice exhibited an age-associated decline in neurogenesis and cognitive performance as reported herein and by others (Billings, et al., 2007, Billings, et al., 2005, Kishimoto, et al., 2001, Rodriguez, et al., 2008, Woodruff-Pak, et al., 1990), APα was effective at different stages in these two cases. APα was more effective in 3xTgAD mice and appeared to be targeting the decline in regenerative capacity associated with AD. It is possible that APα specifically antagonizes the effector or pathway through which AD pathology induces neurogenic and cognitive deficits, such as Aβ. Indeed APα has been shown to delay progression of Aβ burden in 3xTgAD mice as evidenced by reduced intracellular Aβ and extracellular plaques (Chen, 2008).
4.2 Neurosteroids, neurogenesis, and cognitive performance
In summary, regenerative capacity and cognitive function were decreased in a transgenic mouse model of AD. Further, these deficits increased in magnitude with age. APα restored both regenerative capacity and cognitive function in these transgenic AD mice under conditions of intraneuronal Aβ. Transition to extraneuronal deposition of Aβ into plaques was associated with a loss in APα efficacy. Emergence of significant efficacy of APα as a regenerative and restorative cognitive agent was evident in normal aging mice at 15-months of age. In terms of magnitude of response, in every instance, in both 3xTgAD and nonTg mice, where APα had an effect it induced a 100% or more increase in cognitive performance in learning and memory. In 3xTgAD mice where APα was effective, i.e. 6- and 9-month age groups, survival of BrdU+ cells was increased by more than 100% and in 15-month nonTg, survival of BrdU+ cells was increased by 50%.
With any therapeutic intervention there will be responders and non-responders. The data within this experiment and our earlier experiments indicate that there is less variability in the vehicle treated group, i.e. clustering around a common performance level whereas introduction of APα separates the group into responders and nonresponders. The key question is: does APα promote a greater performance capability than nonresponders? When we separated the data into responders versus nonresponders and not simply an average group response, the data show that APα promotes cognitive performance, i.e. within 2/3 of the group. In addition, 1/3 of the animals performed at or below the average of the vehicle group. Because this series of experiments was based on a single exposure to APα, we do not know whether the nonresponders would convert to responders with multiple exposures to APα. These experiments are currently underway.
Our previous results derived from young 3xTgAD mice (Wang, et al., 2010) indicated that APα-induced cognitive improvement was significantly correlated with neurogenesis and integration of these new neurons into the hippocampal learning and memory circuitry. Based on these correlation analyses, the increased survival of newly generated neurons in the aged 3xTgAD and nonTg mice was likely to have contributed to the restoration of hippocampal-dependent associative learning and memory in these animals. From a translational perspective, an optimal therapeutic would target the neurogenic and cognitive deficits as well as the mechanisms leading to AD pathology. A single exposure to APα induced significant therapeutic efficacy to restore regenerative and cognitive capacities while having no impact on AD pathology (Chen, 2008). We therefore have focused our most recent translational analyses to determine a therapeutic regimen that promotes regeneration and cognitive function while simultaneously reducing AD pathology. Results of those analyses indicate that a once per week treatment regimen for six months, initiated prior to and during AD pathology development, results in a significant increase of new neurons, survival of those new neurons and reduction in AD pathology (Chen, 2008). Determination of the impact of this treatment regimen on cognitive performance is currently being evaluated.
Acknowledgements
This research was supported by grants from by the National Institute on Aging U01AG031115, the Alzheimer Drug Discovery Foundation, and the Kenneth T. and Eileen L. Norris Foundation to RDB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: Patents pending on allopregnanolone as a therapeutic for mild cognitive impairment and Alzheimer's disease.
References
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14(7):325–337. doi: 10.1016/j.tics.2010.04.003. 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol. 2008;73:357–365. doi: 10.1101/sqb.2008.73.019. 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28(10):2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE, Robel P, Schumacher M. Neurosteroids: beginning of the story. International Review of Neurobiology. 2001;46:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Salvestroni C, Casarosa E, Nappi RE, Lanzone A, Luisi S, Purdy RH, Petraglia F, Genazzani AR. Aging is associated with changes in allopregnanolone concentrations in brain, endocrine glands and serum in male rats. Eur J Endocrinol. 1998;138(3):316–321. doi: 10.1530/eje.0.1380316. [DOI] [PubMed] [Google Scholar]
- Billings LM, Green KN, McGaugh JL, LaFerla FM. Learning decreases A beta*56 and tau pathology and ameliorates behavioral decline in 3xTg-AD mice. J Neurosci. 2007;27(4):751–761. doi: 10.1523/JNEUROSCI.4800-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Bilsland JG, Haldon C, Goddard J, Oliver K, Murray F, Wheeldon A, Cumberbatch J, McAllister G, Munoz-Sanjuan I. A rapid method for the quantification of mouse hippocampal neurogenesis in vivo by flow cytometry. Validation with conventional and enhanced immunohistochemical methods. J Neurosci Methods. 2006;157(1):54–63. doi: 10.1016/j.jneumeth.2006.03.026. 10.1016/j.jneumeth.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106(32):13594–13599. doi: 10.1073/pnas.0901402106. 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30(4):212–222. doi: 10.1016/j.tips.2008.12.006. 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: Form and function in brain. Front Neuroendocrinol. 2008 doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer's disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3(3):185–190. doi: 10.2174/156720506777632817. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang JM, Irwin RW, Yao J, Liu L, Brinton RD. 2008 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. Chronic administration of allopregnanolone modifies progression of alzheimer's pathology in the triple transgenic alzheimer's disease mouse. Program No 55411 2008 Online. [Google Scholar]
- Chevallier NL, Soriano S, Kang DE, Masliah E, Hu G, Koo EH. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am J Pathol. 2005;167(1):151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J Neurosci Res. 2010;88(10):2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22(1):106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Beres AJ, Barber AE, Svendsen CN. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson's disease. Exp Neurol. 2008;209(1):213–223. doi: 10.1016/j.expneurol.2007.09.022. 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ermini FV, Grathwohl S, Radde R, Yamaguchi M, Staufenbiel M, Palmer TD, Jucker M. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am J Pathol. 2008;172(6):1520–1528. doi: 10.2353/ajpath.2008.060520. 10.2353/ajpath.2008.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Morgan DG, Gordon MN, Woodruff-Pak DS. Associative and motor learning in 12-month-old transgenic APP+PS1 mice. Neurobiol Aging. 2006;27(8):1118–1128. doi: 10.1016/j.neurobiolaging.2005.05.019. 10.1016/j.neurobiolaging.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the Adult Brain. J Neurosci. 2002;22(3):612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago N, El-Etr M, Sananes N, Cadepond F, Samuel D, Avellana-Adalid V, Baron-Van Evercooren A, Schumacher M. 3alpha,5alpha-Tetrahydroprogesterone (allopregnanolone) and gamma-aminobutyric acid: autocrine/paracrine interactions in the control of neonatal PSA-NCAM+ progenitor proliferation. J Neurosci Res. 2004;78(6):770–783. doi: 10.1002/jnr.20348. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195(2):353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29(1):129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shuai B, Cai J, Coksaygan T, Rao MS, Shetty AK. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25(8):2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- Henry S, Bigler S, Wang J. High throughput analysis of neural progenitor cell proliferation in adult rodent hippocampus. Biosci Trends. 2009;3(6):233–238. [PMC free article] [PubMed] [Google Scholar]
- Hong XP, Peng CX, Wei W, Tian Q, Liu YH, Yao XQ, Zhang Y, Cao FY, Wang Q, Wang JZ. Essential role of tau phosphorylation in adult hippocampal neurogenesis. Hippocampus. 2009 doi: 10.1002/hipo.20712. [DOI] [PubMed] [Google Scholar]
- Kim WR, Kim Y, Eun B, Park OH, Kim H, Kim K, Park CH, Vinsant S, Oppenheim RW, Sun W. Impaired migration in the rostral migratory stream but spared olfactory function after the elimination of programmed cell death in Bax knock-out mice. J Neurosci. 2007;27(52):14392–14403. doi: 10.1523/JNEUROSCI.3903-07.2007. 10.1523/JNEUROSCI.3903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Suzuki M, Kawahara S, Kirino Y. Age-dependent impairment of delay and trace eyeblink conditioning in mice. Neuroreport. 2001;12(15):3349–3352. doi: 10.1097/00001756-200110290-00040. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17(15):5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber ME, Lichtensteiger W. Ontogeny of 5 alpha-reductase (type 1) messenger ribonucleic acid expression in rat brain: early presence in germinal zones. Endocrinology. 1996;137(7):2718–2730. doi: 10.1210/endo.137.7.8770891. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Mouthon MA, Gheusi G, de Chaumont F, Olivo-Marin JC, Lamarque S, Abrous DN, Boussin FD, Lledo PM. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One. 2009;4(9):e7017. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010 doi: 10.1016/j.tins.2010.09.003. 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Kim JJ. Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats. J Neurosci. 2004;24(13):3242–3250. doi: 10.1523/JNEUROSCI.5382-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19(6):672–682. doi: 10.1038/cr.2009.56. 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, Ervin JF, Butterfield MI, Blazer DG, Massing MW, Lieberman JA. The Neurosteroid Allopregnanolone Is Reduced in Prefrontal Cortex in Alzheimer's Disease. Biol Psychiatry. 2006;60(12):1287–1294. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002a;13(1):35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Synthesis, regulation, and function of neurosteroids. Endocr Res. 2002b;28(4):463. doi: 10.1081/erc-120016823. [DOI] [PubMed] [Google Scholar]
- Naylor JC, Kilts JD, Hulette CM, Steffens DC, Blazer DG, Ervin JF, Strauss JL, Allen TB, Massing MW, Payne VM, Youssef NA, Shampine LJ, Marx CE. Allopregnanolone levels are reduced in temporal cortex in patients with Alzheimer's disease compared to cognitively intact control subjects. Biochim Biophys Acta. 2010;1801(8):951–959. doi: 10.1016/j.bbalip.2010.05.006. 10.1016/j.bbalip.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niidome T, Taniuchi N, Akaike A, Kihara T, Sugimoto H. Differential regulation of neurogenesis in two neurogenic regions of APPswe/PS1dE9 transgenic mice. Neuroreport. 2008;19(14):1361–1364. doi: 10.1097/WNR.0b013e32830e6dd6. 00001756-200809170-00002 [pii] [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003a;24(8):1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003b;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ohno M, Tseng W, Silva AJ, Disterhoft JF. Trace eyeblink conditioning requires the hippocampus but not autophosphorylation of alphaCaMKII in mice. Learn Mem. 2005;12(3):211–215. doi: 10.1101/lm.90205. [DOI] [PubMed] [Google Scholar]
- Park KI, Himes BT, Stieg PE, Tessler A, Fischer I, Snyder EY. Neural stem cells may be uniquely suited for combined gene therapy and cell replacement: Evidence from engraftment of Neurotrophin-3-expressing stem cells in hypoxic-ischemic brain injury. Exp Neurol. 2006;199(1):179–190. doi: 10.1016/j.expneurol.2006.03.016. 10.1016/j.expneurol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21(2):464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Reddy DS, Shetty AK. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res. 2006a;83(6):1088–1105. doi: 10.1002/jnr.20802. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. Fetal hippocampal CA3 cell grafts enriched with FGF-2 and BDNF exhibit robust long-term survival and integration and suppress aberrant mossy fiber sprouting in the injured middle-aged hippocampus. Neurobiol Dis. 2006b;21(2):276–290. doi: 10.1016/j.nbd.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006c;5(6):545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS ONE. 2008;3(8):e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Verkhratsky A. Impaired cell proliferation in the subventricular zone in an Alzheimer's disease model. Neuroreport. 2009;20(10):907–912. doi: 10.1097/WNR.0b013e32832be77d. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med. 2006;47(4):381–389. doi: 10.1016/j.annemergmed.2005.12.011. 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Parvez S, Wali B, Siemen D, Stein DG. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Res. 2009;1263:165–173. doi: 10.1016/j.brainres.2009.01.045. 10.1016/j.brainres.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: Role of astrocytes. Glia. 2005 doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi N, Niidome T, Goto Y, Akaike A, Kihara T, Sugimoto H. Decreased proliferation of hippocampal progenitor cells in APPswe/PS1dE9 transgenic mice. Neuroreport. 2007;18(17):1801–1805. doi: 10.1097/WNR.0b013e3282f1c9e9. 00001756-200711190-00010 [pii] [DOI] [PubMed] [Google Scholar]
- Taupin P, Ray J, Fischer WH, Suhr ST, Hakansson K, Grubb A, Gage FH. FGF-2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron. 2000;28(2):385–397. doi: 10.1016/s0896-6273(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14(1):58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- Verret L, Jankowsky JL, Xu GM, Borchelt DR, Rampon C. Alzheimer's-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J Neurosci. 2007;27(25):6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330(6001):198. doi: 10.1126/science.1194653. 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Shors TJ. Neurogenesis, learning and associative strength. Eur J Neurosci. 2008;27(11):3020–3028. doi: 10.1111/j.1460-9568.2008.06222.x. 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25(19):4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RF, Brinton RD. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107(14):6498–6503. doi: 10.1073/pnas.1001422107. 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill-Engerer S, David JP, Sazdovitch V, Liere P, Eychenne B, Pianos A, Schumacher M, Delacourte A, Baulieu EE, Akwa Y. Neurosteroid quantification in human brain regions: comparison between Alzheimer's and nondemented patients. J Clin Endocrinol Metab. 2002;87(11):5138–5143. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Finkbiner RG, Sasse DK. Eyeblink conditioning discriminates Alzheimer's patients from non-demented aged. Neuroreport. 1990;1(1):45–48. doi: 10.1097/00001756-199009000-00013. [DOI] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Exp Neurol. 2007;204:77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]