Abstract
Background & Aims
Nuclear receptors such as pregnane X receptor and constitutive androstane receptor (CAR) are important regulators of drug-metabolizing systems such as P450s enzymes and modulate xenobiotic metabolism as well as hepatocellular proliferation. Binding of CAR to NR response elements alone is not sufficient to activate gene expression. Here we investigate the role of steroid receptor coactivator (SRC) family members in CAR-mediated hepatocyte proliferation and drug metabolism.
Methods
The role of SRCs in CAR activation was assessed in cell-based transfection assays and protein-protein interaction assays. The in vivo role of SRCs in CAR-mediated hepatocyte proliferation and drug metabolism was examined by using mice deficient in SRCs.
Results
SRC-3 displayed the highest coactivating activity to CAR compared with SRC-1 and SRC-2 in a cell-based reporter assay. Knockout of SRC-3 in mice attenuated hepatic hyperplasia induced by a CAR agonist 1,4-bis-[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), which was associated with a reduced expression of c-Myc and Foxm-1. In contrast, knockout of SRC-1 or SRC-2 in mice did not affect TCPOBOP-induced hepatic hyperplasia. SRC-3-deficient mice were hypersensitive to zoxazolamine-induced paralysis, but were resistant to acetaminophen hepatotoxicity induced by TCPOBOP, whereas mutant mice deficient in SRC-1 or SRC-2 exhibited severe acetaminophen hepatotoxicity similar to wild-type controls. Accordingly, deficiency in SRC-3, but not SRC-1 or SRC-2, resulted in a reduced CAR-mediated expression of drug metabolism-related genes in the liver.
Conclusions
Our study demonstrates that SRC-3 is the predominant transcriptional coactivator among the three SRC family members for CAR activation to promote hepatocyte proliferation and drug metabolism.
Keywords: SRC-3, CAR, hepatocyte proliferation, drug metabolism
Introduction
The liver responds to xenobiotics by activating the nuclear receptors (NRs) such as pregnane X receptor and constitutive androstane receptor (CAR), which lead to the induction of cytochrome P450s and other enzymes that function in the process of xenobiotic metabolism [1]. In addition to regulating xenobiotic metabolism, CAR also plays important roles in drug-induced hepatocyte proliferation, liver regeneration, and murine hepatocarcinogenesis [2, 3]. CAR is retained in the cytoplasm of untreated hepatocytes in an inactive form but can be activated by Phenobarbital (PB) and a group of agents referred to as “PB-like inducers” such as 1,4-bis-[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), the most potent PB-like inducer and a specific agonistic ligand for murine CAR [3–5]. Activation of CAR by PB or PB-like inducers results in translocation of CAR from the hepatocyte cytoplasm to the nucleus and bind to the NR response elements localized in the promoter of the target genes such as Cyp2B10 [6].
Binding of CAR to NR response elements alone is not sufficient to activate gene expression. In order to activate gene expression, NR has to interact with coactivators to form a multiprotein complex that activates the general transcriptional machinery via processes involving chromatin remodeling, formation of stable preinitiation complexes, and enhanced RNA polymerase II recruitment [7]. The essential role of coactivators such as activating signal cointegrator 2 (ASC-2) and PBP/TRAP220/DRIP205 in hepatic CAR activation has been demonstrated [8, 9], while the role of steroid receptor coactivators (SRCs) in hepatic CAR activation remains to be elucidated.
SRCs are members of the p160 transcriptional coactivator family which includes SRC-1, SRC-2/GRIP1/TIF2, and SRC-3/pCIP/ACTR/AIB1/RAC-3/TRAM-1. SRCs are about 160 kDa in size and share an overall sequence similarity of 50%–55% [10, 11]. All SRCs are expressed in the liver [12]. SRC-3 is overexpressed in 68% of human hepatocellular carcinoma (HCC) and promotes HCC progression by enhancing cell proliferation and invasiveness [13], indicating an important pathophysiological role of SRC-3 in the liver. It has been reported that SRC-1~3 can enhance CAR transactivation in both an in vitro cell-based reporter assay and an in vivo liver-based reporter assay [14–16]. However, it is not clear which SRC family member plays the predominant role in hepatic CAR activation. In this study, we used mutant mice deficient in SRC-1, SRC-2, or SRC-3 to address this question. We found that SRC-3-deficient mice exhibited reduced TCPOBOP-induced hepatocyte hyperplasia, abrogated TCPOBOP-induced APAP hepatotoxicity, and exacerbated zoxazolamine-induced paralysis compared to their wild-type controls. In contrast, both SRC-1- and SRC-2-deficient mice exhibit typical TCPOBOP-induced hepatocyte hyperplasia and severe TCPOBOP-induced APAP hepatotoxicity similar to their wild-type controls. These results indicate that SRC-3 is the predominant SRC coactivator for CAR activation to promote hepatocyte proliferation and drug metabolism.
Materials and Methods
Animals
Mice deficient in SRC-1 (SRC-1−/−) or SRC-2 (SRC-2−/−) are maintained on a C57BL/6 background, and mice deficient in SRC-3 (SRC-3−/−) are maintained on a C57BL/6×129/Sv background. Wild-type littermates served as controls. Adult male mice (6–8 weeks old) were used in all experiments. All animals received human care and animal experiments were performed in accordance with the Laboratory Animal Center of Xiamen University or the Institutional Animal Care and Use Committee at Baylor College of Medicine.
Transient transfection and reporter assays
L-O2, Huh-7, Chang’s or Hepa1-6 cells were transfected with equal amounts of SRC-1/2/3 together with NRs and NR reporter plasmids respectively using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). NR agonists were added at 8 h post-transfection. Cells were harvested at 24 h after NR agonist treatment and luciferase activities were measured. Similar results were obtained from at least three independent experiments.
TCPOBOP and acetaminophen (APAP) treatments
For TCPOBOP treatment, mice were given a single i.p. injection of TCPOBOP at a dose of 3 mg/kg body weight. BrdU was injected i.p. at a dose of 50 mg/kg body weight twice a day. Mice were sacrificed at the indicated time after TCPOBOP injection and their body weight and liver weight were measured. For TCPOBOP-induced APAP hepatotoxicity assay, mice were given a single i.p. injection of APAP (250 mg/kg) at 72 h after a single treatment with TCPOBOP (mice were not starved before APAP treatment). Mice were sacrificed 24 h later and their serum samples were collected for measuring levels of alanine aminotransferase (ALT) by using a GP-transaminase Kit (Jiancheng Biotechnology Institute, Nanjing, China).
Zoxazolamine paralysis experiment
Mice pre-treated with a single dose of corn oil or 3 mg/kg TCPOBOP for 72 h were i.p. injected with zoxazolamine (300 mg/kg), and paralysis duration was recorded as described [17].
Histology, immumohistochemistry, and Western blot analysis
Paraffin sections of livers were cut and stained with hematoxylin and eosin. Immunohistochemistry and Western blot analysis were performed as described [13, 18].
Quantitative real-time reverse transcription (RT)–PCR
Quantitative real-time PCR was performed as described [13, 18]. The primers used for real-time PCR were listed in Supplementary Table 1.
Statistical analysis
Values were shown as the mean + SD from the number of replicates described in the text. Bars in the graph represent SD. The statistical significance of differences between mean values (p < 0.05) was evaluated with the two-tailed Student’s t-test.
Results
SRC-3 is an essential transcriptional coactivator for CAR-mediated transactivation in vitro
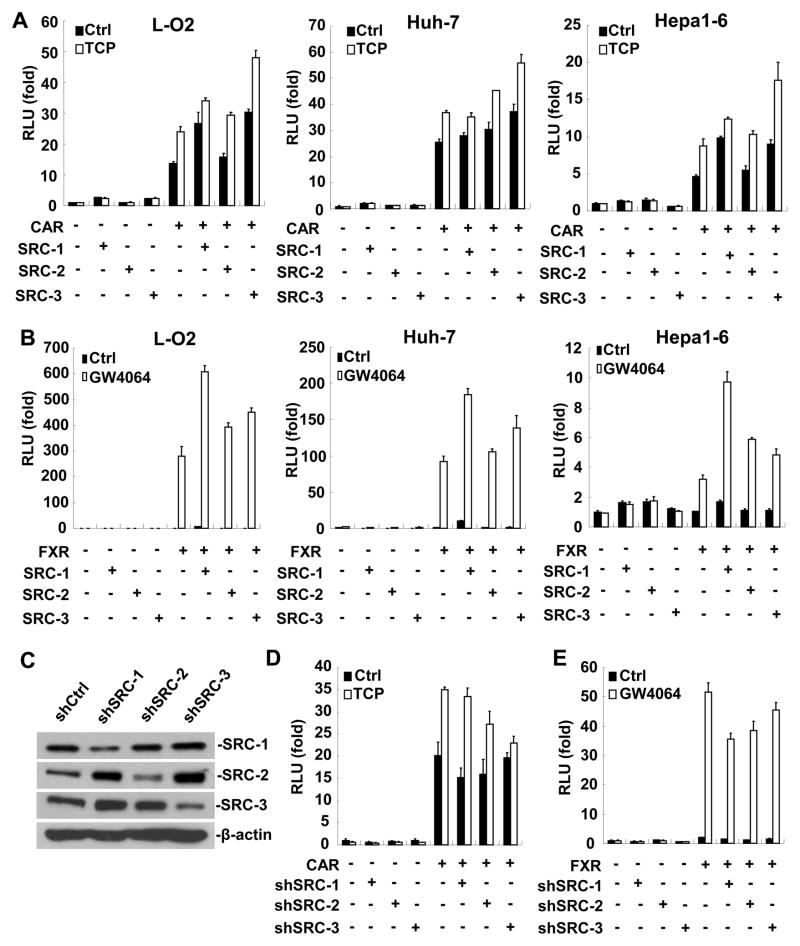
To investigate the involvements of SRC family members in the transactivation process induced by liver-enriched NRs such as CAR and farnesoid X receptor (FXR), we co-transfected three individual SRC family members (SRC-1~3) with CAR or FXR together with their corresponding reporters into human liver cell lines L-O2, Huh-7, Chang’s, and mouse liver cell line Hepa1-6, respectively. As shown in Fig. 1A, B and Supplementary Fig. 1, all SRCs enhanced CAR or FXR mediated reporter activation in the presence of TCPOBOP, an agonist for CAR, or GW4064, a synthetic agonist for FXR. However, SRC family members exhibited different coactivating activities. Among them, SRC-3 displayed the highest coactivating activity to CAR in all cell lines examined, whereas SRC-1 showed the highest coactivating activity to FXR in all cell lines except Chang’s cells. These data suggest that different NRs might have preferences for different SRC family members. To further reveal the role of SRC family members in CAR or FXR activation, we knockdowned the expression of SRC-1, SRC-2 or SRC-3 in Huh-7 cells by shRNA (Fig. 1C). Down-regulation of SRC-3, but not SRC-1 or SRC-2, dramatically abolished the stimulatory effect of TCPOBOP on CAR activation (Fig. 1D), whereas down-regulation of SRC-1 had the most significant reduction effect on FXR transactivation (Fig. 1E). Collectively, these results indicate that SRC-3 is an essential transcriptional coactivator for TCPOBOP-activated CAR, but not for FXR.
Figure 1. SRC-3 is a critical transcriptional coactivator for CAR activation in vitro.
(A) SRC-3 exhibited the highest coactivating activity to CAR. (B) SRC-1 exhibited the highest coactivating activity to FXR. (C) Down-regulation of SRC-1, SRC-2, or SRC-3 by shRNA was confirmed by Western blot analysis. (D) Down-regulation of SRC-3, but not SRC-1 or SRC-2, markedly abrogated the stimulatory effect of TCPOBOP on CAR activation. (E) Down-regulation of SRC-1 had the most significant reduction effect on FXR transactivation. TCP: TCPOBOP.
Knockout of SRC-3 in mice reduces TCPOBOP-induced hepatic hyperplasia
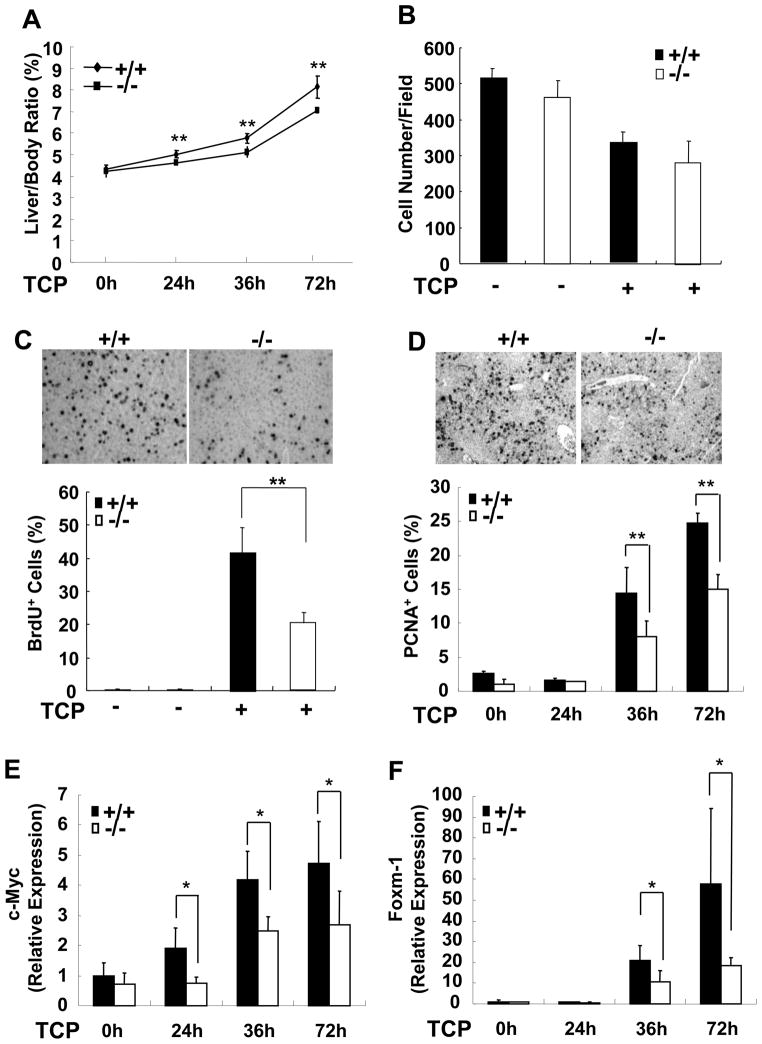
Having demonstrated the dominant role of SRC-3 in CAR activation in vitro, we further investigated whether SRC-3 also plays a major role in CAR-mediated transcription in vivo. TCPOBOP-induced hepatomegaly is a well-established model for examining CAR activation, in which TCPOBOP induces both hepatocyte hypertrophy (increase in cell size and cell growth) and hyperplasia (increase in cellular division and cell number) [3, 17, 19]. As shown in Fig. 2A, liver mass was increased in both wild-type and SRC-3−/− mice after TCPOBOP treatment, however the increasing level of liver mass in SRC-3−/− mice was less than that in wild-type mice. To evaluate the hepatocyte hypertrophy, we examined relative hepatocyte size in wild-type and SRC-3−/− mice by counting hepatocytes per visual field in sections of livers. Fig. 2B showed a similar decrease in hepatocyte number per visual field in both types of mice after TCPOBOP treatment, suggesting that SRC-3 is dispensable for TCPOBOP-induced hepatocyte hypertrophy. To evaluate hepatocyte hyperplasia, BrdU incorporation in hepatocytes and proliferating cell nuclear antigen (PCNA) immunohistochemical staining were performed in liver sections from both types of mice after TCPOBOP treatment. The number of BrdU-positive hepatocytes as well as PCNA-positive hepatocytes in SRC-3−/− livers was significantly less than that in wild-type mice (Fig. 2C, D). To further confirm the function of SRC-3 in hepatocyte proliferation, we measured the expression of another proliferation marker Ki-67, and found that SRC-3−/− mice showed lower levels of hepatic Ki-67 expression than wild-type controls (Supplementary Fig. 2).
Figure 2. SRC-3 deficiency reduces TCPOBOP-induced hepatic hyperplasia.
(A) Relative liver weight of wild-type and SRC-3−/− mice with or without TCPOBOP treatment. (B) Hepatocyte numbers per visual field in liver sections from wild-type and SRC-3−/− mice were similar with or without TCPOBOP treatment (magnification, ×200). (C) Cumulative BrdU-positive hepatocytes in the livers of SRC-3−/− mice were reduced compared with wild-type mice at 72 h after TCPOBOP treatment (1500 -2000 hepatocyte nuclei per section were counted). Inserted figures showed the immunohistochemial staining of BrdU in liver sections (magnification, ×200). (D) The number of PCNA-positive hepatocyte was lower in SRC-3−/− mice than that in wild-type mice at 36 and 72 h after TCPOBOP treatment. Inserted figures showed the immunohistochemial staining of PCNA at 72 h after TCPOBOP treatment (magnification, ×200). (E) Expression of c-Myc in the livers of SRC-3−/− mice was reduced at 24, 36, and 72 h after TCPOBOP treatment compared with wild-type mice. (F) Expression of Foxm-1 in the livers of SRC-3−/− mice was reduced at 36 and 72 h after TCPOBOP treatment compared with wild-type mice. mRNA expression of c-Myc (E) and Foxm-1 (F) was assessed by real-time PCR. Data are the means + SD of five male mice per group. * p<0.05, ** p<0.01.
Because c-Myc, a downstream target of CAR, has been implicated in the process of hepatocyte hyperplasia in response to TCPOBOP treatment [20], we investigated whether SRC-3 regulates hepatocyte proliferation through modulating c-Myc expression. The expression of c-Myc mRNA in the livers of wild-type mice was significantly increased at 24, 36, and 72 h after TCPOBOP treatment, while it showed only a moderate increase in the livers of SRC-3−/− mice (Fig. 2E). Furthermore, we investigated the expression of Foxm-1, one of the direct target genes of c-Myc that functions as an important downstream effecter in TCPOBOP-CAR signaling pathway for the development of hyperplasia [20, 21]. As shown in Fig. 2F, Foxm-1 expression in the livers of wild-type mice was strongly induced at 36 and 72 h after TCPOBOP treatment, while deficiency of SRC-3 significantly attenuated this effect. These results suggest that SRC-3 is involved in TCPOBOP-induced hepatic hyperplastic process through regulating the expression of c-Myc and Foxm-1.
For comparison, we investigated TCPOBOP-induced hepatomegaly in SRC-1−/− and SRC-2−/− mice and found that both SRC-1−/− and SRC-2−/− mice showed reduced liver mass after TCPOBOP treatment compared to wild-type controls (Supplementary Fig. 3A). Further studies revealed that both SRC-1−/− and SRC-2−/− mice exhibited reduced hepatocyte hypertrophy as demonstrated by more hepatocyte counts per visual field in liver sections (Supplementary Fig. 3B), but normal hepatic hyperplasia as demonstrated by similar BrdU immunohistochemical staining (Supplementary Fig. 3C). Consistently, the expression of hepatic c-Myc and Foxm-1 was comparable between mutant mice deficient in SRC-1 or SRC-2 and their wild-type controls (Supplementary Fig. 3D). These results demonstrate that SRC-1 and SRC-2 play minor roles in TCPOBOP-induced hepatic hyperplasia.
In addition to identify the important role of SRC-3 in TCPOBOP-CAR mediated hepatic hyperplasia, we also investigated whether SRC-3 participates in the regulation of hepatocyte proliferation induced by partial hepatectomy (PH). As shown in Supplementary Fig. 4, at 40 h post-PH, SRC-3−/− mice showed significantly lower liver regenerative rate compared with wild-type mice as demonstrated by a reduced PCNA immunohistochemical staining as well as a reduced c-Myc and Foxm-1 expression. These results suggest that SRC-3 also plays an important role in PH-induced liver regeneration.
Knockout of SRC-3 in mice abrogates TCPOBOP-induced APAP hepatotoxicity
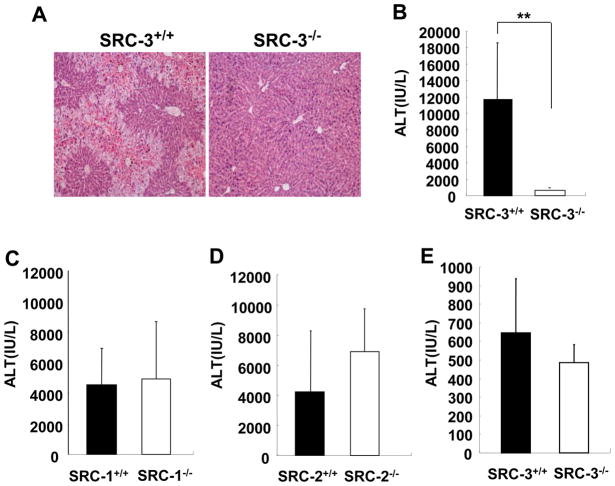
The above results indicate that SRC-3 may enhance the transactivation activity of CAR in vivo. To corroborate this notion, we further investigated the coactivating role of SRC-3 for CAR activation by means of TCPOBOP-induced APAP hepatotoxicity, a well-established in vivo model for studying CAR-regulated drug metabolism [22]. APAP is converted to a virulent form by CYP isoforms induced by CAR activator TCPOBOP to cause hepatic injury, whereas disruption of the CAR gene abrogates TCPOBOP-induced APAP hepatotoxicity [22]. Wild-type and SRC-3−/− mice were given a single i.p. injection of APAP (250 mg/kg) at 72 h after TCPOBOP treatment. The H&E-stained livers 24 h after APAP treatment showed that SRC-3 deficiency dramatically reduced the damaged area in the liver (Fig. 3A). Consistently, serum levels of ALT (an indicator of liver damage) were markedly lower in SRC-3−/− mice compared with wild-type mice (Fig. 3B). These data demonstrate that SRC-3 deficiency abrogates TCPOBOP-induced APAP hepatotoxicity and SRC-3 plays a critical role in CAR-mediated drug metabolism. Attenuation of TCPOBOP-induced APAP hepatotoxicity as demonstrated by elevated serum ALT levels was only observed in SRC-3−/− mice, but not in mutant mice deficient in SRC-1 or SRC-2, the other two closely related members of the SRC coactivator family (Fig. 3C, D). These data indicate that SRC-1 and SRC-2 play a minor role in CAR activation or their functions in CAR activation can be compensated by SRC-3 in the liver.
Figure 3. SRC-3 deficiency reduces TCPOBOP-mediated APAP hepatotoxicity.
(A, B) TCPOBOP-pretreated SRC-3−/− mice exhibited dramatically less severe liver injury after APAP (250 mg/kg) administration compared with wild-type mice. Acute liver injury was determined by H&E staining of sections of livers (A) and by measuring ALT levels in serum from both types of mice at 24 h after APAP administration (B) Data are the means + SD of five male mice per group. (C, D) TCPOBOP-pretreated mice deficient in SRC-1−/− (C) or SRC-2−/− mice (D) exhibited severe liver injury similar to wild-type controls after APAP (250 mg/kg) administration. Liver injury was reflected by measurement of ALT levels in serum. Data are the means + SD of four male mice per group. (E) Without TCPOBOP pre-treatment, wild-type and SRC-3−/− mice exhibited similar liver injury after APAP (600 mg/kg) administration. Data are the means + SD of four male mice per group. ** p<0.01.
To determine whether SRC-3 deficiency can reduce APAP hepatotoxicity when CAR is not activated by TCPOBOP, we i.p. injected wild-type and SRC-3−/− mice with different doses of APAP without TCPOBOP pre-treatment. A single i.p. challenge with low dose of APAP (250 mg/kg) did not cause obvious liver injury in both wild-type and SRC-3−/− mice (data not shown), but challenge with higher dose of APAP (600 mg/kg) resulted in comparable liver injury in both types of mice (Fig. 3E), demonstrating that wild-type and SRC-3−/− mice have similar sensitivity to APAP hepatotoxicity when CAR is not activated by TCPOBOP.
Knockout of SRC-3 in mice exacerbates zoxazolamine-induced paralysis
To determine the critical role of SRC-3 in CAR-mediated drug metabolism is not limited to APAP, we performed zoxazolamine-induced paralysis experiments in wild-type, SRC-3+/−, and SRC-3−/− mice. Zoxazolamine, a muscle relaxant that causes paralysis, can be detoxified by CAR-regulated hepatic CYP enzymes such as CYP1A2 [23], so mice can recover from zoxazolamine-induced paralysis after clearance of zoxazolamine. TCPOBOP pre-treatment further induce these CYP enzymes to accelerate the detoxification of zoxazolamine and eventually reduce the paralysis duration [17]. As shown in Table 1, under normal physiological condition, injection of zoxazolamine caused paralysis on all mice. Most wild-type mice (13/15) showed temporary paralysis, and then recovered. Only 2/15 of mice died. Interestingly, SRC-3+/− mice exhibited a longer paralysis period; 11/18 of these mice recovered from paralysis, while 7/18 of mice died. Strikingly, all SRC-3−/− mice died of paralysis. When mice were pretreated with TCPOBOP, none of the wild-type mice were paralyzed after zoxazolamine injection, but 12/15 of SRC-3+/− and 6/9 of SRC-3−/− mice were paralyzed. Among the paralyzed mice, 11/15 of SRC-3+/− and 5/9 of SRC-3−/− mice could be recovered, while one SRC-3+/− and one SRC-3−/− mouse died. As expected, the expression of hepatic SRC-3 was reduced in SRC-3+/− mice and absent in SRC-3−/− mice as measured by Western blotting (Supplementary Fig. 5). Therefore, wild-type, SRC-3+/−, and SRC-3−/− mice displayed SRC-3 dosage-dependent susceptibility to zoxazolamine-induced paralysis, further demonstrating that SRC-3 plays an important role in CAR-mediated hepatic drug metabolism.
Table 1.
Zoxazolamine paralysis test
| SRC-3+/+ | SRC-3+/− | SRC-3−/− | ||||
|---|---|---|---|---|---|---|
| Control | 8/15 | recovered < 8h | 1/18 | recovered < 8h | / | |
| 4/15 | recovered <12h | 6/18 | recovered <12h | / | ||
| 1/15 | recovered <24h | 4/18 | recovered <24h | / | ||
| 2/15 | died | 7/18 | died | 6/6 | died | |
| TCPOBOP | 18/18 | not paralyzed | 3/15 | not paralyzed | 3/9 | not paralyzed |
| / | 11/15 | recovered< 8h | 5/9 | recovered< 8h | ||
| / | 1/15 | died | 1/9 | died | ||
Mice were pretreated with a single dose of corn oil or TCPOBOP for 72 h, then mice were given a single i.p. injection of zoxazolamine, duration of paralysis was recorded after mice were able to right their bodies repeatedly.
Knockout of SRC-3 impairs CAR-mediated expression of drug metabolism-related genes in the liver
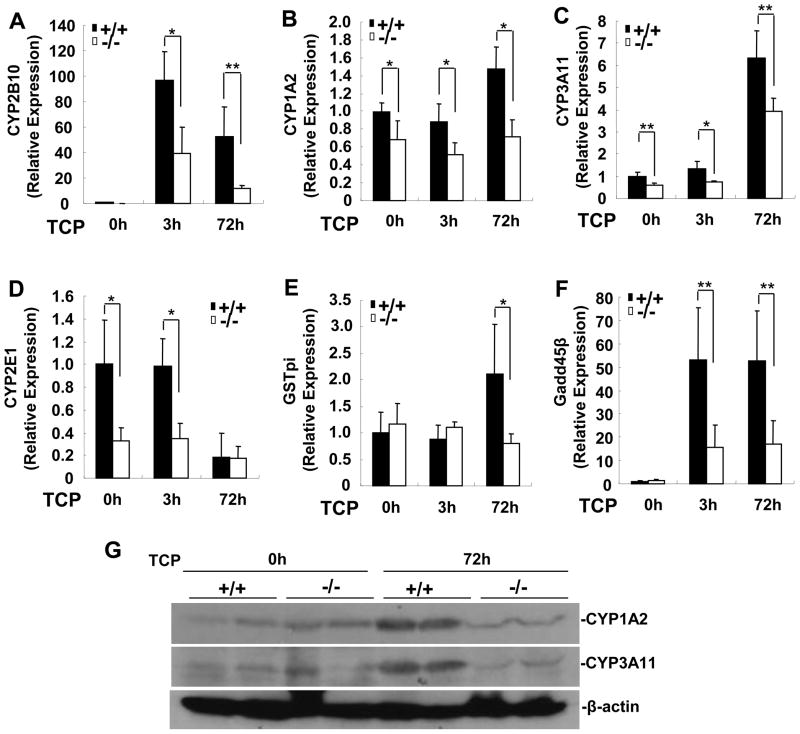
Since SRC-3-deficient mice displayed an impaired CAR-mediated hepatic drug metabolism, reduced expression of CAR target genes involved in drug metabolism in the liver may underlie these phenotypic changes. We chose to examine the expression of those genes at early (3 h) and late (72 h) stage of TCPOBOP treatment. As shown in Fig. 4A~C, the expression of CYP2B10, CYP1A2, and CYP3A11 was significantly lower in SRC-3−/− mice than that in wild-type mice in the absence or presence of TCPOBOP. The expression of CYP2E1 was lower in SRC-3−/− mice in the absence or at an early stage of TCPOBOP treatment, but reduced to similar levels in both types of mice at 72 h after TCPOBOP treatment (Fig. 4D). Although the expression of glutathione S-transferase pi (GSTP1) was similar in both types of mice in the absence or at an early stage of TCPOBOP treatment, it was increased significantly in wild-type mice but not in SRC-3−/− mice at 72 h after TCPOBOP treatment (Fig. 4E). The expression of Gadd45β, an immediate-early response gene induced by TCPOBOP [2, 24], was also significantly lower in SRC-3−/− mice than that in wild-type mice after TCPOBOP treatment (Fig. 4F). In addition, the expression of hepatic CAR mRNA in both types of mice was comparable (Supplementary Fig. 6), ruling out the possibility that the reduced expression of CAR targets in the livers of SRC-3−/− mice is due to a reduced expression of CAR. Consistent with mRNA results, we found that the protein levels of hepatic CYP1A2 and CYP3A11 in SRC-3−/− mice were significantly lower than those of wild-type mice at 72 h after TCPOBOP treatment (Fig. 4G). Altogether, these results demonstrate that disruption of SRC-3 impairs the expression of several CAR target genes, and suggest that SRC-3 is essential for CAR activation in the liver.
Figure 4. SRC-3 deficiency impairs the expression of CAR target genes related to drug-metabolism in the liver.
Wild-type and SRC-3−/− mice were treated with a single dose of corn oil or TCPOBOP for 3 h or 72 h. Total RNA were isolated from livers of both types of mice. (A-F) Gene expression was determined by real-time PCR. Data are the means + SD of five male mice per group. * p<0.05, ** p<0.01. (G) Protein expression of CYP1A2 and CYP3A11 in the livers of wild-type and SRC-3−/− mice.
At 72 h after TCPOBOP treatment, the expression of most CAR target genes involved in drug metabolism was similar between wild-type and SRC-1−/− or SRC-2−/− mice, except relatively lower expression of CYP3A11 in SRC-2−/− mice (Supplementary Fig. 7). These results consisted with the observations that mice deficient in SRC-1 or SRC-2 exhibited severe acetaminophen hepatotoxicity similar to wild-type controls.
Discussion
Nuclear receptor CAR plays important roles in modulating xenobiotic metabolism and hepatocellular proliferation by activating drug metabolism-related and cell proliferation-related gene expression, respectively. Binding of CAR to its NR response elements alone is insufficient to activate gene expression. CAR needs to recruit transcriptional coactivators to its NR response elements for chromatin remodeling and transcriptional initiation. Although the p160 coactivators in the SRC family are known to coactivate CAR, it is unclear which specific member is preferentially used in CAR-dependent transcriptional activation of gene expression. SRC-1, SRC-2, and SRC-3 proteins share 50~55% similarity [10, 11], thus, when overexpressed in transfected cells, all three SRC family members are capable of enhancing transcriptional activation mediated by CAR and other NRs such as androgen receptor (AR), estrogen receptor (ER), and FXR in cell-based reporter assays. However, several studies have also demonstrated the preferences of individual SRC coactivator usage by different NRs, such as AR has a preference for SRC-3 (Supplementary Fig. 8) [25]. In this study, SRC-3 consistently displayed the highest coactivator activity to CAR compared with SRC-1 and SRC-2 in cell-based assays, suggesting that SRC-3 may be a dominant coactivator in the SRC family to support CAR function. Therefore, we further addressed whether SRC-3 is also the predominant SRC coactivator for CAR-regulated hepatocyte proliferation and drug metabolism in the liver.
Gene targeting in mice is a powerful approach for studying gene function in vivo. Mutant mice deficient in SRC-1, SRC-2 or SRC-3 are viable; they are therefore useful for studying the gene function in the liver. Although SRC family members may compensate for each other, a predominant role of individual SRC family member can still be revealed by studying individual knockout mice. Indeed, Jeong et al. reported that the expression of a large number of genes was differentially altered between individual knockout mice of SRC family members, demonstrating that SRC-1, 2 and 3 exhibit a high degree of specific in vivo functions in the liver [12]. Furthermore, Chopra et al. reported that single SRC-2 knockout in mice resulted in a glycogenopathy resembling Von Gierke's disease, and demonstrated that SRC-2 served as a predominant SRC transcriptional coactivator for NR RORα to modulate Glucose-6-phosphtase expression in the liver [26].
We initiated our study to define the in vivo role of SRC family members in CAR activation by using SRC-1, SRC-2, or SRC-3 knockout mice. We found that knockout of SRC-3 in mice reduced TCPOBOP-induced hepatocyte hyperplasia, abrogated TCPOBOP-induced APAP hepatotoxicity, and exacerbated zoxazolamine-induced paralysis. These phenotypes highly resemble that of CAR-deficient mice [17, 22], indicating that SRC-3 plays an important role in CAR activation in the liver. In contrast, both SRC-1 and SRC-2 knockout mice exhibited typical TCPOBOP-induced hepatocyte hyperplasia and severe TCPOBOP-induced APAP hepatotoxicity similar to their wild-type controls, indicating that SRC-1 and SRC-2 are not required for CAR activation for promoting hepatocyte proliferation and drug metabolism. Collectively, our in vivo study demonstrates that SRC-3 is the predominant SRC coactivator for CAR-regulated hepatocyte proliferation and drug metabolism in the liver.
In this study, we found that SRC-3 directly interacted with CAR through S/T and HAT domains, but not receptor interaction domain (RID) (Supplementary Fig. 9), whereas SRC-1 and SRC-2 interact with CAR through their second LXXLL motif in RID [5]. Like SRC-1 and SRC-2, ASC-2 and PBP, two essential coactivators for CAR activation, also use their LXXLL motifs to bind to CAR. Since one CAR can only bind to a single LXXLL motif at once [27], SRC-1 and SRC-2 may compete with ASC-2 and PBP to bind to CAR and subsequently limit the maximal activation potentials of these coactivators; in contrast, SRC-3 and ASC-2 or PBP may simultaneously bind to CAR to allow them to exhibit the maximal coactivation potentials. This hypothesis is supported by the observation that SRC-3 but not SRC-1, cooperated with ASC-2 to enhance CAR transactivation (Supplementary Fig. 10). Therefore, the unique binding properties of SRC-3 to CAR may at least in part explain why SRC-3 exhibits the highest coactivating activity to CAR in vitro, and only SRC-3 is required for CAR-regulated hepatocyte proliferation and drug metabolism in vivo.
Our present study may have some clinical implications. Since SRC-3 is overexpressed in 68% of human hepatocellular carcinoma (HCC) and promotes HCC cell proliferation [13], and CAR plays a critical promoting role in HCC progression [3], SRC-3 overexpression in HCC may activate CAR, resulting in further promotion of HCC progression. In addition, the dose-dependent activation of CAR by SRC-3 may contribute to personal variations in drug sensitivity since the expression of hepatic SRC-3 may vary widely between different individuals. Further study is needed to verify these implications.
In conclusion, our study highlights the crucial role of SRC-3 in CAR-regulated gene expression and CAR-promoted hepatocyte proliferation and drug metabolism. Our findings suggest that modulation of SRC-3 coactivator activity may represent a future therapeutic strategy for CAR-related abnormalities.
Supplementary Material
Acknowledgments
We thank Victor Chun-Kong Yu (National University of Singapore) for critical reading of the manuscript, Hongwu Chen (University of California at Davis) for providing pSRC-3-RID-AAA plasmid, and Xiaoliang Wang for technical assistance.
Financial support:
This work was supported by grants from the Natural Science Foundation of Fujian Province of China (2008J0110 and 2010J06014), the Fundamental Research Funds for the Central Universities (2010121085), Research Fund for the Doctoral Program of Higher Education of China (20100121110004), the Science Planning Program of Fujian Province (2009J1010), and the R01 DK058242 grant to J.X. from National Institutes of Health, United States of America.
Abbreviations
- SRC
Steroid receptor coactivator
- NR
Nuclear receptor
- CAR
Constitutive androstane receptor
- TCPOBOP
1,4-bis-[2-(3,5-dichloropyridyloxy)] benzene
- ASC-2
Activating signal cointegrator 2
- FXR
Farnesoid X receptor
- PCNA
Proliferating cell nuclear antigen
- APAP
Acetaminophen
- ER
Estrogen receptor
- AR
Androgen receptor
- RID
Receptor interacting domain
Footnotes
Conflict of interest:
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stanley LA, Horsburgh BC, Ross J, Scheer N, Wolf CR. PXR and CAR: nuclear receptors which play a pivotal role in drug disposition and chemical toxicity. Drug Metab Rev. 2006;38:515–597. doi: 10.1080/03602530600786232. [DOI] [PubMed] [Google Scholar]
- 2.Locker J, Tian J, Carver R, Concas D, Cossu C, Ledda-Columbano GM, et al. A common set of immediate-early response genes in liver regeneration and hyperplasia. Hepatology. 2003;38:314–325. doi: 10.1053/jhep.2003.50299. [DOI] [PubMed] [Google Scholar]
- 3.Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, et al. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol. 2005;19:1646–1653. doi: 10.1210/me.2004-0520. [DOI] [PubMed] [Google Scholar]
- 4.Waxman DJ, Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem J. 1992;281 ( Pt 3):577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol. 2000;20:2951–2958. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith CL, O'Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 8.Choi E, Lee S, Yeom SY, Kim GH, Lee JW, Kim SW. Characterization of activating signal cointegrator-2 as a novel transcriptional coactivator of the xenobiotic nuclear receptor constitutive androstane receptor. Mol Endocrinol. 2005;19:1711–1719. doi: 10.1210/me.2005-0066. [DOI] [PubMed] [Google Scholar]
- 9.Jia Y, Guo GL, Surapureddi S, Sarkar J, Qi C, Guo D, et al. Transcription coactivator peroxisome proliferator-activated receptor-binding protein/mediator 1 deficiency abrogates acetaminophen hepatotoxicity. Proc Natl Acad Sci U S A. 2005;102:12531–12536. doi: 10.1073/pnas.0506000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 2003;17:1681–1692. doi: 10.1210/me.2003-0116. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Wu RC, O'Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong JW, Kwak I, Lee KY, White LD, Wang XP, Brunicardi FC, et al. The genomic analysis of the impact of steroid receptor coactivators ablation on hepatic metabolism. Mol Endocrinol. 2006;20:1138–1152. doi: 10.1210/me.2005-0407. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Chen Q, Li W, Su X, Chen T, Liu Y, et al. Overexpression of transcriptional coactivator AIB1 promotes hepatocellular carcinoma progression by enhancing cell proliferation and invasiveness. Oncogene. 2010;29:3386–3397. doi: 10.1038/onc.2010.90. [DOI] [PubMed] [Google Scholar]
- 14.Xia J, Liao L, Sarkar J, Matsumoto K, Reddy JK, Xu J, et al. Redundant enhancement of mouse constitutive androstane receptor transactivation by p160 coactivator family members. Arch Biochem Biophys. 2007;468:49–57. doi: 10.1016/j.abb.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright E, Vincent J, Fernandez EJ. Thermodynamic characterization of the interaction between CAR-RXR and SRC-1 peptide by isothermal titration calorimetry. Biochemistry. 2007;46:862–870. doi: 10.1021/bi061627i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min G, Kemper JK, Kemper B. Glucocorticoid receptor-interacting protein 1 mediates ligand-independent nuclear translocation and activation of constitutive androstane receptor in vivo. J Biol Chem. 2002;277:26356–26363. doi: 10.1074/jbc.M200051200. [DOI] [PubMed] [Google Scholar]
- 17.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 18.Yu C, Wang F, Jin C, Wu X, Chan WK, McKeehan WL. Increased carbon tetrachloride-induced liver injury and fibrosis in FGFR4-deficient mice. Am J Pathol. 2002;161:2003–2010. doi: 10.1016/S0002-9440(10)64478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Columbano A, Ledda-Columbano GM, Pibiri M, Piga R, Shinozuka H, De Luca V, et al. Increased expression of c-fos, c-jun and LRF-1 is not required for in vivo priming of hepatocytes by the mitogen TCPOBOP. Oncogene. 1997;14:857–863. doi: 10.1038/sj.onc.1200891. [DOI] [PubMed] [Google Scholar]
- 20.Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, et al. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology. 2008;48:1302–1311. doi: 10.1002/hep.22475. [DOI] [PubMed] [Google Scholar]
- 21.Ledda-Columbano GM, Pibiri M, Cossu C, Molotzu F, Locker J, Columbano A. Aging does not reduce the hepatocyte proliferative response of mice to the primary mitogen TCPOBOP. Hepatology. 2004;40:981–988. doi: 10.1002/hep.20403. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298:422–424. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- 23.Liang HC, Li H, McKinnon RA, Duffy JJ, Potter SS, Puga A, et al. Cyp1a2(−/−) null mutant mice develop normally but show deficient drug metabolism. Proc Natl Acad Sci U S A. 1996;93:1671–1676. doi: 10.1073/pnas.93.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Columbano A, Ledda-Columbano GM, Pibiri M, Cossu C, Menegazzi M, Moore DD, et al. Gadd45beta is induced through a CAR-dependent, TNF-independent pathway in murine liver hyperplasia. Hepatology. 2005;42:1118–1126. doi: 10.1002/hep.20883. [DOI] [PubMed] [Google Scholar]
- 25.Zhou XE, Suino-Powell KM, Li J, He Y, Mackeigan JP, Melcher K, et al. Identification of SRC3/AIB1 as a preferred coactivator for hormone-activated androgen receptor. J Biol Chem. 2010;285:9161–9171. doi: 10.1074/jbc.M109.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, et al. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McInerney EM, Rose DW, Flynn SE, Westin S, Mullen TM, Krones A, et al. Determinants of coactivator LXXLL motif specificity in nuclear receptor transcriptional activation. Genes Dev. 1998;12:3357–3368. doi: 10.1101/gad.12.21.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.