Abstract
The exact biological role of the cytokine Tumor Necrosis Factor (TNF) in the central nervous system (CNS) is not well understood; but overproduction of TNF by activated microglia has been implicated in neuronal death, suggesting that TNF inhibition in the CNS may be a viable neuroprotective strategy. We investigated the role of TNF signaling in regulation of microglia effector functions using molecular, cellular, and functional analyses of postnatal and adult microglia populations in the CNS. No differences were found by flow cytometric analyses in the basal activation state between TNF-null and wild type mice. Although TNF-null microglia displayed an atypical morphology with cytoplasmic vacuoles in response to stimulation with lipopolysaccharide (LPS), the phagocytic response of TNF-null microglia to E.coli particles in vitro was normal and there were no signs of enhanced caspase 3 activation or apoptosis. Functionally, conditioned media from LPS-stimulated TNF-null microglia was found to have significantly reduced levels of IL-10, IL-6, IL-1β, IL-12 and CXCL1 relative to wild type microglia and exerted no cytotoxic effects on neurally differentiated dopaminergic MN9D cells. In contrast, incubation of wild type microglia with TNF inhibitors selectively depleted the levels of soluble TNF and its cytotoxicity on MN9D cells. To distinguish whether reduced cytotoxicity by LPS-activated TNF-null microglia could be attributed to deficient autocrine TNF signaling, we employed primary microglia deficient in one or both TNF receptors (TNFR1 and TNFR2) in co-culture with MN9D cells and found that neither receptor is required to elicit LPS-evoked TNF production and cytotoxicity on dopaminergic cells.
INTRODUCTION
Brain microglia are responsible for immune surveillance in the CNS. Tumor necrosis factor (TNF) is a Th1-class cytokine produced by activated microglia and macrophages. TNF is involved in regulation of the immune system, protection from bacterial infections, cell growth, and viral replication and is a major player in many autoimmune and neurodegenerative diseases (Aggarwal 2000a). TNF-null mice are viable and fertile, but histological analysis revealed that their splenic architecture is severely disrupted (Pasparakis et al. 1996). In addition, TNF-null mice fail to develop monocyte-derived follicular dendritic cell clusters and germinal centers which compromises their immune system and ability to fight systemic Listeria monocytogenes bacterial infections (Aggarwal 2000a). Because brain microglia derive from primitive myeloid progenitors (Ginhoux et al. 2010) and are ontogenically distinct from peripheral monocytes and macrophages, the primary goal of our study was to determine whether TNF is also important for development and function of brain microglia, the monocyte-derived macrophage population responsible for the innate immune response in the brain. Microglia make up about 10–20% of the total glial cell population (Carson et al. 2006) and are recruited to sites of injury or infection where they engulf invading pathogens and extracellular debris by phagocytosis (Ransohoff and Perry 2009). Microglia secrete a number of inflammatory cytokines including TNF, interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) in response to inflammatory stimuli.
In recent years, chronic neuroinflammation (sustained microglial activation and overproduction of pro-inflammatory cytokines such as TNF) has been implicated in the pathophysiology of several neurodegenerative diseases including Parkinson’s disease (PD), Alzheimer’s disease (AD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD) (Frank-Cannon et al. 2009; Whitton 2007). Studies with mice lacking TNF or TNF receptors aimed at determining whether TNF was required for the death of dopaminergic (DA) neurons in the midbrain substantia nigra induced by 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP), the mitochondrial complex I inhibitor commonly used to induce experimental parkinsonism in rodents, yielded conflicting results likely due to differences in dosing regimens (Ferger et al. 2004; Rousselet et al. 2002; Sriram et al. 2002; Sriram et al. 2006b). Importantly, follow-up studies suggested differences in microglia activation between adult TNF-null mice and wild type mice may have influenced the neuroinflammatory and neurodegenerative responses to MPTP (Sriram et al. 2006a). In agreement with genetic studies demonstrating reduced sensitivity to MPTP toxicity in mice lacking functional TNF signaling (Ferger et al. 2004; Sriram et al. 2002; Sriram et al. 2006b), chronic in vivo inhibition of soluble TNF signaling with dominant negative TNF (DN-TNF) inhibitor proteins significantly attenuated neurotoxin- or endotoxin-induced DA neuron death and microglial activation (McCoy et al. 2006; McCoy et al. 2008), directly implicating soluble TNF in the progressive loss of nigral DA neurons and pathophysiology of PD. Given the potential contribution of microglial activation to neurodegenerative diseases and the suggestion that anti-TNF therapies in the CNS may exert neuroprotective effects on vulnerable neuronal populations, a secondary goal of these studies was to determine the role of TNF in regulating brain microglia effector functions as a way to gauge the potential for collateral damage of anti-TNF therapies on microglia function. Our findings directly establish the importance of TNF in a select subset of microglia effector functions and have several important implications for studies involving TNF-null mice and anti-TNF therapies.
METHODS
Animals
TNF-null (B6;129S6-Tnftm1Gkl/J; stock number 003008); TNFR1-null (C57BL/6-Tnfrsf1atm1Imx/J; stock number 003242); TNFR2-null (B6.129S2-Tnfrsf1btm1Mwm/J; stock number 002620); and TNFR1/R2-null (B6,129S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J; stock number 003243) mice along with non-transgenic background strains as controls were obtained from Jackson Labs (Bar Harbor, ME). Experimental procedures involving the use of animals or animal tissue were performed in accordance with the NIH Guidelines for Animal Care and Use and approved by the Institutional Animal Care and Use Committee at The University of Texas Southwestern Medical Center at Dallas and Emory University School of Medicine. Animals were housed in a climate-controlled facility with a 12-hour light/dark cycle.
Primary Microglia
Postnatal Mouse Microglia Harvesting and Culture
For immunocytochemistry, phagocytosis, and target effector assays microglia were isolated from postnatal day 3–5 (P3–P5) TNF-null or wild type C57/Bl6 mouse pups according to published protocols (Lee et al. 2008) with minor modifications. Briefly, whole brains were isolated, minced, and placed in ice-cold dissociation media containing sterile filtered DNase1 (1µL/mL, Invitrogen, Carlsbad, CA), Dispase II (1.2 U/mL, Roche, Indianapolis, IN), and Papain (1 mg/mL, Sigma-Aldrich, St. Louis, MO) dissolved in DMEM/F12 (Invitrogen). Cells were dissociated for 30 minutes at 37°C with agitation every 10 minutes. Following mechanical and chemical dissociation, the population of mixed glial cells from approximately 4–6 pups per genotype was filtered through a 40 µm-pore filter (BD Falcon) and plated on T75 flasks in DMEM/F12 supplemented with 20 % heat-inactivated fetal bovine serum (Sigma), 1 % penicillin/streptomycin (Sigma), and 1% L-glutamine (Sigma). Mixed glial cultures were maintained in culture in a humidified incubator at 37°C and 5 % CO2 for 14–18 days and media was replenished every 3–4 days. Once cultures reached confluence, primary microglial cells were isolated from the astroglial cell bed by mechanical agitation on an orbital shaker (150 rpm) for 1 hour at 37°C. Following isolation, cells were plated in DMEM/F12 supplemented with 10 % heat-inactivated fetal bovine serum (Sigma) supplemented with 1 % penicillin/streptomycin, and 1 % L-glutamine a density of 50,000 cells/well in a 24-well plate for multiplexed immunoassay experiments, immunocytochemical analysis and target effector assays or plated at a density of 100,000 cells/well in a 96-well plate for phagocytosis experiments. For flow cytometry, primary microglia were isolated from postnatal day 5 (P5) pups by MACS® neural dissociation and OctoMACS® CD11b magnetic bead separation according to the manufacturer’s protocol (Miltenyi Biotech). For flow cytometry, isolated microglia cells were labeled with fluorescently conjugated anti-F4/80 and anti-CD68 antibodies, fixed with 4 % paraformaldehyde in PBS, and sorted according to published protocols (Lee et al. 2008).
Adult Mouse Microglia Harvesting and Culture
Adult (4–6 month old) mice were sacrificed with an overdose of anesthetic and perfused with ice-cold glucose-containing buffered saline supplemented with 2 units/mL of heparin. The brain was aseptically removed and carefully freed from the meninges. Brains were discarded when not appropriately exsanguinated. Each dissected brain was placed in 3 ml of ice-cold DMEM/F12 and finely minced with a scalpel then mixed with 3 mL of 2X enzymatic dissociation medium containing sterile filtered DNase1 (1µL/mL, Invitrogen, Carlsbad, CA), Dispase II (1.2 U/mL, Roche, Indianapolis, IN), and Papain (1 mg/mL, Sigma-Aldrich, St. Louis, MO) dissolved in DMEM/F12 (Invitrogen). The minced brain tissue was incubated at 37°C with continuous agitation. After a 30 min incubation, tissue was transferred to a 50 mL-conical tube and the enzymatic reaction was quenched by the addition of 5 mL of 20 % heat-inactivated fetal bovine serum (FBS) in Hanks balanced salt solution (HBSS) (Invitrogen). After centrifugation at 200 × g for 10 min, cells were pipetted up and down with fire-polished Pasteur pipettes of decreasing diameter.
After rinsing with 1X HBSS, freshly isolated adult microglial cells were prepared for flow cytometry analysis. Cells from each brain were resuspended in 4 ml of 70 % isotonic Percoll (Sigma, Saint Louis, MO) and centrifuged for 30 min at 500 × g on a 30:37:70 (4 mL of each in 15-mL conical tube) Percoll gradient then 2 mL of HBSS was layerd on the top of the gradient. Microglia were obtained from the 37:70 % interface, washed and resuspended in DMEM/F12 media supplemented with 20 % heat-inactivated fetal bovine serum (Sigma), 1 % penicillin/streptomycin (Sigma), and 1 % L-glutamine (Sigma). Isolated adult microglia cells (the approximate yield was 2–3 × 105 cells from one adult mouse) were labeled with CD68-FITC antibodies (Serotec) then fixed with 4 % paraformaldehyde in PBS.
To maximize their viability in culture for studies involving LPS-evoked inflammatory factor production, adult microglia were dissociated as described in (Moussaud and Draheim 2010) then placed in long-term glial cultures. Briefly, the cells were resuspended in 20 mL of 20 % isotonic Percoll (Sigma, Saint Louis, MO) and overlayed with 20 mL of 1X HBSS. A mixed glial cell population containing microglia and astrocytes was obtained by centrifugation for 30 min at 500 × g with slow acceleration and no brake. The material at the interphase which contained myelin and cell debris was discarded, the mixed glial cell population in the pellet was washed twice in HBSS and suspended in DMEM/F12 supplemented with 20 % heat-inactivated fetal bovine serum (Sigma), 1% penicillin/streptomycin (Sigma), 1% L-glutamine (Sigma) and recombinant mouse (rm) GM-CSF (0.5 ng/mL) as described in (Moussaud and Draheim 2010). The cell suspension from one mouse brain was plated in a T75 flask coated with poly-D-lysine and the medium was replenished twice a week and maintained in culture for 14–16 days. Adult microglia that detached from the feeder cell layer with mild mechanical agitation were collected from the supernatant fraction. For multiplexed immunoassay experiments to measure inflammatory factor production, cells were plated at a density of 3,000 cells per well in 96-well plates and put in rmGM-CSF-free medium for 3 days before LPS stimulation. Adult microglia purity was found to be >98 % as measured by CD68 and GFAP immunocytochemistry.
Flow Cytometry
Primary microglial cells were isolated from postnatal day 3 (P3) wild type or TNF-null mice using the MACS neural dissocation kit followed by CD11b magnetic bead separation as per the manufacturer’s instructions (Miltenyi Biotech). Immediately following isolation, microglial cells were immunolabeled with anti-F4/80-TxRed and anti-CD68-FITC (Serotec) for 15 min on ice in the dark. Cells were washed 3 times with PBS containing 0.01 % NaN3, 0.05 % BSA, and fixed with 4 % PFA for 10 min on ice in the dark. TxRed and FITC-conjugated monoclonal antibodies (mAbs) with irrelevant specificity were used as negative controls. Cells were resuspended in wash buffer for flow cytometric analysis within 48 hours after fixation. A total of 104 cells with light scatter characteristics of cells of each sample were analyzed using a FACSCalibur instrument (BD Biosciences).
Alamar Blue Proliferation Assay
Cell viability and proliferation of primary microglia was measured using the Alamar Blue reagent (Invitrogen). Cells were seeded at a density of 2000 cells/well in a 96-well plate. Alamar Blue was added as per the manufacturer instructions 2 hr before the first time-point; absorbance was read at 570 nm and 595 nm. Plates were returned to a humidified incubator at 37°C and 5 % CO2 between readings.
Fluorescence Immunocytochemistry
Fluorescence immunocytochemistry was performed as previously described (McCoy et al. 2006). Anti-CD45 (Serotec) was used at 1:250 and the nuclear counterstain bisbenzamide or Hoechst 33258 (Invitrogen) at 1:20,000. Appropriate Alexa-conjugated secondary antibodies (Invitrogen) were used at a dilution of 1:1000. All digital images were acquired on an Olympus BX61 fluorescence microscope with a CoolSnap CCD ES monochromatic camera and analyzed with Metamorph software.
Quantification of Primary Microglia in vitro
Primary microglia cells from TNF-null or C57BL/6 mice were plated into 96-well black-wall Falcon plates (BD Biosciences) at the density of 8,000 cells/well in plating media. Twenty-four hours after seeding, cells in one plate were fixed with 4% paraformaldehyde (t = 0 hours). Cells in another two plates were fixed after treatment with vehicle or 1ug/ml of LPS for 24h (t = 24 hours) and 48h (t = 48 hours). Fixed cells were stained with 0.5ug/mL Hoechst 33342 (Invitrogen). Images were captured using the IMAGEXPRESS 5000A automated cellular imaging and analysis system in the Chemical Biology Discovery Center of Emory University. Cell counts and statistics were performed with MetaXpress software.
Phagocytosis Assay
Primary microglia were isolated and plated at a density of 50,000 cells/well in 96-well plates and allowed to adhere for 8 hours. Upon adherence, cells were treated overnight with low LPS (10 ng/mL) or high LPS (1 µg/mL). Phagocytic activity of fluorescent E. coli bioparticles was measured using a Vybrant Phagocytosis Assay kit (Invitrogen).
Multiplexed Immunoassays
Conditioned media was collected and analyzed for inflammatory factor production on an MSD 7-spot pro-inflammatory immunoassay per the manufacturer’s instructions (Meso Scale Discovery). For measurements in primary microglia, cells from WT or TNF-null mice were plated at a density of 50,000 cells/well in a 24-well plate and treated for 24 hours as indicated (n = 3 for each condition) for each of three independent experiments. Because primary microglia do not proliferate under these in vitro conditions, the number of microglia present at the beginning of the assay was the same as the number at the end of the assay and did not differ between genotypes, as confirmed by Alamar Blue proliferation assays measured for 3 consecutive days in vitro (Figure 2). Values shown are mean + S.E.M. representative of three independent experiments.
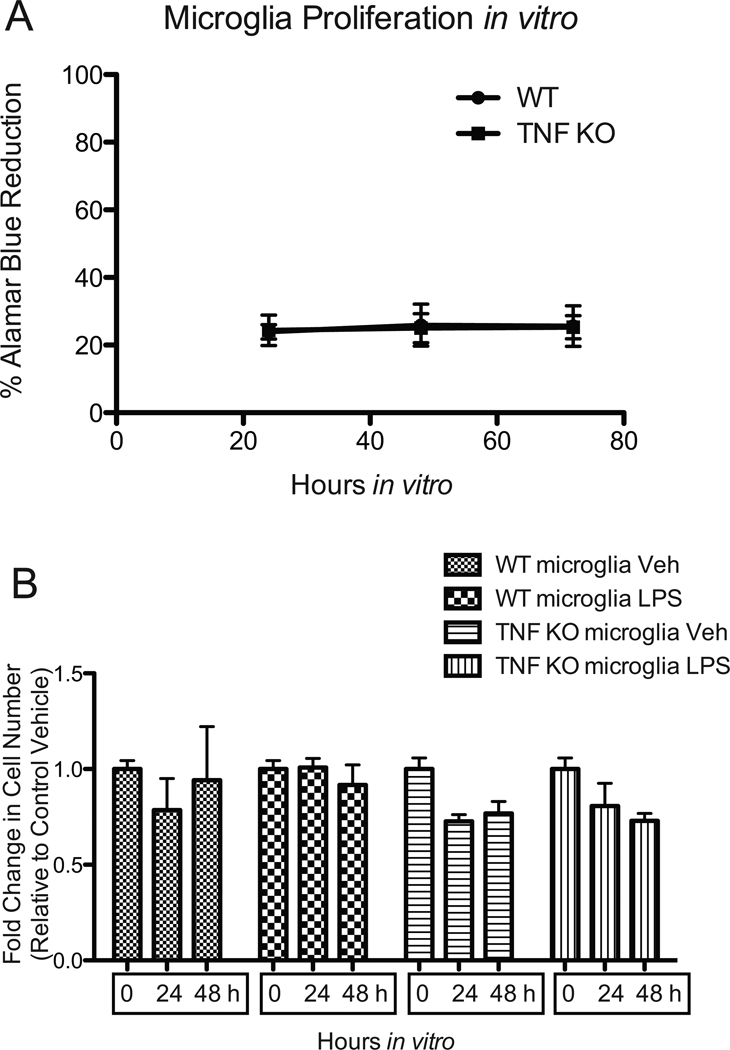
Figure 2. The proliferative potential of primary microglia in vitro is limited and is not regulated by TNF.
(A) Proliferation of cells in culture was measured by Alamar Blue assay as per the manufacturer’s instructions. (B) Primary microglia cells from TNF-null or WT C57BL/6 mice were plated into 96-well black-wall Falcon plates (BD Biosciences) at the density of 8,000 cells/well in growth medium. Twenty-four hours after seeding, cells in one plate were fixed with 4% paraformaldehyde for the 0 hour time point. Cells in two plates were treated with vehicle or 1ug/mL of LPS for 24 hours or 48 hours and then fixed with 4% paraformaldehyde. Fixed cells were incubated with 0.5ug/mL Hoechst 33342 to counterstain all nuclei. Images were captured on an IMAGEXPRESS 5000A automated cellular imaging system and analyzed with MetaXpress software.
Target-Effector Assays (MTS and LDH Cytotoxicity Assays)
The dopaminergic neuron-like cell line derived from mouse ventral mesencephalon MN9D (Choi et al. 1991) was provided by Dr. Michael Zigmond at the University of Pittsburgh. Cells were maintained in DMEM supplemented with 10 % Fetal Clone III (Hyclone) and 1% penicillin/streptomycin (Sigma). For neural differentiation, MN9D cells were plated at a density of 7,000 cells/well in 96-well plates and allowed to adhere overnight. Twenty-four hours later, MN9D cells were terminally differentiated as per published protocols (Lee et al. 2008) in serum-free DMEM supplemented with 5 mM valproic acid (Sigma) and N2 supplement (Invitrogen) for 72 hours. In parallel with the MN9D cell differentiation period, primary microglia were isolated and plated at a density of 50,000 cells/well in 24-well plates and treated with PBS, low LPS (10 ng/mL) or high LPS (1 ug/mL) for 24 hours. Following treatment, microglia conditioned media were harvested and clarified of cell debris by centrifugation. Microglial cells were fixed in 4 % paraformaldehyde in PBS (pH 7.4). For target-effector assays or LDH cytotoxicity assay, MN9D cell media was removed and replaced with primary microglia conditioned media (CM) supplemented with 5 mM valproic acid (to maintain the MN9D differentiated state) for 48 hours. MN9D metabolic activity was assayed using the MTS reagent in the CellTiter 96 Aqueous Assay reagent (Promega, Madison, WI). MN9D cytotoxicity was assayed using an LDH release assay (Clontech Laboratories Inc., Mountain View, CA) as per manufacturer’s instructions. MTS or LDH reactions were measured at a wavelength of 492 nm on an absorbance plate reader (Thermo Lab Systems Multiskan Ascent) and the values plotted and analyzed with GraphPad software. Values shown are mean ± S.E.M. representative of three independent experiments.
Microglia-Dopaminergic Neuroblastoma Cell Co-culture and TH+ Cell Survival
MN9D cells were plated at a density of 7,500 cells/well in 96-well black-wall Falcon plates (BD Biosciences) coated with PLL (Sigma) and allowed to adhere. Twenty-four hours later, MN9D cells were terminally differentiated as per published protocols (Lee et al. 2008) in serum-free DMEM supplemented with 5 mM valproic acid (Sigma) and N2 supplement (Invitrogen) for 72 hours. Prior to experiments, media were replaced with fresh differentiation media. Differentiated MN9D cells were pretreated with 200 ng/mL of etanercept (or saline vehicle) for 1 hour prior to addition of primary microglia to the cultures (at a density of 5,000 cells/well). To activate microglia, co-cultures were treated with 1 ug/mL LPS (Sigma) or saline vehicle for an additional 24 hours. To measure the effects of activated microglia on survival of neurally differentiated dopaminergic MN9D cells, co-cultures were fixed with 4% paraformaldehyde then stained with anti-TH primary antibody (Chemicon) and Alexa fluor -594 conjugated secondary antibody (Invitrogen). Fluorescence intensity was quantified using a Fluostar Omega plate reader (BMG labtech) at excitation wavelength 584 nm and emission 620 nm. The data were normalized to vehicle control.
Brain Tissue
Quantitative Polymerase Chain Reaction
Real-time quantitative polymerase chain reaction (QPCR) was performed according to previously published protocols (Lee et al. 2007, Frank-Cannon et al. 2007, Kurrasch et al., 2004). Total RNA was isolated from freshly-dissected brain tissue and treated with DNAse I (Invitrogen, CA); 1 ug of RNA was reverse transcribed into cDNA using Superscript II RNAse H-reverse transcriptase (Invitrogen, CA). QPCR was performed on an ABI Prism 7000 Detection System (Applied Biosystems). All reactions were performed in a 384-well format with 50 ng cDNA, 10 uL SYBR green PCR mastermix, and 150 nM each forward and reverse oligonucleotide primers. Oligonucleotide primers for Tumor Necrosis Factor Receptor 1 (TNFR1), TNF Receptor 2 (TNFR2), MAC1, Macrosialin (CD68), and cyclophilin (CPH2), were obtained from Integrated DNA Technologies (Coralville, IA). Primer sequences are available on request. All reactions were performed in triplicate and mRNA levels for the mouse gene of interest were normalized to the housekeeping gene CPH2.
Tissue Processing for Immunohistochemistry
Adult (6–8 month old) WT and TNF-null animals were deeply anesthetized with Euthasol (Butler, Dublin, OH) (100 mg in 500 µL sterile saline) and transcardially perfused with 250 mL of heparinized (1mL/L) phosphate buffered saline (PBS) pH 7.4 followed by 500 mL of 4 % paraformaldehyde (PFA) in PBS pH 7.4. Brains were post-fixed for 24 hours in 4 % PFA and then dissected out and placed into a 20 % sucrose solution in PBS pH 7.4 for 26–28 hours. Brains were cryosectioned coronally on a Leica1650 cryostat (cut thickness: 30 µM); sections were collected serially throughout the brain into a tissue collection solution (Phosphate buffered saline with 0.01 % Sodium Azide) and stored at 4 °C until further analysis.
Fluorescence Immunohistochemistry
Free-floating coronal brain sections sections were double labeled with anti-Iba-1 diluted 1:10,000 (Wako) and anti-CD68 diluted 1:1000 (Serotec) overnight at room temperature. Following an overnight incubation with primary antibody, endogenous peroxidases were quenched with 1 % hydrogen peroxide solution in PBS pH 7.4 for 30 minutes. Sections were incubated at room temperature for 4 hours in appropriate biotin-conjugated secondary antibodies diluted 1:200 (Jackson Labs). For fluorescent signal amplification, sections were treated with the Vector Elite avidin-biotin complex according to manufacturer’s instructions (Vector Laboratories) and incubated with fluorescently-conjugated TSA diluted 1:50 in TSA buffer (PerkinElmer Life Sciences Boston, MA) for 12 minutes. Sections were then mounted onto glass slides and coverslipped using BioMeda Gel-Mount. Images were captured with an Olympus DP70 digital camera and analyzed with Olympus DP Software.
Quantification of Microglia in Adult Brain Sections
Quantification of Iba-1 and CD68-positive microglia was performed on images captured under 20× objective lens on a Nikon 90i fluorescence microscope using thresholding analysis on Nikon Elements 5 software. Values represent the mean ± S.E.M. of CD68 or Iba-1-positive microglia per field calculated from six separate brain sections (5 random fields per section) within SNpc or a region of equal size in the entorhinal cortex from 3 animals per genotype.
Statistics
Values shown are means ± S.E.M. Statistical analyses were performed as indicated in the figure legend for each experiments using Graph Pad Prism Software (La Jolla, CA).
RESULTS
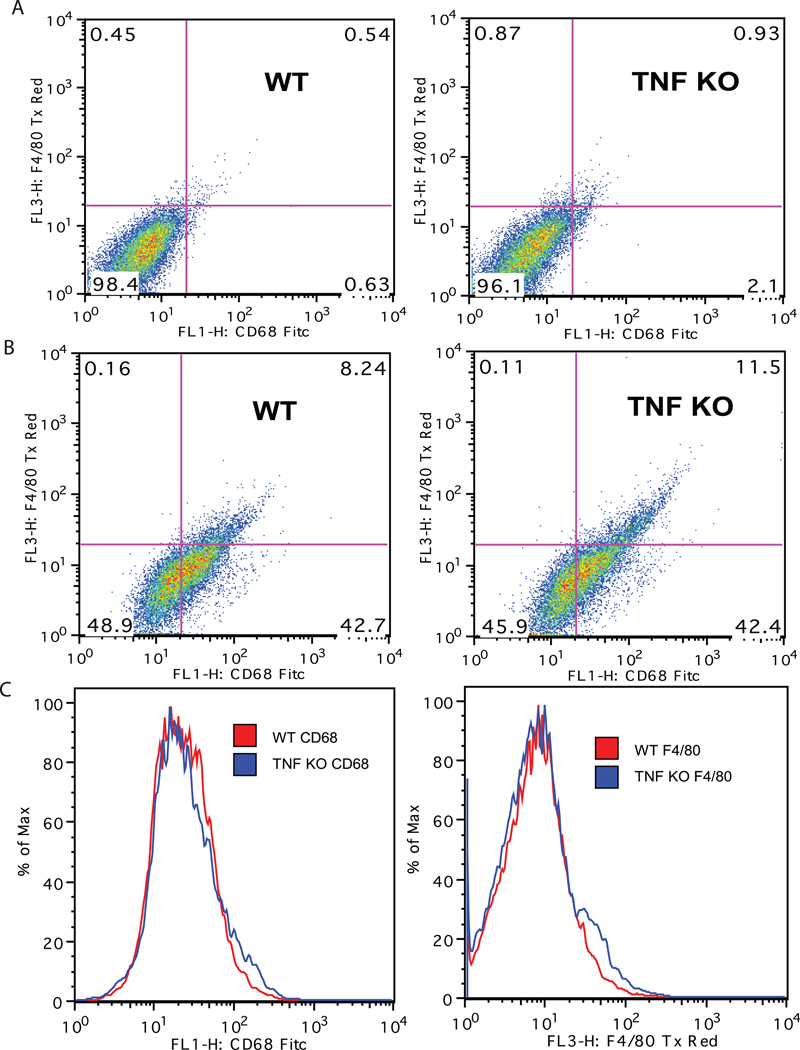
Because brain microglia are derived from primitive myeloid progenitors (Ginhoux et al. 2010) and are ontogenically distinct from peripheral monocytes and macrophages, it is of importance to determine whether TNF is also important for development and function of brain microglia. We measured the surface expression of the myeloid marker macrosialin (CD68) and the activation marker F4/80 on freshly isolated microglia from the postnatal brains of wild type or TNF-null mice by FACS analysis. There were no significant differences in the mean fluorescence intensity of CD68+ or F4/80+ microglia between genotypes (Figure 1). Similar results were obtained with primary peritoneal macrophages from young adult wild type or TNF-null mice (data not shown). Next, we sought to determine whether TNF signaling was critical for growth and expansion of microglia. To address this question in vitro, we isolated primary microglia from postnatal wild type or TNF-null mouse pups and plated them in culture at equal densities and monitored cell proliferation using Alamar Blue reduction for 72 hrs. Also, we performed cell counts of primary microglia treated with saline or LPS for 48 hours to measure LPS-induced changes in microglia number in either genotype (Figure 2). Using two different approaches to measure the proliferative potential of microglia in vitro, we found that primary microglia from both wild type and TNF-null mice did not increase in number under our culture conditions nor displayed robust proliferative responses by LPS (Figure 2).
Figure 1. TNF signaling is not required during development for expression of CD68 and F4/80 by brain microglia.
Primary microglia were isolated from postnatal day 3–5 pups by MACS® neural dissociation and positively selected using CD11b magnetic bead separation (Miltenyi Biotec). Isolated microglia cells were double-labeled with fluorescently conjugated F4/80-Texas Red and CD68-FITC antibodies, fixed and sorted by flow cytometry. Cells stained with non-immune IgG-Texas Red and IgG-FITC antibodies as negative control (A); cells double-labeled with F4/80-Texas Red and CD68-FITC (B); histogram plots for single-labeled populations (C). FACs analysis of the live cell population revealed no difference in the mean fluorescence intensity between genotypes.
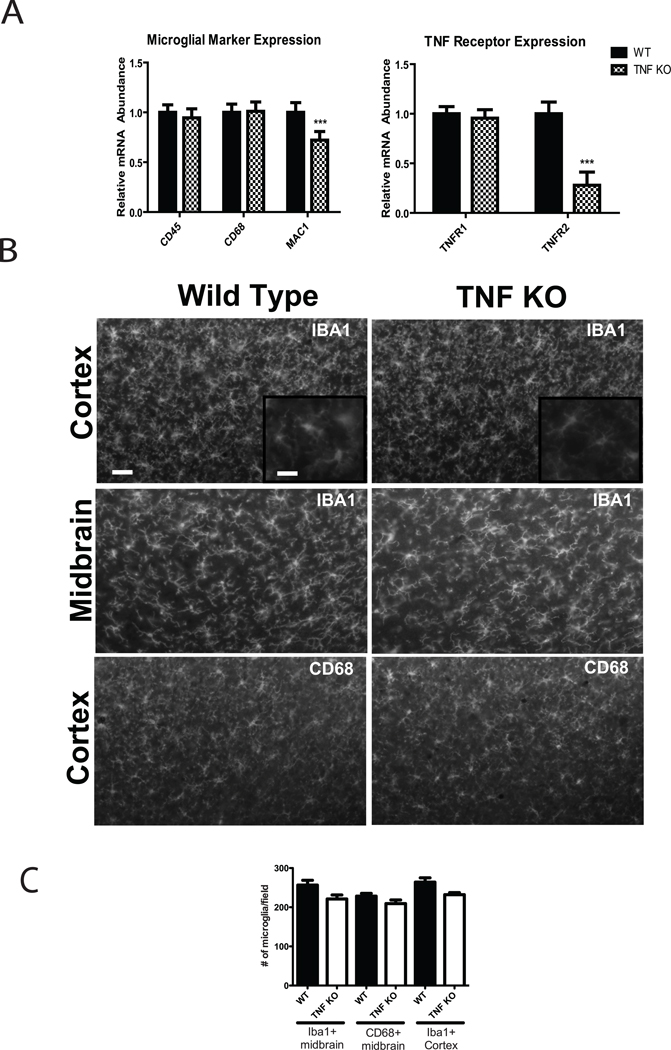
To confirm and extend the results obtained on cell surface markers in postnatal microglia and determine whether TNF signaling is critical for expansion of microglia in vivo, we performed QPCR to measure the mRNA levels of microglia cell surface markers (CD68, CD45 and MAC-1) and TNF receptors (TNFR1 and TNFR2) from whole brain homogenates. We also quantified the number of microglia on the brain sections from wild type or TNF-null adult mice by immunohistochemistry analysis using anti-CD68 and Iba-1 antibodies… In agreement with results from flow cytometry results, there were no significant differences between genotypes in the levels of CD68 or CD45 expression (Figure 3A) or in the numbers of CD68+ and Iba-1-positive microglia (Figure 3B, C), suggesting that TNF is dispensable for normal expression of these genes in vivo during microglia development and in adult brain. However, we found a statistically significant decrease in expression of TNFR2 and in expression of Macrophage Attack Complex (MAC)-1 in TNF-null mouse brains compared to wild type mouse brains, suggesting that TNF may be required for developmental expression of these and perhaps other genes of functional importance to microglia.
Figure 3. TNF signaling is not required for expansion of brain microglia but is critical for expression of Mac-1 and TNFR2 in adult brain.
(A) Brains (n = 4 mice/genotype) were rapidly removed and microdissected. Total RNA was extracted and reverse transcribed into cDNA for real-time PCR analysis of microglial surface markers or TNF receptors. Expression of CD45, CD68, and TNFR1 mRNA was not significantly different between genotypes but a significant reduction in expression of MAC1 and TNFR2 mRNAs was detectable in brain of adult TNF-null mice. Unpaired t-test, *p < 0.05, ***p < 0.001. (B) Immunohistochemical analysis of microglial markers Iba-1 and CD68 in midbrain or cortex of adult WT and TNF-null mice. Scale bar = 100 µm in low magnification images and 50 µm in the inset images. (C) Quantification of Iba-1 and CD68-positive cells per field in midbrain and cortex (See Methods) revealed no significant differences between genotypes.
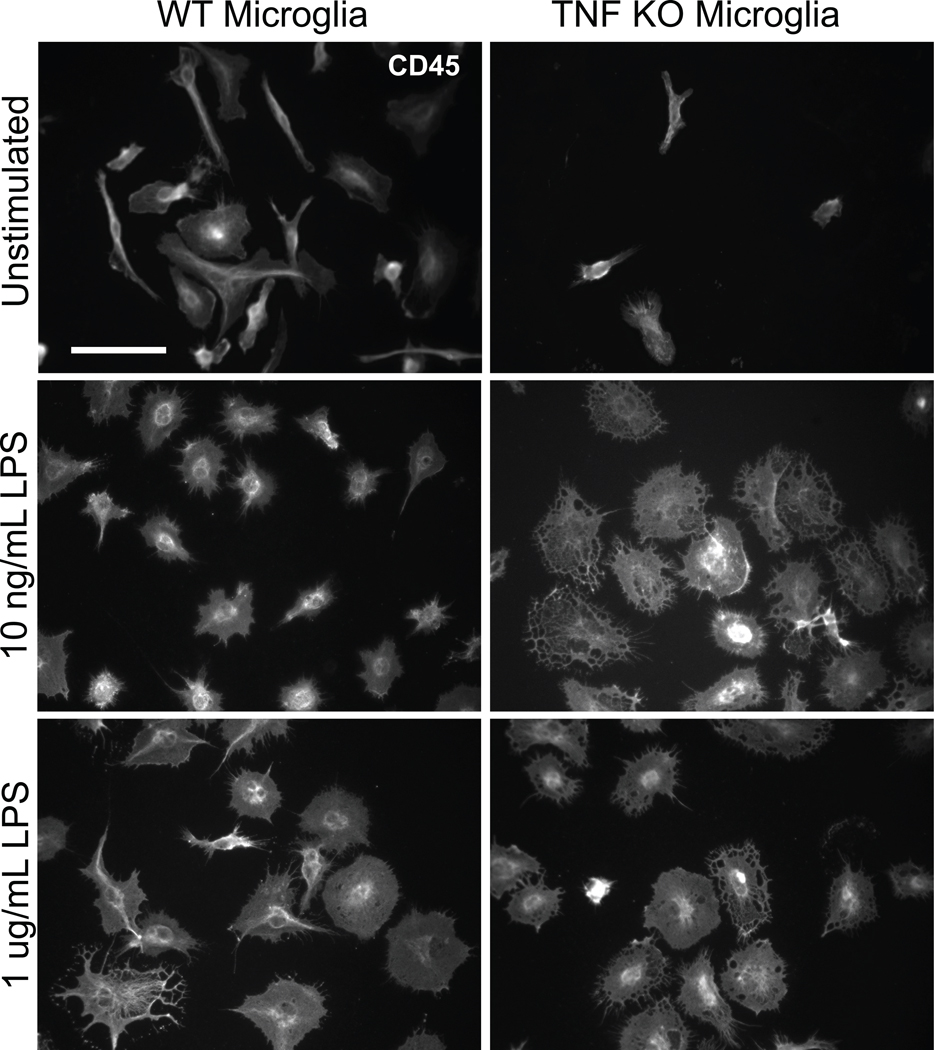
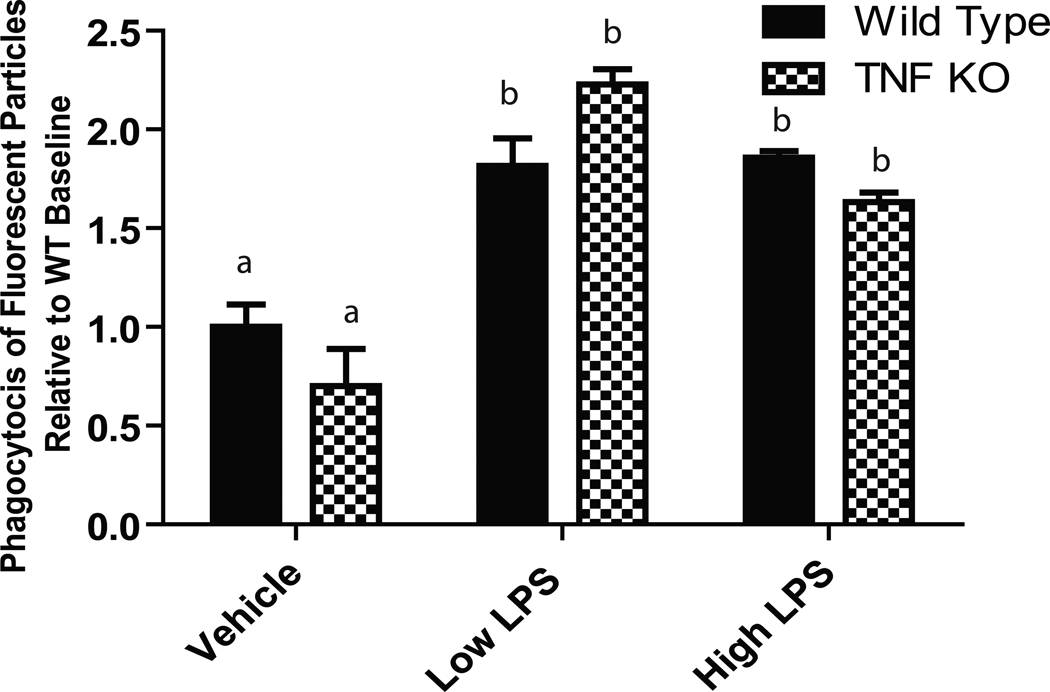
To investigate the extent to which TNF regulates microglia effector functions, we tested LPS-evoked morphological changes and phagocytic responses. Primary microglia were treated with low (10 ng/mL) or high (1 µg/mL) LPS for 24 hours. We then performed immunocytochemistry using an anti-CD45 antibody. TNF-null microglia displayed increased cytoplasmic vacuole-formation in response to LPS, the functional significance of which is unclear at this time. TNF-null microglia also displayed attenuated perinuclear localization of CD45 compared to wild type microglia (Figure 4), an effect that was more prominently detected after low LPS treatment. To rule out that the morphological alterations in TNF-null microglia were associated with apoptotic signaling, we performed immunostaining for pyknotic nuclei by bisbenzimide (Hoechst 33258) counterstain. We found no evidence of condensed or pyknotic nuclei (Supplemental Figure S1). However, the atypical morphology of activated TNF-null microglia suggested that other microglia effector functions involving motility and remodeling of the cytoskeleton such as phagocytosis might also be impaired in TNF-null microglia. Therefore, we investigated the ability of TNF-null and wild type microglia to phagocytose E. coli particles. Our results suggest that there are no differences between genotypes in LPS-induced phagocytosis (Figure 5).
Figure 4. Morphological analysis reveals atypical morphology of activated microglia isolated from TNF-null mice.
Primary microglia isolated from wild type or TNF-null postnatal day 3–5 (P3–5) pups were treated 24-hours post-plating with 10 ng/mL or 1 µg/mL lipopolysaccharide (LPS). Cells were fixed and stained with an anti-CD45 antibody to assess microglia morphology. Scale bar = 50 µm.
Figure 5. Microglia from TNF-null mice display normal phagocytic responses to E.coli particles.
Primary microglia cells were isolated from wild type or TNF-null postnatal day 3–5 pups, plated and treated overnight with low (10 ng/mL) or high (1 ug/mL) LPS. Phagocytic activity was measured using the Vybrant Phagocytosis Assay (Invitrogen). Two-way ANOVA and Bonferroni’s post hoc test. Bars with different letters are statistically significantly different from each other at p<0.05.
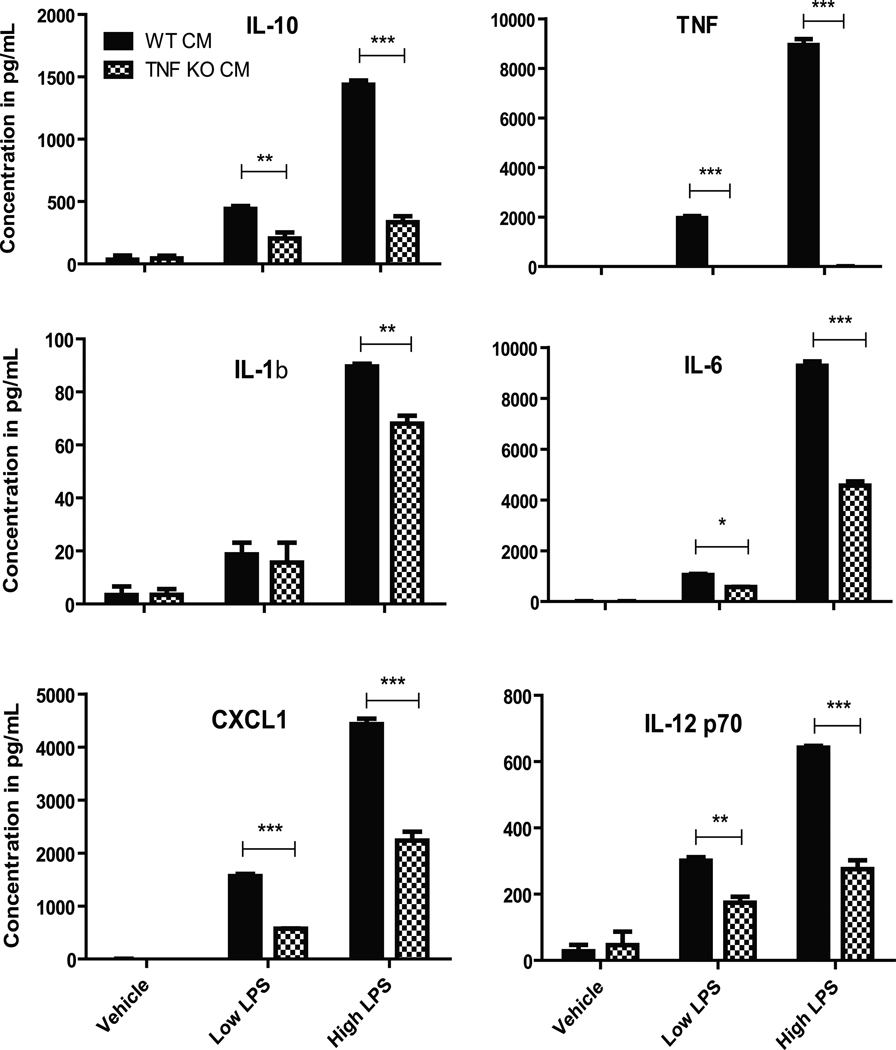
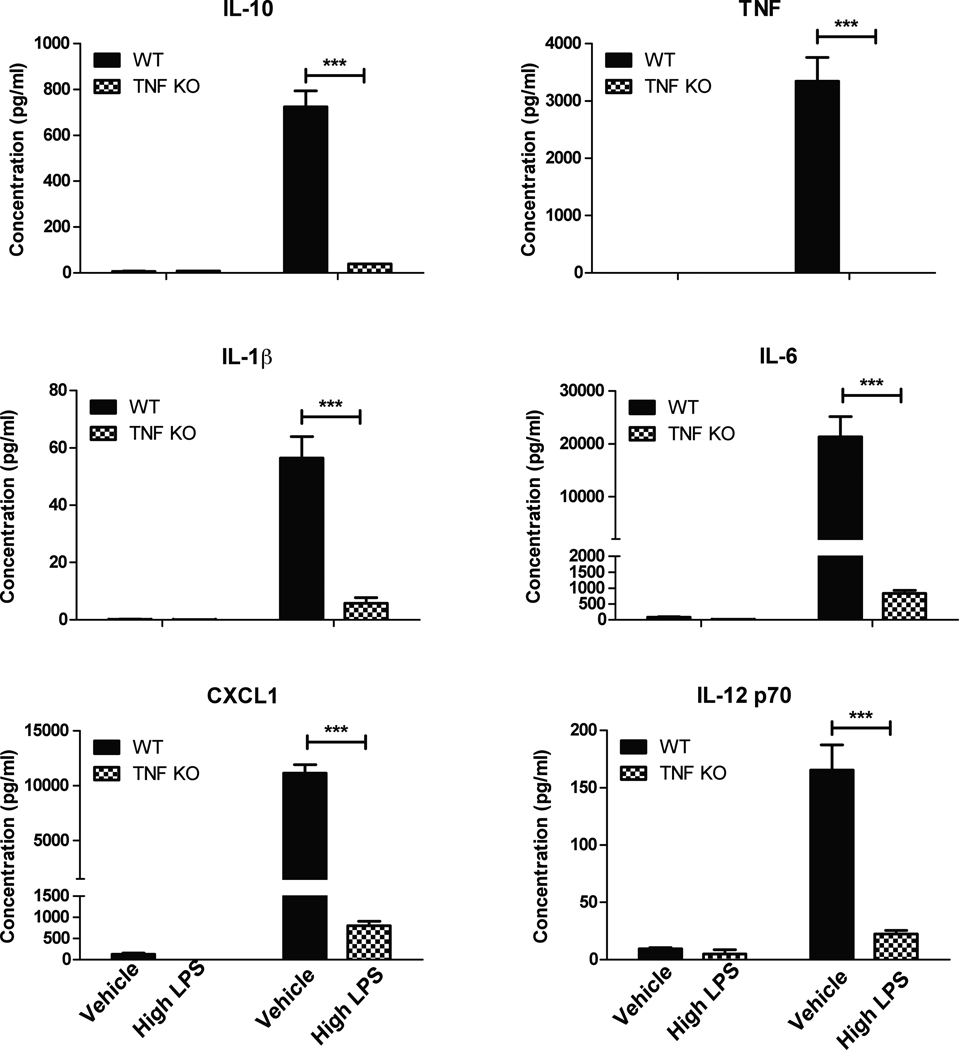
Next, we investigated the extent to which TNF deficiency during development affected expression and production of inflammatory mediators by microglia under resting or stimulated conditions. Multiplexed immunoassays analyses of conditioned media from resting or LPS-stimulated primary microglia harvested from wild type or TNF-null postnatal mice revealed significantly lower levels of TNF as well as other cytokines and chemokines including IL-1β, IL-6, IL-10, IL-12 and CXCL1 upon stimulation with LPS in postnatal TNF-null microglia compared to wild type microglia (Figure 6). Similar results were obtained with microglia harvested from adult TNF-null mice (Figure 7) and with peritoneal macrophages from adult TNF-null mice (data not shown). Together these findings indicate that TNF is critical for expression and/or secretion of a number of cytokines and chemokines in brain microglia consistent with its role in macrophages and dendritic cells (Douni et al. 1995).
Figure 6. Microglia from postnatal TNF-null mice display deficits in LPS-evoked inflammatory production.
Primary microglia cells isolated from wild type or TNF-null postnatal day 3–5 pups were plated and treated with low (10 ng/mL) or high (1 µg/mL) LPS for 24 hrs. Conditioned media was collected and analyzed for inflammatory factor production by multiplexed immunoassay (MesoScale). Two-way ANOVA with Bonferroni’s post hoc test. *p < 0.05, **p <0.01,***p < 0.001.
Figure 7. Microglia from adult TNF-null mice display deficits in LPS-evoked inflammatory factor production.
Primary microglia cells isolated from wild type or TNF-null adult (4–6 month) mice were plated and treated with LPS (1 µg/mL) for 24 hrs. Conditioned media was collected and analyzed for inflammatory factor production by multiplexed immunoassay (MesoScale). Two-way ANOVA with Bonferroni’s post hoc test. ***p <0.001.
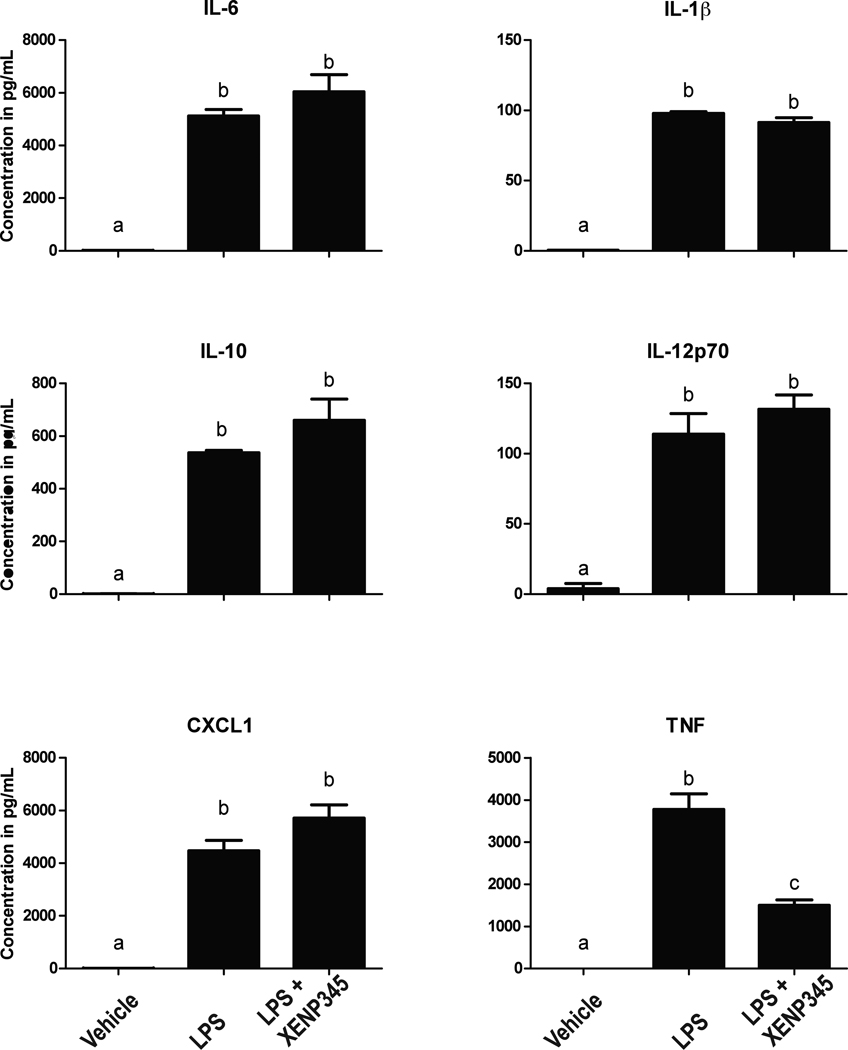
In order to determine if the blunted cytokine secretion by LPS-stimulated TNF-null microglia was due to developmental changes in gene transcription as a result of TNF deficiency or due to the lack of acute TNF signaling during the LPS stimulus, we treated primary wild type microglia overnight with the DN-TNF inhibitor XENP345 prior to LPS (1 µg/mL) stimulus. We found that pre-treatment with XENP345 only reduced the amount of detectable soluble TNF in the supernatant and did not affect secretion of all other cytokines (Figure 8). Together, these data indicate that TNF deficiency during microglia development impairs the normal increase in gene expression and production of inflammatory mediators during the microglia activation response, but acute exposure to TNF inhibitors does not interfere with this process.
Figure 8. Neutralization of solTNF with DN-TNF inhibitor XENP345 neutralizes solTNF but not other cytokines.
Primary microglia isolated from wild type postnatal day 3–5 pups were plated and treated overnight with saline or XENP345 (200ng/mL) and LPS (1 µg/mL) for 24 hours. Media was collected and run on an MSD 7-spot pro-inflammatory ELISA, n = 3 for each treatment condition. One-way ANOVA and Bonferroni's post hoc test. Bars with different letters are statistically significantly different from each other at p<0.05.
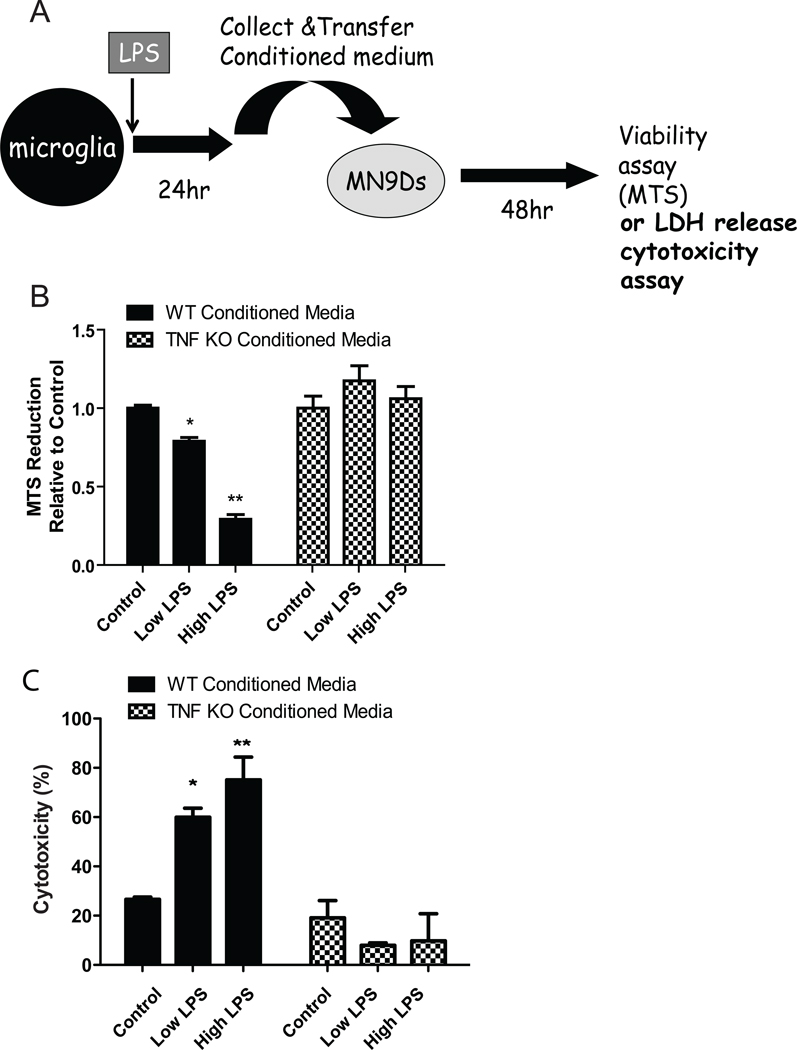
Next we investigated the extent to which TNF ablation or TNF inhibitors affected the cytotoxic phenotype of activated microglia. Our previous studies have demonstrated that soluble TNF is a critical component of endotoxin- and oxidative neurotoxin-induced death of dopaminergic neurons (Harms et al. 2011; McCoy et al. 2006; McCoy et al. 2008). Here, we investigated whether conditioned media (CM) from activated postnatal TNF-null microglia exerted decreased cytotoxicity on dopaminergic MN9D cells by performing target-effector assays. CM from resting or LPS-stimulated microglia are transferred directly onto terminally differentiated dopaminergic MN9D cells which do not express the LPS receptor, toll like receptor (TLR)4 (Figure 9A). In agreement with previous work (Lee et al. 2008; Tran et al. 2008), we found that CM of wild type microglia stimulated with LPS reduced metabolic activity of differentiated MN9D cells in a dose-dependent manner as measured by MTS reduction (Figure 9B) and exerted increased cytotoxicity on MN9D cells as measured by LDH release (Figure 9C). In contrast, CM of TNF-null microglia stimulated with LPS did not reduce MN9D cell viability under any LPS concentration tested. Given the fact that the reduced cytotoxicity of TNF-null microglia could be due to lack of TNF production but also due to reduced levels of several cytokines upon treatment with LPS (Figure 6), we next measured the direct effect of recombinant mouse (rm)-TNF, rm-IL-6 and rm-IL-1β on differentiated MN9D dopaminergic cell viability based on the rationale that all three of these cytokines have been implicated in degeneration of DA neurons. We found there was a dose-dependent decrease in MN9D cell metabolic activity upon treatment with soluble rm-TNF but not with either rm-IL-6 or rm-IL-1β treatment (Supplemental Figure S2). These findings are consistent with previous findings from our group demonstrating that neutralization of soluble TNF in the CM of LPS-stimulated microglia with the soluble decoy receptor etanercept or the dominant-negative TNF inhibitor XENP345 was sufficient to attenuate the cytotoxic effects of LPS-stimulated microglia on primary DA neurons in vitro and in vivo (McCoy et al. 2006).
Figure 9. TNF-null microglia display reduced cytotoxicity on differentiated dopaminergic MN9D cells.
Schematic of the target-effector assay (A); Primary microglia isolated from wild type or TNF-null postnatal day 3–5 pups were plated and treated for 24 hrs with low (10 ng/mL) or high (1 µg/mL) LPS. Conditioned media (CM) from resting or treated microglia were collected and transferred directly onto cultures of differentiated dopaminergic MN9D cells for 48 hrs. Metabolic activity of MN9D dopaminergic cell line was assayed using an MTS reduction assay. Two-way ANOVA with Bonferroni’s post hoc test. *p < 0.05, **p < 0.01 (B); Cytotoxicity of CM on MN9D cells was assayed using an LDH release assay. Two-way ANOVA with Bonferroni’s post hoc test. *p < 0.05, **p < 0.01 (C).
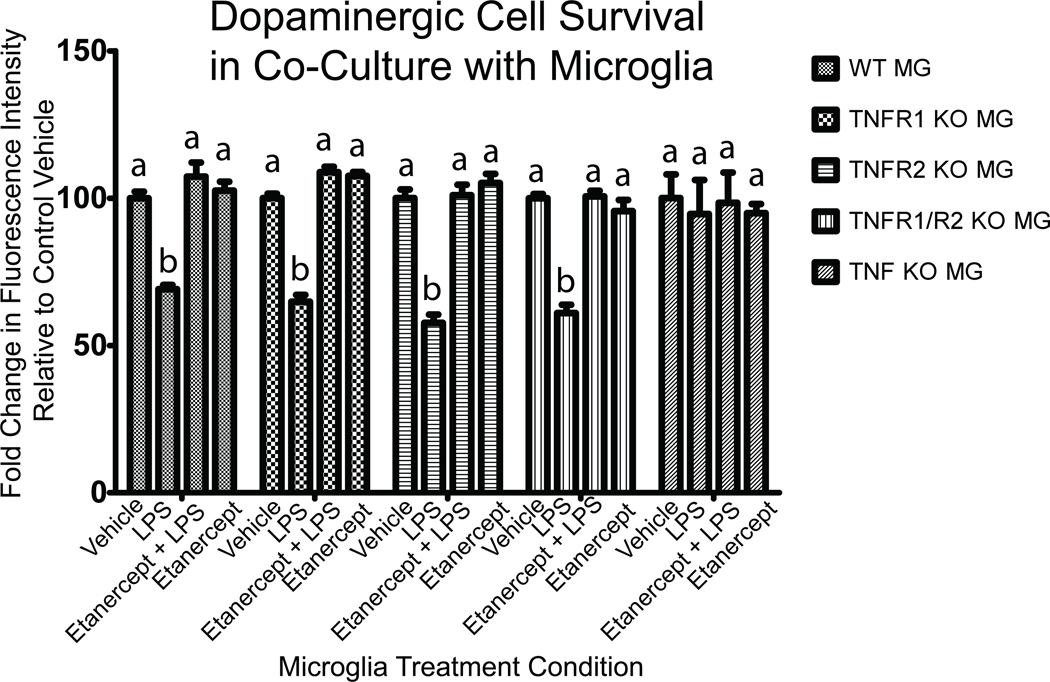
To determine the extent to which the LPS-induced cytotoxicity of activated microglia requires autocrine TNF signaling via TNFRs on microglia, we performed co-culture experiments with dopaminergic MN9D cells and microglia deficient in TNF or one or both TNF receptors. We found no difference between genotypes in the degree of cytotoxicity of resting microglia on MN9D cells (Figure 10); however, LPS stimulation of microglia of all genotypes except TNF-null microglia (which cannot produce TNF) led to reduced viability of co-cultured MN9D cells measured after 24 hours and this cytotoxicity could be blocked with pre-treatment of MN9D cultures with the TNF decoy receptor etanercept for 1 hour before addition of microglia. These findings indicate that LPS-evoked soluble TNF production is required for eliciting microglia cytotoxicity on dopaminergic cells but does not require activation of TNF receptors on microglia for autocrine propagation of the LPS signal and evoked response.
Figure 10. LPS-induced TNF secretion by primary microglia but not autocrine TNF signaling is required for microglial toxicity on dopaminergic cells.
Primary microglia from postnatal TNF-null, TNFR1-null, TNFR2-null, or TNFR1/R2 null mice were put in co-culture with neurally differentiated dopaminergic MN9D cells. Twenty-four hours after stimulation of co-cultures with LPS (1 ug/mL or saline vehicle in the presence or absence of the TNF decoy receptor etanercept (200 ng/mL) MN9D cell survival was assessed by quantifying the number of tyrosine hydroxylase (TH)-positive cells as described under Methods. A two-way ANOVA and Bonferroni's post hoc test. Columns with different letters are statistically significantly different from each other at p<0.05.
DISCUSSION
Although the role of TNF in the CNS has not been explored extensively, genetic and pharmacological studies have strongly implicated TNF and microglial activation in DA neuron death and seem to provide compelling rationale for use of TNF inhibitors in the CNS as a neuroprotective strategy in PD. However, TNF signaling has been shown to participate in a form of synaptic plasticity termed synaptic scaling (Stellwagen and Malenka 2006), glutamatergic neurotransmission, regulation of blood-brain barrier (BBB), and injury-mediated microglial and astrocyte activation (reviewed in (McCoy and Tansey 2008). Therefore, we aimed to elucidate the role of TNF signaling in microglial effector function to ascertain the potential for harmful bystander effects of such approaches on microglia function. In this study we provide detailed molecular, cellular, and functional analyses of the effects of TNF ablation and TNF inhibitors on postnatal and adult microglia populations. The significance of our studies is several-fold. First, our findings directly establish the importance of TNF in a select subset of microglia effector functions; second, our findings have several important implications for studies involving TNF-null mice and on the use of anti-TNF therapies to neutralize microglial-derived TNF.
Monocyte-derived dendritic cell populations display arrested development in TNF-null mice (Pasparakis et al. 1996). Although recent lineage analysis indicated brain microglia derive from primitive myeloid precursors and are ontogenically distinct from peripheral monocytes (Ginhoux et al. 2010), it was of great interest to investigate the extent to which brain microglia from TNF-null mice displayed developmental arrest or other deficits in effector functions. Using multiple and complementary approaches, we found that TNF is not critical for growth and expansion of brain microglia number, their basal activation state, or phagocytic responses. In contrast, our analyses indicate that the presence of TNF in microglia during development is critical for expression and secretion of several cytokines (TNF, IL-1β, IL-6, IL-12 p70, IL-10) and chemokines (CXCL1). The functional consequence of TNF ablation is a drastic reduction in the production of key cytokines and chemokines by activated microglia that is evident in neonatal mice and persists into adulthood. This finding is not entirely unexpected given the known role of TNF as a priming cytokine at the apex of inflammatory cascades in immune cells (Aggarwal 2000b) and the lineage similarities between brain microglia and peripheral macrophages (Ginhoux et al. 2010). However, one clear implication from these findings is that results involving use of TNF-null mice to investigate the direct role of TNF in the CNS under normal conditions or in disease models should be interpreted with caution because it is clear that their brains will display significant alterations in microglia-derived inflammatory factors as a result of TNF ablation. Moreover, previous work from our group demonstrated that co-addition of TNF inhibitors in vitro (McAlpine et al. 2009; McCoy et al. 2006) attenuated LPS-induced microglial activation transiently raising the possibility that pharmacological inhibition of TNF could phenocopy the effects of genetic ablation of TNF and indirectly affect production of other cytokines and chemokines. However, the direct comparison of inflammatory factor production by TNF-null microglia versus wild type microglia treated with the soluble TNF-selective DN-TNF (XENP345) inhibitor indicates this is not the case and treatment of microglia with TNF inhibitors selectively and specifically affects TNF bioactivity and does not affect production of other inflammatory mediators by activated microglia in an acute manner. Anti-TNF biologics are an FDA approved, safe treatment for a number of peripheral inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease (Tansey and Szymkowski 2009) and our group has used them to investigate the role of TNF signaling in pre-clinical models of neurodegeneration (McAlpine et al. 2009; McCoy et al. 2006). However, the extent to which long-term use of such TNF inhibitors affects microglial function remains to be determined. Importantly, the studies presented here demonstrate that while genetic ablation of TNF affects normal production of cytokines by activated microglia, prolonged exposure to TNF inhibitors has no such effects on microglia behavior. One obvious difference between TNF-null microglia and microglia treated with TNF inhibitors is that the latter do not completely abolish all TNF signaling; thus, any residual soluble TNF may be sufficient to maintain intact autocrine feedback loops that are important for production of additional cytokines. Because our results provide direct evidence that selectively neutralization of microglial-derived soluble TNF activity does not interfere with production of other inflammatory cytokines, our findings suggest that anti-TNF biologics are likely to afford potent neutralization of TNF-dependent processes without significantly affecting other important aspects of microglia activation and effector function.
Brain microglia are responsible for immune surveillance in the CNS; but in recent years chronic microglial activation and overproduction of pro-inflammatory cytokines has been implicated in the pathophysiology of several neurodegenerative diseases including Parkinson’s disease (PD), Alzheimer’s disease (AD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD) (Frank-Cannon et al. 2009). In PD patients, TNF mRNA and protein levels are elevated and microglial activation is widespread, in particularly in the substantia nigra pars compacta (SNpc), the midbrain area most affected by degeneration in this disease (Duke et al. 2007). Using a murine dopaminergic neuron-like cell line derived from mouse ventral mesencephalon (Choi et al. 1991), here we demonstrate that TNF is the critical microglial-derived factor that compromises the viability of these cells. Other studies have suggested that IL-1β may also compromise DA neuron survival (Godoy et al. 2008); however, while both IL-1β and IL-6 are produced by activated microglia in our assays, neither have direct toxic effects on MN9D dopaminergic cells. Nevertheless, it has been demonstrated that in vivo inhibition of IL-1β affords neuroprotective effects in part derived from the indirect effects of reduced TNF production (Koprich et al. 2008). While it cannot be ruled out that TNF may be acting with one or more additional factors such as reactive oxygen species (ROS) and nitric oxide (NO) to compound the neurotoxic effects on dopaminergic cells, the peak of ROS/RNS production typically occurs 4 hrs after stimulation with LPS (Gao et al., 2002) which makes it unlikely that oxidative damage to DA cells via synergy with ROS/RNS accounts for the loss of viability measured in our assays at 24 hrs post LPS stimulation. Based on these findings and those from previous studies indicating that anti-TNF biologics added to CM from LPS-stimulated microglia attenuated loss of viability of dopaminergic cells (Lee et al. 2008, Tran et al. 2006), we conclude that direct neutralization of TNF is sufficient to spare DA neurons in an inflamed microenvironment.
In summary, these findings establish the relative importance of TNF in a number of microglia effector functions. TNF is not critical for development of microglia number, their basal activation state, or their phagocytic responses to E.coli particles but may be required for morphological changes and for production of several important cytokines and chemokines that typically occur upon LPS stimulation. Importantly, although activated microglia produce a number of pro-inflammatory cytokines that may affect neuronal survival, TNF seems to be the critical microglial-derived factor that compromises survival of DA neuron-like cells. Together with previous observations that chronic TNF signaling inhibition in vivo affords neuroprotection to nigral DA neurons (Harms et al. 2011; McCoy et al. 2006; McCoy et al. 2008), the studies presented here strengthen the notion that TNF inhibition in the CNS may be a viable therapeutic approach to attenuate the progressive loss of nigral DA neurons that occurs in PD without compromising microglia effector functions in the CNS which are critical for immune surveillance and debris clearance.
Supplementary Material
Primary microglia from TNF-null mice do not display pyknotic nuclei characteristic of apoptotic cells in response to LPS stimulation. Microglia were isolated from wild type or TNF-null postnatal day 3 pups and placed in cell culture conditions as described for CD45 immunostaining. Scale bar = 50 µm.
Differentiated dopaminergic MN9D cells were treated for 48 hours with TNF (A), TNF plus the decoy receptor etanercept (200 ng/mL) (B), IL-1β (C), or IL-6 (D). Cell viability of MN9D dopaminergic cell line was measured by MTS reduction assay. One way ANOVA with Bonferroni’s post hoc test, *p<0.05, **p<0.01, ***p<0.001.
Acknowledgements
We thank members of the Tansey lab for helpful discussions. Funding support for these studies was provided by grant 5R01NS094933 (M.G.T) from NIH/NINDS.
References Cited
- Aggarwal BB, Samanta A, Feldmann M. TNFα. In: Oppenheim JJaF M, editor. Cytokine Reference. London: Academic Press; 2000a. pp. 414–434. [Google Scholar]
- Aggarwal BB, Samanta A, Feldmann M. TNFa. In: J. J. a. F. Oppenheim ML, editor. Cytokine Reference. Academic Press; 2000b. pp. 414–434. [Google Scholar]
- Carson MJ, Thrash JC, Walter B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin Neurosci Res. 2006;6(5):237–245. doi: 10.1016/j.cnr.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Won LA, Kontur PJ, Hammond DN, Fox AP, Wainer BH, Hoffmann PC, Heller A. Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res. 1991;552(1):67–76. doi: 10.1016/0006-8993(91)90661-e. [DOI] [PubMed] [Google Scholar]
- Douni E, Akassoglou K, Alexopoulou L, Georgopoulos S, Haralambous S, Hill S, Kassiotis G, Kontoyiannis D, Pasparakis M, Plows D, et al. Transgenic and knockout analyses of the role of TNF in immune regulation and disease pathogenesis. J Inflamm. 1995;47(1–2):27–38. [PubMed] [Google Scholar]
- Duke DC, Moran LB, Pearce RK, Graeber MB. The medial and lateral substantia nigra in Parkinson's disease: mRNA profiles associated with higher brain tissue vulnerability. Neurogenetics. 2007;8(2):83–94. doi: 10.1007/s10048-006-0077-6. [DOI] [PubMed] [Google Scholar]
- Ferger B, Leng A, Mura A, Hengerer B, Feldon J. Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem. 2004;89(4):822–833. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4(1):47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson's disease. Brain. 2008;131(Pt 7):1880–1894. doi: 10.1093/brain/awn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Barnum CJ, Ruhn KA, Varghese S, Trevino I, Blesch A, Tansey MG. Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson's disease. Mol Ther. 2011;19(1):46–52. doi: 10.1038/mt.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness CL, da Silva RP, Fawcett J, Gordon S, Simmons DL. Macrosialin, a mouse macrophage-restricted glycoprotein, is a member of the lamp/lgp family. J Biol Chem. 1993;268(13):9661–9666. [PubMed] [Google Scholar]
- Koprich JB, Reske-Nielsen C, Mithal P, Isacson O. Neuroinflammation mediated by IL-1 beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson's disease. J Neuroinflammation. 2008;5(1):8. doi: 10.1186/1742-2094-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, McCoy MK, Harms AS, Ruhn KA, Gold SJ, Tansey MG. Regulator of G-protein signaling 10 promotes dopaminergic neuron survival via regulation of the microglial inflammatory response. J Neurosci. 2008;28(34):8517–8528. doi: 10.1523/JNEUROSCI.1806-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine FE, Lee JK, Harms AS, Ruhn KA, Blurton-Jones M, Hong J, Das P, Golde TE, LaFerla FM, Oddo S, et al. Inhibition of soluble TNF signaling in a mouse model of Alzheimer's disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol Dis. 2009;34(1):163–177. doi: 10.1016/j.nbd.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26(37):9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Ruhn KA, Martinez TN, McAlpine FE, Blesch A, Tansey MG. Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats. Mol Ther. 2008;16(9):1572–1579. doi: 10.1038/mt.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaud S, Draheim HJ. A new method to isolate microglia from adult mice and culture them for an extended period of time. J Neurosci Methods. 2010;187(2):243–253. doi: 10.1016/j.jneumeth.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184(4):1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rousselet E, Callebert J, Parain K, Joubert C, Hunot S, Hartmann A, Jacque C, Perez-Diaz F, Cohen-Salmon C, Launay JM, et al. Role of TNF-alpha receptors in mice intoxicated with the parkinsonian toxin MPTP. Exp Neurol. 2002;177(1):183–192. doi: 10.1006/exnr.2002.7960. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. Faseb J. 2002;16(11):1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-alpha. Faseb J. 2006a;20(6):670–682. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- Sriram K, Miller DB, O'Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem. 2006b;96(3):706–718. doi: 10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Szymkowski DE. The TNF superfamily in 2009: new pathways, new indications, and new drugs. Drug Discov Today. 2009;14(23–24):1082–1088. doi: 10.1016/j.drudis.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Tran TA, McCoy MK, Sporn MB, Tansey MG. The synthetic triterpenoid CDDO-methyl ester modulates microglial activities, inhibits TNF production, and provides dopaminergic neuroprotection. J Neuroinflammation. 2008;5:14. doi: 10.1186/1742-2094-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150(8):963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary microglia from TNF-null mice do not display pyknotic nuclei characteristic of apoptotic cells in response to LPS stimulation. Microglia were isolated from wild type or TNF-null postnatal day 3 pups and placed in cell culture conditions as described for CD45 immunostaining. Scale bar = 50 µm.
Differentiated dopaminergic MN9D cells were treated for 48 hours with TNF (A), TNF plus the decoy receptor etanercept (200 ng/mL) (B), IL-1β (C), or IL-6 (D). Cell viability of MN9D dopaminergic cell line was measured by MTS reduction assay. One way ANOVA with Bonferroni’s post hoc test, *p<0.05, **p<0.01, ***p<0.001.