Abstract
Background
Conceptual models and recent evidence indicate that neural response to reward is altered in depression. Taking a developmental approach to investigating reward function in adolescent depression can elucidate the etiology, pathophysiology, and course of depression, a disorder that typically begins during adolescence and has high rates of recurrence.
Methods
This conceptual review describes the what, when, and how of altered reward function in adolescent depression. With the goal of generating new, testable hypotheses within a developmental affective neuroscience framework, we critically review findings and suggest future directions. Peer-reviewed empirical papers for inclusion in this critical review were obtained by searching PubMed, PsycInfo, and ScienceDirect for the years 1990–2010.
Results
A pattern of low striatal response and high medial prefrontal response to reward is evident in adolescents and adults with depression. Given the salience of social stimuli for positive affect and depression, reward function might be especially disrupted in response to social rewards. Because of changes in the dopamine system and reward function with aging, altered reward function in depression might be more evident during adolescence than later in life; however, low reward function may also be a stable characteristic of people who experience depression. Mechanisms of altered reward function in depression could include disrupted balance of corticostriatal circuit function, with disruption occurring as aberrant adolescent brain development.
Conclusions
Future studies should examine responses to social rewards; employ longitudinal and prospective designs; and investigate patterns of functional connectivity in reward circuits. Understanding altered reward function in depression has potential implications for treatment development. A more rigorous approach to investigating anhedonia, threat-reward interactions, and comorbid anxiety will be valuable to future progress in describing the role of reward function in the pathophysiology of depression.
Keywords: depression, development, reward, brain function
Neural aspects of reward function have been conceptualized as critical to the etiology, development, pathophysiology, and treatment of depression (e.g., Forbes & Dahl, 2005; Hasler, Drevets, Manji, & Charney, 2004). This direction of research helps to shift the field away from a narrow focus on increased negative affect in depression and toward consideration of diminished positive affect, and it contributes to the search for mechanisms underlying mood and subjective experience.
A developmental psychopathology perspective could be especially valuable to understanding the role of reward function in depression and to reconciling seemingly conflicting findings on this topic, such as mixed results on low striatal response to reward. Because depression typically begins during adolescence (Kessler, Avenevoli, & Merikangas, 2001), and adolescent depression confers a high risk of recurrence in adulthood (Lewinsohn, Rohde, Klein, & Seeley, 1999), adolescent depression provides a valuable scientific opportunity. Examining reward function during adolescent depression can elucidate the larger course of the disorder and place its etiology within the context of typical, dramatic changes in reward function that occur during this developmental period (Somerville, Jones, & Casey, 2010).
This review examines the evidence for what in particular about reward function is disrupted in adolescent depression, when in the development of depression reward function is disrupted, and how disruption in neural reward circuits occurs. We propose testable hypotheses to guide future work on this compelling topic, and we briefly consider how a deeper understanding of these issues may inform intervention strategies. To obtain material for this conceptual review, we searched for peer-reviewed empirical papers in English published between 1990 and 2010 using PubMed, PsycInfo, and ScienceDirect indices, with terms such as depress*, reward, decision-making, dopamine, fMRI, striatum, and adolescen* (with the * wild card allowing retrieval of terms with the same stem, such as depressed and depression). Papers identified by the search were included if (1) they focused on topics relevant to the focus of the review (e.g., depression and striatal or medial prefrontal cortex (mPFC) response to reward; depression and decision-making behavior; adolescence and reward function; dopamine and depression), (2) used fMRI with a reward processing paradigm; (3) reported results of statistical tests; and (4) compared currently depressed and healthy control groups (e.g., not remitted depressed). Extending the stance of our previous reviews (Forbes, 2011; Forbes & Dahl, 2005; Forbes, et al., 2009), the specific goals of this review are to integrate recent neuroimaging findings; provide an overview of key related topics, such as reward-related decision-making; and develop an explicit foundation for future research.
What about Reward Function Is Disrupted?
This section reviews three literatures relevant to neural aspects of reward function in adolescent depression—functional magnetic resonance imaging (fMRI) of reward, reward-related decision-making behavior, and dopamine function—and then discusses methodological issues important to the neurobiology of reward function.
Neural Response to Reward
The neural circuitry of reward includes regions such as the striatum, orbitofrontal cortex, and amygdala (Haber & Knutson, 2010). Notably, because the dopamine neuromodulatory system plays a critical role in reward (Haber & Knutson, 2010), target regions of midbrain dopamine neurons, such as the striatum and mPFC, have been a focus of research on reward function in depression (see Figure 1).
Figure 1.
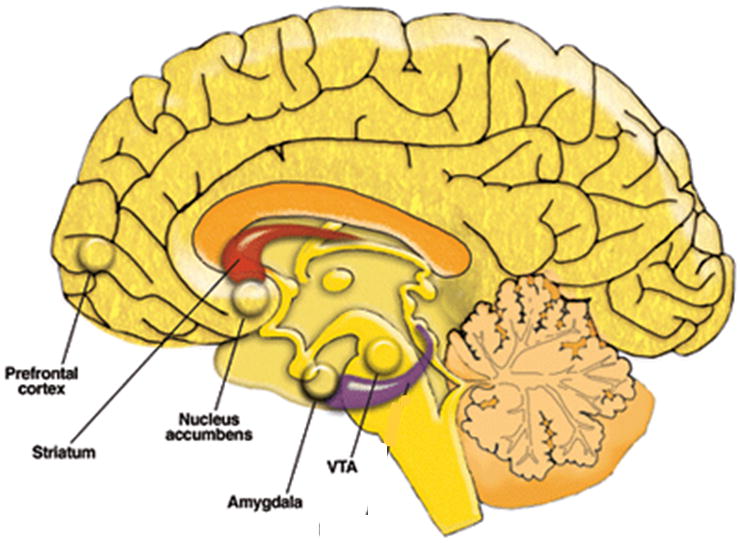
Neural reward circuitry, with an emphasis on regions found to have altered function in depression. Adapted from Clapp et al., 2008.
Adolescents and adults with depression exhibit altered brain function in key reward-related areas in response to rewarding experiences (Table 1). In particular, findings have begun to converge on low reactivity to monetary reward in the striatum, a brain region associated with many aspects of reward processing (Balleine, Delgado, & Hikosaka, 2007; Haber & Knutson, 2010). Adolescents with depression exhibit reduced reactivity in the striatum in response to decision-making, anticipation, and outcome involving monetary reward (Forbes, et al., 2009; Forbes, et al., 2006). Adults with depression exhibit reduced reactivity in the striatum during decision-making (Smoski, et al., 2009), anticipation (Pizzagalli, et al., 2009; Smoski, et al., 2009), and outcome (Pizzagalli, et al., 2009; Smoski, et al., 2009) involving monetary reward; in response to pleasant words (Epstein, et al., 2006) and pleasant facial expressions (Surguladze, et al., 2005); and when attempting to enhance positive affective state (Heller, et al., 2009).
Table 1.
Studies of Depression and Neural Response to Reward
| Authors | Task | Findings | Supports Low Reward Function? | ||
|---|---|---|---|---|---|
| Striatum | mPFC | Other | |||
| Studies with Adolescents | |||||
| Forbes et al., 2006 | Monetary guessing task | MDD < comparison during decision- making/anticipation and outcomea | MDD < comparisona | amygdala: MDD > comparison during outcome | Yes |
| Forbes et al., 2009 | Monetary guessing task | MDD < comparison during anticipation and outcome (both: −6, 18, 3) | MDD > comparison during outcome (−4, 60, 3) | Yes | |
| Studies with Adults | |||||
| Davey et al., 2011 | Social feedback about likeability | No group difference | MDD > comparison for faces vs. fixation (−14, 50, −2); (6, 32, −2) | amygdala: MDD > comparison (72, −36, −12) | No |
| Epstein et al., 2006 | Pleasant words | MDD < comparison (−15, 15, −6) (15, 6, −3) | MDD < comparisonb | Yes | |
| Heller et al., 2009 | Positive affect regulation task | MDD < comparison (−9, 12, 0) | N.A. | Yes | |
| Keedwell et al., 2005a | Pleasant autobiographical memory | MDD > comparison (10, 51, 4) (−1, 33, 51) | |||
| Keedwell et al., 2005b | Pleasant autobiographical memory | MDD < comparison (18, −4, 20) | MDD > comparison (−25, 4, 42); (−22, −7, 42) | Yes | |
| Knutson et al., 2008 | Monetary Incentive Delay task |
No group difference | MDD > comparison during anticipation (−11, 11, 34) and outcome (8, 40, 4) | Mixed | |
| Pizzagalli et al., 2009 | Monetary Incentive Delay task |
MDD < comparison during anticipation (−28, −13, −2) and outcomeb | N.A. | Yes | |
| Smoski et al., 2009 | Wheel of Fortune monetary reward task | MDD < comparison during decision- making (−8, 6, 6) and anticipation (−18, −16, 22) | MDD < comparison during decision- making (−14, 44, 48) | orbitofrontal cortex: MDD > comparison during decision-making (26, 30, −16) | Yes |
| Surguladze et al., 2006 | Pleasant facial expressions | MDD < comparison (22, 11, −2) | No group difference | Yes | |
peak voxel not reported because analyses tested group differences in mean function of anatomically defined regions of interest
peak voxel coordinates not reported, although both papers depicted clusters in figures. Pizzagalli et al. stated regions as bilateral caudate and left nucleus accumbens; Epstein et al. stated regions as left dorsomedial frontal gyrus
Note. MDD: major depressive disorder. Coordinates for the peak voxel of the cluster for group difference results are noted under each finding. All coordinates are in Talairach space, except for those for the studies by Pizzagalli et al., 2009 and Davey et al., 2011, which are in Montreal Neurological Institute space. N.A. Not applicable because the region was not included in analyses of group differences.
In parallel with low striatal reactivity, some reward studies have reported that people with depression exhibit high reactivity in mPFC areas that are postulated to play a role in regulating reward response, including the anterior cingulate cortex (Knutson, et al., 2008) and nearby mPFC in adults (Keedwell, et al., 2005a) and adolescents with depression (Forbes, et al., 2009). Some studies, however, have reported that depression is associated with low response in this region (Epstein et al., 2006; Forbes et al., 2006; Smoski et al., 2009). Most recently, a study has reported that adult depression is associated with enhanced amygdala response to social reward (Davey et al., 2011). This finding is consistent with a finding on response to monetary reward in adolescent depression (Forbes et al., 2006). Findings on altered mPFC and amygdala response to rewarding stimuli in depression also could have implications for adolescent depression. Similarly, the developmental decrease in striatal function during attention to happy faces (Lindstrom et al., 2009) suggests that development of striatal response to other classes of reward could influence differences in adolescent depression.
Reward and Decision-Making
Reward function plays a critical role in decision-making because it influences motivation and learning. Consistent with reduced striatal response to reward described above, many behavioral studies have found that depression is associated with reduced sensitivity to rewarding outcomes during decision-making (Table 2). During gambling or monetary-reward tasks, adults with depression make decisions that are more conservative (Corwin, Peselow, Feenan, Rotrosen, & Fieve, 1990), slower (Kaplan, et al., 2006), and less flexible in the face of shifting contingencies (Cella, Dymond, & Cooper, 2010). Depression—and anhedonia in particular—is associated with failure to exhibit a response bias toward rewarded stimuli in signal detection tasks, in which one set of stimuli is subtly rewarded more frequently than another (Pizzagalli, Iosifescu, Hallet, Ratner, & Fava, 2008; Pizzagalli, Jahn, & O’Shea, 2005). In adolescents with depression, there is less distinction of high-magnitude and low-magnitude rewards under high-probability conditions (Forbes, Shaw, & Dahl, 2007) and less improvement in cognitive control with reward (Hardin, Schroth, Pine, & Ernst, 2007; Jazbec, McClure, Hardin, Pine, & Ernst, 2005).
Table 2.
Studies of Depression and Decision-Making under Reward Conditions
| Authors | Tasks | Findings | Supports Low Reward Function? |
|---|---|---|---|
| Forbes et al., 2007 | Reward-contingent decision task | Choice of “risky” option: depression group showed no difference between high-probability/low- magnitude and high-probability/high-magnitude conditions | Yes |
| Hardin et al., 2007 | Reward antisaccade task | Performance: MDD < comparison Latency: improved with reward in comparison group but not MDD |
Yes |
| Jazbec et al., 2005 | Reward antisaccade task | Inhibitory efficiency: improved with reward in comparison but not MDD | Yes |
| Kyte et al., 2005 | Decision making task | Speed of decisions when betting points: MDD > comparison | No |
| Cella et al., 2010 | Iowa gambling task | Contingency-shift phase: MDD less perceptive of shifts from punishment to reward contingencies | Yes |
| Chase, Frank, et al., 2010 | Probabilistic selection task | RT: MDD < comparison Blunted learning during practice: positive correlation with anhedonia |
Yes |
| Chase, Michael, et al., 2010 | Cued reinforcement reaction time task | Total points: MDD > comparison Likelihood of reaching threshold: MDD > comparison Errors: MDD < comparison |
No |
| Corwin et al., 1990 | Signal detection task | Response bias: MDD more conservative than comparison | Yes |
| Kaplan et al., 2006 | Cambridge Gamble Task | Decision-making latency: MDD > comparison | Yes |
| Lempert & Pizzagalli, 2010 | Intertemporal choice | Delay discounting: negative association with anhedonia | Yes |
| Pizzagalli et al., 2008 | Signal detection task | Response bias toward rewarded stimuli: MDD < comparison | Yes |
| Pizzagalli et al., 2005 | Signal detection task | Response bias toward rewarded stimuli: negatively correlated with depressive symptoms | Yes |
| Takahashi et al., 2008 | Intertemporal choice task | Impulsivity: MDD > comparison | No |
Note. MDD: major depressive disorder. RT: reaction time.
Findings on decision-making in both adolescent depression and adult depression also have seeming inconsistencies. Adults with depression exhibit greater discounting of future rewards in intertemporal choice tasks (Takahashi, et al., 2008), which involve decisions between immediate, smaller-magnitude and delayed, larger-magnitude rewards and are thought to assess impulsivity. They may also exhibit shorter reaction time or better performance in choice tasks (Chase, Michael, Bullmore, Sahakian, & Robbins, 2010). Notably, adolescents with depression can appear to make faster, more impulsive decisions (Kyte, Goodyer, & Sahakian, 2005). Rather than enhanced reward function, however, such impulsivity could instead reflect pessimistic or inaccurate anticipation of future events, as well as altered PFC function, given the putative role of the PFC in planning and goal pursuit (Mushiake, et al., 2009). Depressive features must also be considered. For instance, probabilistic reversal reward learning in elderly adults with depression is disrupted for suicide attempters but not suicide ideators, with suicide attempters giving more weight to the last trial rather than to earlier patterns of outcomes (Dombrovski, et al., 2010). Also, blunting during reinforcement learning in a probabilistic decision-making task is positively associated with level of anhedonia (Chase, Frank, et al., 2010; Lempert & Pizzagalli, 2010). Thus, it appears that in depression, there is difficulty integrating future rewards into current decisions.
Dopamine
At a molecular level, altered neural response to reward and altered decision-making behavior under rewarded conditions are likely to be subserved by changes in dopamine function. In fact, depression is postulated to be associated with disrupted dopamine signaling (see Dunlop & Nemeroff, 2007; Nestler & Carlezon, 2006 for reviews). Specifically, functioning in the mesolimbic pathway of dopamine neurons from the ventral tegmental area to the nucleus accumbens in the ventral striatum is proposed to play an etiologic role in depression (Nestler & Carlezon, 2006). Dopamine is implicated in typical affective response to reward and in rewarded behavior through projections of midbrain dopamine neurons to the striatum and medial prefrontal cortex (Haber & Knutson, 2010). Dopamine release is postulated to facilitate learning and goal-directed behavior by engaging both of these neural regions. A useful distinction in explaining the influence of dopamine neurons on reward-related behavior is that between tonic dopamine transmission, which provides a steady baseline level of dopamine regardless of external stimuli, and phasic dopamine transmission, which occurs in response to a stimulus. Goal-directed behavior has been associated with reduced phasic dopamine transmission in response to non-receipt of reward, with the phasic change serving to engage prefrontal regions in the service of changing current behavior (Sesack & Grace, 2010). In depression, difficulties with regulating mood flexibly or low behavioral activation could reflect reduced dopamine signaling. Evidence for this perspective includes findings from positron emission tomography and single photon emission computerized tomography studies, which can measure the density of dopamine receptors to infer the availability of dopamine in relevant regions such as the striatum (Cannon, et al., 2009). Animal models also provide evidence for this hypothesis: greater firing of ventral tegmental area dopamine neurons in rodents accompanies improvement in depressive-like behavior (Friedman, Friedman, Dremencov, & Yadid, 2008).
Intriguing findings from pharmacologic challenge studies provide an opportunity to illustrate claims about dopamine system function in depression. Seemingly at odds with postulated low dopamine function in depression, depression has been associated with greater sensitivity to stimulant drugs, which increase available dopamine. During amphetamine challenge, adults with depression report experiencing greater subjective rewarding effects (Tremblay, Naranjo, Cardenas, Herrmann, & Busto, 2002; Tremblay, et al., 2005) but exhibit less striatal response than healthy adults (Tremblay, et al., 2005). While these findings might suggest enhanced dopamine responding, differences in tonic and phasic dopamine neuron activity (Goto, Otani, & Grace, 2007) could also lead to the interpretation that depression involves low tonic dopamine levels, which disrupt the phasic dopamine response to reward. Alternatively, depression could alter dopamine response to different classes of rewarding stimuli, with lower response to natural rewards but enhanced response to drug rewards. The authors of these studies propose two possible neural mechanisms for these findings: (1) unusual glutamate transmission, which has been observed in depression (McCullumsmith & Meador-Woodruff, 2002) and which mediates dopamine response to amphetamine (Paladini, Fiorillo, Morikawa, & Williams, 2001); and (2) dopamine system compensation for low dopamine function (Tremblay, et al., 2005). Dopamine system changes reported in adult depression include increased density of D2 receptors—which is also associated with response to tricyclic and selective serotonin reuptake inhibitor (SSRI) antidepressant treatment (D’Haenen & Bossuyt, 1994; Ebert, Feistel, Loew, & Pirner, 1996; Klimke, et al., 1999)—and lower density of dopamine transporter (Klimek, Schenck, Han, Stockmeier, & Ordway, 2002; Meyer, et al., 2001). While the specific type of dopamine system disruption is not revealed by these findings, they are consistent with altered dopamine function in depression.
Given the evidence for involvement of the dopamine system in depression, it can appear somewhat surprising that the most effective and widely used pharmacologic treatment for depression is selective serotonin re-uptake inhibitor medications (SSRIs; Masi et al., 2010). Medications targeting serotonin can “reverberate” in other monoamine systems, suggesting that dopamine function may be influenced indirectly by SSRIs (El Mansari et al., 2010). Several antidepressant medications such as pramipexole influence dopamine function directly (Dunlop & Nemeroff, 2007; El Mansari et al., 2010). Another pharmacologic issue worth mentioning in this context is that stimulant treatment for attention-deficit/hyperactivity disorder, which increases dopamine function, can induce depression in a small subgroup of children.
Methodological Issues
Which types of rewards?
A compelling possibility is that reward function in depression is particularly disrupted in response to social rewards. Conceptually, depressed mood is seen as having a social function, for example by reducing one’s dependence on the social group during times of strong competition for resources (Allen & Badcock, 2003). Developmental psychopathology and affective neuroscience perspectives emphasize the social changes of adolescence as facilitating the onset of depression (Davey, Yucel, & Allen, 2008). Social rewards elicit positive affect, which plays a central role in affiliation and social status (Bora, Yucel, & Allen, 2009), and reactivity in reward-related brain areas (Davey, Allen, Harrison, Dwyer, & Yucel, 2009). Notably, depression has an important association with social functioning, with loss of a romantic relationship as a typical triggering event for first episodes (Monroe, Rohde, Seeley, & Lewinsohn, 1999). Social stressors are viewed as strong influences on the development and course of depression in adolescents (Sheeber, Hops, & Davis, 2001).
Few studies of reward function in depression have focused on social rewards. This trend appears to be changing, with recent studies reporting that depression is associated with differences in neural response to autobiographical memory (Keedwell, et al., 2005b) and to face-processing during positive social feedback (Davey et al., 2011). Monetary reward is understandably more straightforward to study and can be understood in the context of reward paradigms used across species. But without examining the rewards that are most fundamental to human functioning, we may be missing a chance to understand the mechanisms and extent of reward dysfunction in depression. On a similar note, it will be important to gain enough insight into the normal function of the brain’s reward systems and the commonalities of response to monetary and social rewards to achieve an understanding of how reward function might differ in response to different classes of rewards in depression.
fMRI paradigms
Differences in fMRI reward paradigms could explain the two notable exceptions to the pattern of low striatal response to reward in depression: one study reported no difference in striatal response (Knutson, Bhanji, Cooney, Atlas, & Gotlib, 2008) and another reported greater striatal response (Remijnse, et al., 2009). Paradigms differ in whether reward is contingent on performance (as in the Monetary Incentive Delay task) and in the way that trials are structured (e.g., anticipation, decision-making, and motor preparation might all occur in the same trial component). Importantly, developmental influences on reward function could also explain differences in findings. As described below, depression-related alterations in reward function might be more evident in adolescents than in adults.
Sample characteristics
Discrepant findings could occur also occur because of participant characteristics. Adolescents with depression differ in their clinical presentation, with variability in the experience of symptoms, such as anhedonia, or other forms of psychopathology, such as anxiety. As discussed in Conclusions below, considering these features, as well as integrating research on reward function and threat function, will provide a more thorough picture of reward function.
Multi-method assessment
Assessing reward function across its components is critical to capturing it comprehensively and elucidating its role in depression. Like other affective processes, reward function includes physiological (e.g., brain function), behavioral (e.g., displayed positive affect), and subjective components (e.g., experience of pleasant mood; e.g., McManis et al., 2001). Differences in neural aspects of reward function are most powerfully understood when they are examined in the context of their associations with mood (e.g., feeling happy) or behavior (e.g., performance on a computer task with prizes). For example, we recently reported that greater reactivity in a region of the striatum associated with adolescent depression was correlated with higher levels of subjective positive affect measured in natural environments (Forbes, et al., 2009). Another innovative developmental study found that adolescents whose mothers displayed more punitive behavior in response to adolescents’ positive affect had larger orbitofrontal cortex volume (Whittle, et al., 2009). Both of these studies also illustrate the value of emphasizing assessment of reward in ecologically valid, personally relevant ways. It will be exciting for future studies to investigate the links between neural components of reward function in adolescent depression and well-documented behavioral characteristics, such as less-frequent expression of positive affect (Sheeber, et al., 2009) and lower subjective positive affect in laboratory and natural settings (Joiner & Lonigan, 2000).
When in Development Is Reward Function Altered?
Depression and the Normal Development of Reward Function
Adolescence may be the developmental period at which reward function in depression is most strikingly disrupted. First episodes of depression are likely to occur during adolescence (Lewinsohn, Clarke, Seeley, & Rohde, 1994), when normative levels of reward function are higher than during childhood or adulthood (Somerville, et al., 2010). Behavioral and phenomenological findings both suggest that it could be value to place altered reward function in depression into a context of normal development. Specifically, altered reward function is more characteristic of early than later episodes of depression (Nandrino, Dodin, Martin, & Henniaux, 2004), adolescent anhedonia predicts later major depressive disorder (Pine, Cohen, Cohen, & Brook, 1999), and low positive affect during adolescents’ depressive episodes predict recurrence (Joiner, Lewinsohn, & Seeley, 2002).
Normal Development of Reward Function during Adolescence
Adolescence is a sensitive period for the development of depression and, for reward function, a time of more and less. On one hand, adolescents tend to engage in more high-risk, reward-seeking behaviors than either children or adults (see Somerville, et al., 2010), to report more sensation-seeking (Steinberg, et al., 2008), and to experience rewards more intensely (Ernst, et al., 2005; Steinberg, 2008). These findings are echoed in rodent research (e.g., Laviola, Macri, Morley-Fletcher, & Adriani, 2003). On the other hand, adolescents have low levels of momentary positive affect e.g., (Larson, Moneta, Richards, & Wilson, 2002), are often bored, and show substantial, increasing levels of depressive symptoms (Sawyer, et al., 2009). Adolescents show altered striatal response to reward in comparison to children or adults (Bjork, et al., 2004; Ernst, et al., 2005; Forbes, Ryan, et al., 2010; Galvan, et al., 2006), and greater neural response to reward in regions implicated in social cognition and self-perception, such as the mPFC (Bjork, et al., 2004; Forbes, Ryan, et al., 2010). Adolescents’ greater striatal response to reward prediction error, which is mediated by dopamine neurons (Cohen, et al., 2010), suggests that the development of dopamine function is critical to changes in reward function. Similarly, the simultaneous development of the dopamine system and neural reward circuits during adolescence has been postulated to trigger depression in those who are vulnerable (Davey, et al., 2008). Puberty, with its influence on behavioral (Forbes & Dahl, 2010) and neural (Forbes, Ryan, et al., 2010) changes in reward function—as well as on the development of depression (Angold & Costello, 2006)—likely contributes to this developmental pattern.
Changes in Reward Function during Adulthood
In contrast to adolescence, adulthood may involve decreases in reward function. Aging is associated with difficulty learning reward associations (Mell, et al., 2005), decreased total earnings in reward decision-making (Brown & Ridderinkhof, 2009), and low subjective positive affect (e.g., Kunzmann, 2008). The dopamine system undergoes reductions in receptor density, and there are shifts in patterns of reward-related brain function and the association between midbrain and prefrontal activation (Dreher, Meyer-Lindenberg, Kohn, & Berman, 2008). Striatal function in particular appears to decline (Dreher, et al., 2008; Mell, et al., 2009; Schott, et al., 2007). These aging-related changes could close the reward function gap between people with depression and people without depression during late adulthood, obscuring differences.
However, based on two other perspectives, depression and aging could interact to produce the opposite pattern: greater divergence in reward function between healthy and depressed adults with aging. Adults with depression undergo more rapid aging and higher rates of aging-related illness and cognitive decline (Rapp, et al., 2010). In addition, the kindling hypothesis (Post, 2007)—which postulates that episodes occur more spontaneously and are more severe with the progression of affective disorders—could predict that reward function becomes more unusual with clinical course. Future longitudinal studies are critical for settling this issue.
Depression and Individual Differences in Reward Function
From a different perspective, stable individual differences in reward function could lead some people to have low reward function throughout the lifespan, regardless of episode status. Low reward function has been proposed as a candidate endophenotype of depression, reflecting evidence that it is a risk characteristic that is likely to be heritable, not immediately evident in behavior, present regardless of illness state (i.e., before, during, and after depressive episodes), and predictive of onset (Hasler, et al., 2004). Consistent with this view, nondepressed offspring of depressed parents, a population at high risk of developing depression (Goodman, 2007), exhibit reduced reward-related brain function in response to monetary (Gotlib, et al., 2010) and social reward (Monk, et al., 2008). Reward-related decision-making predicts later depressive disorders in adolescents, even when adjusting for previous depression (Forbes, et al., 2007). In those with depression, reward-related brain function does not seem to improve appreciably between episodes or after treatment (McCabe, Cowen, & Harmer, 2009), and behavioral sensitivity to catecholamine depletion is greater (Hasler, et al., 2009). Prospective studies and studies of high-risk groups are needed to disentangle stable individual differences and episodic changes in reward function. The seeming paradox between low reward function as both a stable individual difference and a characteristic more evident in depression during adolescence is illustrated by Figure 2, which depicts level of reward function with development and indicates that normal developmental change in reward function could reveal depression-related differences most clearly during adolescence.
Figure 2.
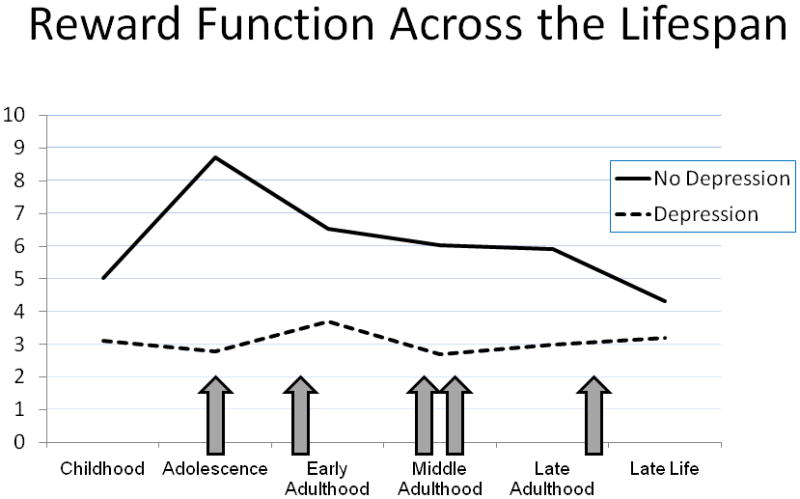
Conceptual model of reward function across the lifespan, depicted separately for depressed and non-depressed populations. Arrows indicate timing of hypothetical depressive episodes. Between-group differences are most evident during adolescence, when levels of reward function are particularly high in the non-depressed group. At the same time, reward function in the depressed group is fairly stable across development and clinical course. Units are arbitrary.
How Is Reward Function Disrupted in Adolescent Depression?
One clue about the mechanisms by which reward function may be disrupted in depression is the pattern, described above, of frontostriatal function: low reactivity in the striatum plus high reactivity in the mPFC. As indicated by our preliminary findings on treatment response in adolescent depression, this pattern also appears to be predictive of worse response to treatment (Forbes, Olino, et al., 2010). In addition, the effortful regulation of positive affect reduces frontostriatal connectivity in adults with depression (Heller, et al., 2009), and behavioral activation therapy alters neural response in both the striatum and mPFC (Dichter, et al., 2009).
There are several possible patterns of disruption in neural circuits including the striatum and mPFC in depression. One possibility, suggested by findings that depressed adolescents tend to dampen positive affect (Feldman, Joormann, & Johnson, 2008), is that a typical initial response to reward occurs in the striatum but is over-regulated by the mPFC, resulting in attenuated intensity and duration. Relatedly, adults with depression exhibit negative, top-down functional connectivity between the ventromedial prefrontal cortex and the amygdala while processing happy faces (Almeida, et al., 2009). A second possibility is that the striatum is less reactive to reward, and increased mPFC function reflects signaling to enhance neural response or elicit dopamine release. Support for low striatal reactivity includes findings of low dopamine release in depression (Martin-Soelch et al., 2008, cf Price & Drevets, 2010). Support for the mPFC’s role in dopamine release includes rodent findings that stimulation of the mPFC elicits greater activity in the ventral tegmental area (e.g. Taber & Fibiger, 1993). A third possibility is that both regions have altered function: the initial striatal response to reward is diminished and, in parallel, the mPFC is excessively engaged in self-processing. Given the mPFC’s role in processing self-relevant material (Passingham, Bengtsson, & Lau, 2010) and in the default mode network that is more active in depressed than healthy adults at rest (Sheline, et al., 2009) and during affective processing (Grimm, et al., 2009), the mPFC might be engaged in the negative, self-focused ruminations that are considered a hallmark of the development of depression (Hankin, et al., 2009). This tendency could lead to difficulty shifting away from self-focused processing in the presence of external rewards. Characterizing the association of these two reward-related brain areas will require additional functional connectivity studies, as well as prospective and longitudinal designs.
Another issue is how this pattern of neural function arises. Developmentally, the typical changes in reward function during adolescence could lead to altered function in frontostriatal circuits that persists over time and launches some adolescents onto a trajectory of depression. Consistent with this view, achieving balance among prefrontal and subcortical affective regions is considered a key developmental task of adolescence (Ernst & Fudge, 2009), and the rapid changes in both reward and executive function systems during adolescence has been conceptualized as etiologically relevant for depression (Davey, et al., 2008).
Conclusions
Treatment Implications
While far from providing practical guidance for diagnosis or intervention, findings on reward function in adolescent depression ideally have potential clinical implications by suggesting targets for treatment. To illustrate, the three proposed neural mechanisms leading to altered reward-related brain function suggest different and possibly promising approaches. If reward function is disrupted through over-regulation of striatal response by mPFC, an mindfulness intervention, as applied to chronic adult depression, might aim to reduce over-regulation (Kuyken, et al., 2008). If reward function is disrupted through low initial striatal response to reward, perhaps initial reactivity could be strengthened through pharmacologic means. If mPFC is ineffective at enhancing striatal response, this circuit could be targeted through training to savor pleasant experiences (McMakin, Siegle, & Shirk, In Press).
Consideration of reward function in adolescent depression raises the issue of personalized interventions. Reward function is not uniformly disrupted in adolescents with depression (Forbes, et al., 2009), and response to treatment is also variable (Kennard, et al., 2006). Greater attention to potential endophenotypes and biomarkers can contribute to understanding which adolescents will respond to which treatments (Brent & Maalouf, 2009). Investigating reward function can be a model for this approach. Recently, we have reported in a small sample of adolescents with depression that reward-related brain function before treatment with either cognitive behavioral therapy or cognitive behavioral therapy plus selective serotonin reuptake inhibitors predicted both severity and symptoms post-treatment and rate of anxiety symptom reduction during treatment (Forbes, Olino, et al., 2010). This is the first study to identify a potential biomarker of treatment response in adolescent depression.
We do not intend to suggest that the study of reward function in depression has led the field to a point at which we will use fMRI scans to diagnose depression or to select the most effective treatment in adolescents. In addition, findings on brain function have yet to lead to efficacious treatments. Instead, we make the optimistic prediction that understanding the neural mechanisms underlying altered reward function can lead us to a better description of the pathophysiology and, ultimately, to treatments addressing the mechanisms of depression.
Recommendations for Future Research
There is much to be done to understand the role of reward function in the development of depression. An important example is the need for a more rigorous, empirical definition of anhedonia and a conceptual model for its association with reward function in depression. Anhedonia is considered a core symptom of depression (American Psychiatric Association, 1994) is present in 76% of adolescents with depression (Lewinsohn, Pettit, Joiner, & Seeley, 2003), may have important predictive value for depression in young people, and is associated with reduced striatal response in adult depression (Keedwell, et al., 2005a). But the construct’s definition is inconsistent, with varying attention to physical, social, and motivational features (Chapman, Chapman, & Raulin, 1976), as well as to its reflection of unusual appetitive or consummatory processes of reward function (Snaith, 1993). Anhedonia is often understood to reflect a complete absence of the experience of pleasure, but clinical impressions indicate that such an affective state is rare in adolescents and that low motivation to obtain reward—rather than low experience of pleasure once reward is obtained—might be a more appropriate focus. As an example of a fruitful approach, a recent review has proposed that anhedonia occurs in depression through alterations of decision-making processes that are associated with motivational or “wanting” aspects of reward function and mediated by dopamine function (Treadway & Zald, 2010). Defining anhedonia in terms of neural, behavioral, and subjective aspects of reward function; examining its association with different components of reward processing; understanding it in developmental context; and improving its measurement could strengthen the foundation for research on reward function in depression.
The interplay of threat and reward systems is a critical topic, given the role of positive affect in regulating negative affect (Garland, et al., 2010) and the emphasis of early studies on depression’s association with bias toward feedback about failure (Elliott, et al., 1997). Peer-reared monkeys, who exhibit behavior similar to human anxiety and depression, exhibit heightened behavioral response to reward (Nelson, et al., 2009). This finding was interpreted as evidence of a possible attempt to regulate negative affect with reward-related behavior. Similarly, the recent report that genes relevant to endocannabinoid function are associated with both reduced neural response to threat and enhanced neural response to reward (Hariri, et al., 2009) point to common genetic pathways for influencing the two affective systems. Also, the context of altered reward function could be considered more carefully. For instance, we have recently examined depression effects on striatal response to reward anticipation based on the outcome of the previous trial (Olino, et al., in press).
Finally, the role of comorbid anxiety in reward function in depression has been ignored, despite the frequent co-occurrence of the two types of affective disturbance (Kessler, et al., 2008). Both types of disorders are postulated to involve disrupted threat processing (Clark & Watson, 1991), and neuroimaging evidence supports this claim (Britton et al., 2011; Savitz & Drevets, 2009). Although affective models of psychopathology claim that only depression is associated with altered reward function (Clark & Watson, 1991), recent evidence indicates that reward function is also altered in anxiety. Contrary to depression, adolescent anxiety is associated with greater striatal response to reward anticipation (Bar-Haim, et al., 2009). Anxiety could thus involve enhanced sensitivity to potential feedback or uncertainty. Because the amygdala, a key region in threat circuits, influences the nucleus accumbens (McGinty & Grace, 2008), and adolescents’ reward-related striatal function predicts reduction in anxiety symptoms with treatment (Forbes, Olino, et al., 2010), enhanced striatal reactivity in anxiety could reflect vigilance for threat. With this intriguing pattern of findings on reward and anxiety, further research on reward function has the potential to provide insights on the differences between anxiety and depression.
Summary
We hope that our attempts to interpret the extant literature and to identify central themes will provoke thought and discussion and will, ideally, inspire future studies. While our hypotheses have necessarily gone beyond the implications of the present evidence, we believe that they can have some value for conceptualizing and investigating reward function in depression.
Key Points.
Altered reward function is postulated to be critical to the development and pathophysiology of depression.
This review proposes that (1) physiological, behavioral, and subjective aspects of reward function are disrupted; (2) altered reward function is most evident in adolescent depression and is stably low in those with depression; and (3) altered reward function is related to the pattern of reactivity of the striatum and medial prefrontal cortex.
A more comprehensive understanding of mechanisms of reward function in adolescent depression will help to develop and personalize treatments.
Future research should include longitudinal and cross-sectional designs employed prospectively and across clinical course.
Acknowledgments
This work was supported by NIH K01 MH74769 (PI: Erika E. Forbes), NIDA R01 DA026222 (PIs: Erika E. Forbes and Daniel S. Shaw), NIH P50 MH080215 (PI: Neal D. Ryan), NIDA R01 DA018910 (PI: Ronald E. Dahl), and a NARSAD Young Investigator Award (PI: Erika E. Forbes). We thank Eric Rodriguez for assistance with references.
Abbreviations
- mPFC
medial prefrontal cortex
References
- Allen NB, Badcock PBT. The social risk hypothesis of depressed mood: Evolutionary, psychosocial, and neurobiological perspectives. Psychological Bulletin. 2003;129(6):1–28. doi: 10.1037/0033-2909.129.6.887. [DOI] [PubMed] [Google Scholar]
- Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biological Psychiatry. 2009;66(5):451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders (DSM-IV) 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child and Adolescent Psychiatric Clinics of North America. 2006;15(4):919–937. doi: 10.1016/j.chc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, et al. Neural Correlates of Reward Processing in Adolescents With a History of Inhibited Temperament. Psychological Science. 2009 doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. Journal of Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, Allen NB. Neurobiology of human affiliative behaviour: implications for psychiatric disorders. Current Opinion in Psychiatry. 2009;22(3):320–325. doi: 10.1097/YCO.0b013e328329e970. [DOI] [PubMed] [Google Scholar]
- Brent DA, Maalouf FT. Pediatric depression: is there evidence to improve evidence-based treatments? Journal of Child Psychology and Psychiatry. 2009;50(1–2):143–152. doi: 10.1111/j.1469-7610.2008.02037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: the role of threat appraisal and fear learning. Depression and Anxiety. 2011;28(1):5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SB, Ridderinkhof KR. Aging and the neuroeconomics of decision making: A review. Cognitive, Affective, & Behavioral Neuroscience. 2009;9(4):365–379. doi: 10.3758/CABN.9.4.365. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Klaver JM, Peck SA, Rallis-Voak D, Erickson K, Drevets WC. Dopamine type-1 receptor binding in major depressive disorder assessed using positron emission tomography and [11C]NNC-112. Neuropsychopharmacology. 2009;34(5):1277–1287. doi: 10.1038/npp.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Dymond S, Cooper A. Impaired flexible decision-making in Major Depressive Disorder. Journal of Affective Disorders. 2010;124(1–2):207–210. doi: 10.1016/j.jad.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chase HW, Frank MJ, Michael A, Bullmore ET, Sahakian BJ, Robbins TW. Approach and avoidance learning in patients with major depression and healthy controls: relation to anhedonia. Psychological Medicine. 2010;40(3):433–440. doi: 10.1017/S0033291709990468. [DOI] [PubMed] [Google Scholar]
- Chase HW, Michael A, Bullmore ET, Sahakian BJ, Robbins TW. Paradoxical enhancement of choice reaction time performance in patients with major depression. Journal of Psychopharmacology. 2010;24(4):471–479. doi: 10.1177/0269881109104883. [DOI] [PubMed] [Google Scholar]
- Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: A pharmacological perspective. Alcohol Research and Health. 2008;31(4):310–339. [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, et al. A unique adolescent response to reward prediction errors. Nature Neuroscience. 2010;13(6):669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin J, Peselow E, Feenan K, Rotrosen J, Fieve R. Disorders of decision in affective disease: an effect of beta-adrenergic dysfunction? Biological Psychiatry. 1990;27(8):813–833. doi: 10.1016/0006-3223(90)90463-c. [DOI] [PubMed] [Google Scholar]
- D’Haenen HA, Bossuyt A. Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biological Psychiatry. 1994;35(2):128–132. doi: 10.1016/0006-3223(94)91202-5. [DOI] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yucel M. Being liked activates primary reward and midline self-related brain regions. Human Brain Mapping. 2009 doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biological Psychiatry. 2009;66(9):886–897. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Clark L, Siegle GJ, Butters MA, Ichikawa N, Sahakian BJ, et al. Reward/Punishment reversal learning in older suicide attempters. The American Journal of Psychiatry. 2010;167(6):699–707. doi: 10.1176/appi.ajp.2009.09030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(39):15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Loew T, Pirner A. Dopamine and depression--striatal dopamine D2 receptor SPECT before and after antidepressant therapy. Psychopharmacology. 1996;126(1):91–94. doi: 10.1007/BF02246416. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Herrod JJ, Robbins TW, Paykel ES. Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. Journal of Neurology, Neurosurgery and Psychiatry. 1997;63(1):74–82. doi: 10.1136/jnnp.63.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mansari M, Guiard BP, Chernoloz O, Ghanbari R, Katz N, Blier P. Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neuroscience & Therapeutics. 2010;16(3):e1–17. doi: 10.1111/j.1755-5949.2010.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. The American Journal of Psychiatry. 2006;163(10):1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33(3):367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Feldman GC, Joormann J, Johnson SL. Responses to positive affect: A self-report measure of rumination and dampening. Cognitive Therapy and Research. 2008;32:507–525. doi: 10.1007/s10608-006-9083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE. FMRI studies of reward processing in adolescent depression. Neuropsychopharmacology. 2011;36(1):372–373. doi: 10.1038/npp.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain and Cognition. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American Journal of Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, May JC, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. Journal of Child Psychology and Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, et al. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cognitive, Affective, & Behavioral Neuroscience. 2010;10(1):107–118. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, et al. Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:162–172. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biological Psychiatry. 2007;61(5):633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Friedman A, Friedman Y, Dremencov E, Yadid G. VTA dopamine neuron bursting is altered in an animal model of depression and corrected by desipramine. Journal of Molecular Neuroscience. 2008;34(3):201–209. doi: 10.1007/s12031-007-9016-8. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Fredrickson B, Kring AM, Johnson DP, Meyer PS, Penn DL. Upward spirals of positive emotions counter downward spirals of negativity: Insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review. 2010 doi: 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annu Rev Clin Psychol. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry. 2010;67(4):380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharmacology. 2007;53(5):583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34(4):932–843. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Oppenheimer C, Jenness J, Barrocas A, Shapero BG, Goldband J. Developmental origins of cognitive vulnerabilities to depression: review of processes contributing to stability and change across time. Journal of Clinical Psychology. 2009;65(12):1327–1338. doi: 10.1002/jclp.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. Journal of Child Psychology and Psychiatry. 2007;48(5):446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biological Psychiatry. 2009;66(1):9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004 doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hasler G, Luckenbaugh DA, Snow J, Meyers N, Waldeck T, Geraci M, et al. Reward processing after catecholamine depletion in unmedicated, remitted subjects with major depressive disorder. Biological Psychiatry. 2009;66(3):201–205. doi: 10.1016/j.biopsych.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biological Psychiatry. 2005;58(8):632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Jr, Lewinsohn PM, Seeley JR. The core of loneliness: Lack of pleasurable engagement -- more so than painful disconnection -- predicts social impairment, depression onset, and recovery from depressive disorders among adolescents. Journal of Personality Assessment. 2002;79:472–491. doi: 10.1207/S15327752JPA7903_05. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Jr, Lonigan CJ. Tripartite model of depression and anxiety in youth psychiatric inpatients: Relations with diagnostic status and future symptoms. Journal of Clinical Child Psychology. 2000;29(3):372–382. doi: 10.1207/S15374424JCCP2903_8. [DOI] [PubMed] [Google Scholar]
- Kaplan JS, Erickson K, Luckenbaugh DA, Weiland-Fiedler P, Geraci M, Sahakian BJ, et al. Differential performance on tasks of affective processing and decision-making in patients with Panic Disorder and Panic Disorder with comorbid Major Depressive Disorder. Journal of Affective Disorders. 2006;95(1–3):165–171. doi: 10.1016/j.jad.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biological Psychiatry. 2005a;58(6):495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in Major Depressive Disorder. Biological Psychiatry. 2005b;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kennard B, Silva S, Vitiello B, Curry J, Kratochvil C, Simons A, et al. Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS) Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(12):1404–1411. doi: 10.1097/01.chi.0000242228.75516.21. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Merikangas K. Mood disorders in children and adolescents: An epidemiologic perspective. Biological Psychiatry. 2001;49(12):1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychological Medicine. 2008;38(3):365–374. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biological Psychiatry. 2002;52(7):740–748. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- Klimke A, Larisch R, Janz A, Vosberg H, Muller-Gartner HW, Gaebel W. Dopamine D2 receptor binding before and after treatment of major depression measured by [123I]IBZM SPECT. Psychiatry Research. 1999;90(2):91–101. doi: 10.1016/s0925-4927(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63(7):686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann U. Differential age trajectories of positive and negative affect: further evidence from the Berlin Aging Study. Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2008;63(5):P261–270. doi: 10.1093/geronb/63.5.p261. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76(6):966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Kyte ZA, Goodyer IM, Sahakian BJ. Selected executive skills in adolescents with recent first episode major depression. Journal of Child Psychology and Psychiatry. 2005;46(9):995–1005. doi: 10.1111/j.1469-7610.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- Larson RW, Moneta G, Richards MH, Wilson S. Continuity, stability, and change in daily emotional experience across adolescence. Child Development. 2002;73(4):1151–1165. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neuroscience and Biobehavioral Reviews. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lempert KM, Pizzagalli DA. Delay discounting and future-directed thinking in anhedonic individuals. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41(3):258–264. doi: 10.1016/j.jbtep.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major depression in community adolescents: Age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33(6):809–818. doi: 10.1097/00004583-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Pettit JW, Joiner TE, Jr, Seeley JR. The symptomatic expression of major depressive disorder in adolescents and young adults. Journal of Abnormal Psychology. 2003;112(2):244–252. doi: 10.1037/0021-843x.112.2.244. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Klein DN, Seeley JR. Natural course of adolescent major depressive disorder: I. Continuity into young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(1):56–63. doi: 10.1097/00004583-199901000-00020. [DOI] [PubMed] [Google Scholar]
- Lindstrom KM, Guyer AE, Mogg K, Bradley BP, Fox NA, Ernst M, et al. Normative data on development of neural and behavioral mechanisms underlying attention orienting toward social-emotional stimuli: an exploratory study. Brain Research. 2009;1292:61–70. doi: 10.1016/j.brainres.2009.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi G, Liboni F, Brovedani P. Pharmacotherapy of major depressive disorder in adolescents. Expert Opinion on Pharmacotherapy. 2010;11(3):375–386. doi: 10.1517/14656560903527226. [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205(4):667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullumsmith RE, Meador-Woodruff JH. Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology. 2002;26(3):368–375. doi: 10.1016/S0893-133X(01)00370-0. [DOI] [PubMed] [Google Scholar]
- McGinty VB, Grace AA. Selective activation of medial prefrontal-to-accumbens projection neurons by amygdala stimulation and Pavlovian conditioned stimuli. Cerebral Cortex. 2008;18(8):1961–1972. doi: 10.1093/cercor/bhm223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin D, Siegle G, Shirk S. Positivie Affect Stimulation and Sustainment (PASS) module for depressed mood: A preliminary investigation of treatment-related effects. Cognitive Therapy and Research. doi: 10.1007/s10608-010-9311-5. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManis MH, Bradley MM, Berg WK, Cuthbert BN, Lang PJ. Emotional reactions in children: verbal, physiological, and behavioral responses to affective pictures. Psychophysiology. 2001;38(2):222–231. [PubMed] [Google Scholar]
- Mell T, Heekeren HR, Marschner A, Wartenburger I, Villringer A, Reischies FM. Effect of aging on stimulus-reward association learning. Neuropsychologia. 2005;43(4):554–563. doi: 10.1016/j.neuropsychologia.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Mell T, Wartenburger I, Marschner A, Villringer A, Reischies FM, Heekeren HR. Altered function of ventral striatum during reward-based decision making in old age. Frontiers in Human Neuroscience. 2009;3:34. doi: 10.3389/neuro.09.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Kruger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, et al. Lower dopamine transporter binding potential in striatum during depression. Neuroreport. 2001;12(18):4121–4125. doi: 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology. 2008;20(4):1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. The American Journal of Psychiatry. 2008;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Rohde P, Seeley JR, Lewinsohn PM. Life events and depression in adolescence: Relationship loss as a prospective risk factor for first onset of major depressive disorder. Journal of Abnormal Psychology. 1999;108(4):606–614. doi: 10.1037//0021-843x.108.4.606. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Sakamoto K, Saito N, Inui T, Aihara K, Tanji J. Involvement of the prefrontal cortex in problem solving. International Review of Neurobiology. 2009;85:1–11. doi: 10.1016/S0074-7742(09)85001-0. [DOI] [PubMed] [Google Scholar]
- Nandrino JL, Dodin V, Martin P, Henniaux M. Emotional information processing in first and recurrent major depressive episodes. Journal of Psychiatric Research. 2004;38(5):475–484. doi: 10.1016/j.jpsychires.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Herman KN, Barrett CE, Noble PL, Wojteczko K, Chisholm K, et al. Adverse rearing experiences enhance responding to both aversive and rewarding stimuli in juvenile rhesus monkeys. Biological Psychiatry. 2009;66(7):702–704. doi: 10.1016/j.biopsych.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Dahl RE, Ryan ND, Birmaher B, Axelson DA, et al. “I won, but I’m not getting my hopes up”: Depression moderates the relationship between winning and reward anticipation. Psychiatry Research: Neuroimaging. doi: 10.1016/j.pscychresns.2011.04.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Fiorillo CD, Morikawa H, Williams JT. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nature Neuroscience. 2001;4(3):275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Bengtsson SL, Lau HC. Medial frontal cortex: from self-generated action to reflection on one’s own performance. Trends in Cognitive Science. 2010;14(1):16–21. doi: 10.1016/j.tics.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: Moodiness or mood disorder? The American Journal of Psychiatry. 1999;156(1):133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallet LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. Journal of Psychiatric Research. 2008;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biological Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena. Neuroscience and Biobehavioral Reviews. 2007;31(6):858–873. doi: 10.1016/j.neubiorev.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Wysocki M, Guerrero-Berroa E, Grossman HT, Heinz A, et al. Cognitive Decline in Patients With Dementia as a Function of Depression. American Journal of Geriatric Psychiatry. 2010 doi: 10.1097/JGP.0b013e3181e898d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, van Balkom AJ, Hendriks GJ, Hoogendijk WJ, Uylings HB, et al. Differential frontal-striatal and paralimbic activity during reversal learning in major depressive disorder and obsessive-compulsive disorder. Psychological Medicine. 2009;39(9):1503–1518. doi: 10.1017/S0033291708005072. [DOI] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164(1):300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer MG, Pfeiffer S, Spence SH. Life events, coping and depressive symptoms among young adolescents: a one-year prospective study. Journal of Affective Disorders. 2009;117(1–2):48–54. doi: 10.1016/j.jad.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Schott BH, Niehaus L, Wittmann BC, Schutze H, Seidenbecher CI, Heinze HJ, et al. Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain. 2007;130(Pt 9):2412–2424. doi: 10.1093/brain/awm147. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeber, Allen NB, Leve C, Davis B, Shortt JW, Katz LF. Dynamics of affective experience and behavior in depressed adolescents. Journal of Child Psychology and Psychiatry. 2009;50(11):1419–1427. doi: 10.1111/j.1469-7610.2009.02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeber, Hops H, Davis B. Family processes in adolescent depression. Clinical Child and Family Psychology Review. 2001;4(1):19–35. doi: 10.1023/a:1009524626436. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders. 2009;118(1–3):69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith P. Anhedonia: a neglected symptom of psychopathology. Psychological Medicine. 1993;23(4):957–966. doi: 10.1017/s0033291700026428. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A neurobehavioral perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Electrical stimulation of the medial prefrontal cortex increases dopamine release in the striatum. Neuropsychopharmacology. 1993;9(4):271–275. doi: 10.1038/npp.1993.63. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Oono H, Inoue T, Boku S, Kako Y, Kitaichi Y, et al. Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects--an analysis based on Tsallis’ statistics. Neuroendocrinology Letters. 2008;29(3):351–358. [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews. 2010 doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: altered response to dextroamphetamine. Archives of General Psychiatry. 2002;59(5):409–416. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of General Psychiatry. 2005;62(11):1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yap MB, Yucel M, Sheeber L, Simmons JG, Pantelis C, et al. Maternal responses to adolescent positive affect are associated with adolescents’ reward neuroanatomy. Cognitive, Affective, & Behavioral Neuroscience. 2009;4(3):247–256. doi: 10.1093/scan/nsp012. [DOI] [PMC free article] [PubMed] [Google Scholar]


