Abstract
OBJECTIVE
Oral infection is considered to play a critical role in the pathogenesis of bisphosphonate-related osteonecrosis of the jaw (BRONJ) and antibiotic therapy has become a mainstay of BRONJ therapy. This study was aimed to investigate the effect of antibiotics on bacterial diversity in BRONJ tissues.
MATERIALS AND METHODS
The bacterial profile from soft tissues associated with the BRONJ lesion was determined using 16S rRNA-based denaturing gradient gel electrophoresis (DGGE) and sequencing. Twenty BRONJ subjects classified as stage 0 to 2 were enrolled in this study and patient groups were divided into an antibiotic cohort (n=10) treated with systemic antibiotic and a non-antibiotic cohort (n=10) with no prior antibiotic therapy.
RESULTS
The DGGE fingerprints indicated no significant differences in bacterial diversity of BRONJ tissue samples. Patients on antibiotics had higher relative abundance of phylum Firmicutes with bacterial species, Streptococcus intermedius, Lactobacillus gasseri, Mogibacterium timidum and Solobacterium moorei whereas patients without antibiotics had greater amounts of Parvimonas micra, and S. anginosus. Thirty percent of bacterial populations were uncultured (yet-to be cultured) phylotypes.
CONCLUSION
This study using limited sample size indicated that oral antibiotic therapy may have a limited efficacy on the bacterial population associated with BRONJ lesions.
Keywords: BRONJ, antibiotics, DGGE, microbial diversity
Introduction
Bisphosphonate related osteonecrosis of the jaw (BRONJ) is most frequently defined by current or previous treatment with bisphosphonates (BPs), exposed bone in the maxillofacial region for more than 8 weeks and no history of radiation therapy to the jaws (Ruggiero et al., 2009). These drugs are widely used for the prevention and treatment of osteoporosis (Kamel, 2007) and skeletal complications associated with metastatic cancer, breast cancer and multiple myeloma (Coleman, 2008). These cancer patients are immune-compromised and have reduced regenerative capability due to cancer treatment whereas osteoporotic patients have disturbed level of bone remodeling (Ruggiero, 2011). These osteoporotic patients often suffer from other chronic diseases and have increased risk of developing opportunistic infections which may relate to reduce bone mineral density and oral alveolar bone loss, premature teeth loss and increased severity of periodontal diseases (Kyrgidis et al., 2011). A causal relationship between BPs and BRONJ has not been well understood (Dodson, 2009) and current information on incidence and prevalence is inconclusive (Gliklich & Wilson, 2009). Therefore, understanding the underlying pathogenesis and risk are of paramount importance. The general risk factors may be classified as local (e.g., poor oral hygiene, dental extraction, infection), systemic (e.g., cancer, metastasis, chemotherapy, smoking, obesity and malnutrition) and drug-related (e.g., duration of BP therapy, steroid use) (Gebara & Moubayed, 2009, Ruggiero et al., 2009, Wessel et al., 2008).
Common dilemmas arise because discontinuing BP therapy prior to dental procedures does not prevent or improve BRONJ (Krueger et al., 2007, Diel et al., 2007) and poor oral hygiene or periodontal disease may not correlate with risk assessment (Estilo et al., 2008). Patient management is further complicated because recommendations have focused on avoiding dental extractions (Allam et al., 2011, Fleisher et al., 2008) while BRONJ may be triggered by infection(Hoefert & Eufinger, 2011, Kaplan et al., 2009, Kumar et al., 2010, Abraham et al., 2009, Wong et al., 2010). Effective treatment regimens for BRONJ have not been well established (Migliorati et al., 2005). While nonsurgical conservative therapy such as oral rinses, antibiotics, analgesics, and discontinuing BP therapy have been suggested (Van den Wyngaert et al., 2007), patients may not respond to these approaches (Hoff et al., 2008, Williamson, 2010, Otto et al., 2009).
Several possible mechanisms of BRONJ pathogenesis have been suggested which includes ischemia, reduced bone turnover, toxicity to bone, toxicity to soft tissue, microcracks, inflammation and infection (Hoefert et al., 2010, Kumar et al., 2010, Lesclous et al., 2009, Reid, 2009). Oral infection and inflammation are believed to play a critical role in the pathogenesis of BRONJ. According to Reid, (2009), bone necrosis in patients taking BPs transpires as a result of pre-existing dental infection that is odontogenic or periodontal and eventually spreads to the bone culminating into bisphosphonate-related ONJ (BRONJ). Sedghizadeh et al., (2008) have reported that microbial biofilms may play a role in the etiopathogenesis of 65% to 80% of the chronic human infections.
More than 750 species are reported to inhabit the human oral cavity (Jenkinson & Lamont, 2005) and most of the oral microbiota are organized in complex multispecies biofilms attached to the surfaces of teeth and oral soft tissues (Smoot et al., 2005). A biofilm is a complex community of sessile microbes attached to a substrate (Donlan & Costerton, 2002, Kuramitsu et al., 2007, Sedghizadeh et al., 2009). As reports of histological assessments of BRONJ become more numerous, evidence links specific microbial infection to BRONJ and Actinomyces representing phylum Actinobacteria is a common finding (Kaplan et al., 2009, Kumar et al., 2010, Naik & Russo, 2009). Confirmation of the infectional theory of the condition might result in more rational treatment with antibiotics, with a special reference to the efficient system of application of antibiotics to the hypovascular and hypocellular bone (Kos & Luczak, 2009). The use of oral antimicrobial rinses in combination with oral systemic antibiotic therapy; penicillin, metronidazole, quinolones, clindamycin, doxycycline, erythromycin is indicated for Stages I and II of BRONJ (Ruggiero et al., 2009, Vescovi & Nammour, 2010). Here we report the effect of antibiotic administered for treating infection after the onset of BRONJ. Using 16S rDNA molecular technique we determined and compared the changes in the bacterial population in soft tissues of BRONJ lesion collected from: an antibiotic cohort treated with antibiotic and non-antibiotic cohort with no prior antibiotic treatment.
Materials and methods
Subjects and specimen collection
A total of 20 patients (16 females and 4 males) undergoing BRONJ treatment at New York University College of Dentistry were recruited for this study. The age range was 49 – 84 years, mean 67.05 years. The study was approved by the Institutional Review Board of New York University for human subjects and subjects agreed to participate with their informed consent. These subjects were patients with breast cancer, renal/rectal cancer, multiple myeloma on intravenous BPs (n=14) and those with osteoporosis on oral BPs (n=6). BRONJ subjects were classified as either stage 0, I or II as defined by Ruggiero et al., (2009) and divided into two cohorts: an antibiotic cohort (n=10) treated with systemic antibiotic therapy and non-antibiotic cohort (n=10) with no prior antibiotic therapy (Table 1). For antibiotic cohort, we selected BRONJ subject population on a range of antibiotics. The subjects treated with systemic antibiotics were administered either one of these; tetracycline, doxycycline, ciprofloxacin or amoxicillin for two weeks (Table 1). Specimens were collected from soft tissues associated with the BRONJ lesion and transported in sterile vials containing Tris-EDTA buffer and stored at −20°C.
Table 1.
Data of patients with BRONJ
| Sample name | Age | Sex | Staging of BRONJ | Type of antibiotic | History | BP | Location |
|---|---|---|---|---|---|---|---|
| OT 1 | 69 | M | I | Tetracycline | Renal cancer | IV | Mx |
| OT 2 | 72 | F | I | Tetracycline | Multiple myeloma | IV | Mn |
| OT 3 | 81 | F | II | Tetracycline | Breast cancer | IV | Mn |
| OT 4 | 49 | M | I | Ciprofloxacin | Breast cancer | IV | Mx |
| OT 5 | 84 | F | II | Amoxicillin (discontinued 1 week prior to Tx) | Breast cancer | IV | Non-restorable tooth #18 |
| OT 6 | 56 | F | I | Breast cancer | IV | Mn | |
| OT 7 | 79 | F | I | Osteoporosis | O | Mn | |
| OT 8 | 72 | F | I | Osteoporosis | O | Mn | |
| OT 9 | 73 | F | II | Osteoporosis | O | Mx | |
| OT 10 | 57 | M | I | Rectal cancer | IV | Mx | |
| OT 11 | 62 | F | 0 | Breast cancer | IV | Mn | |
| OT 12 | 56 | F | 0 | Breast cancer | IV | Non-restorable tooth #18 | |
| OT 13 | 72 | F | II | Osteoporosis | O | Mx | |
| OT 14 | 53 | M | I | Tetracycline | Multiple myeloma | IV | Mn |
| OT 15 | 76 | F | II | Tetracycline | Colon cancer | IV | Mn |
| OT 16 | 71 | F | II | Osteoporosis | O | Palatal tori | |
| OT 17 | 65 | F | II | Doxycycline | Multiple myeloma | IV | Mn |
| OT 18 | 57 | F | I | Doxycycline | Breast cancer | IV | Mn |
| OT 19 | 56 | F | I | Doxycycline | Breast cancer | IV | Mn |
| OT 20 | 72 | F | II | Osteoporosis | O | Mn |
F-Female; M- Male; IV-Intravenous: O-Oral; Mn-Mandible; Mx-Maxilla
Regimen/Formulation/Dosages: Tetracycline 250 mg QID × 2 weeks; Doxycycline 100 mg BID × 2 weeks; Ciprofloxacin 100 mg × 2 weeks
DNA extraction from BRONJ tissues
Each specimen was suspended in 500 μl of sterile phosphate-buffered saline (PBS), vortexed for 30 sec and sonicated for 5 and 10 sec respectively. Further, pretreated with Proteinase K (2.5 μg/ml) with overnight incubation at 55°C, and if required, homogenized with sterile disposable pestle and vortexed (Hooper et al., 2007). Bacterial genomic DNA was extracted by modified Epicentre protocol (Epicentre Biotechnologies, Madison, WI) and purified by phenol-chloroform extraction (Li et al., 2007, Pushalkar et al., 2011). Qualitative and quantitative analysis of samples was done by NanoDrop ND 1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE). The samples were stored at −20°C till analyzed further. The DNA concentration was adjusted to 10 ng/μl for all experiments.
16S rDNA amplification
16S rDNA genes from bacterial DNA were amplified with universal eubacterial primers, forward primer 8f (5′-AGTTGATCCTGGCTCAG-3′) and reverse primer 1492r (5′-ACCTTGTTACGACTT-3′) (Lane, 1991) followed by nested PCR targeting V4-V 5 hypervariable region with primers prbac 1 (5′-CGCCCGGGGCGCGCCCCGGGCGGGGCGGGGGCACGGGGGGA-CTACGTGCCAGCAGCC-3′) with 40-nucleotide GC clamp at 5′ end of bac1 (Sheffield et al., 1989) and prbac2 (5′-GGACTACCAGGGTATCTAATCC-3′) (Rupf et al., 1999). Each PCR reaction mix (50 μl) consisted of 1 μl DNA, 5 U Taq DNA polymerase (Invitogen, Carlsbad, CA), 5 μl 10x PCR buffer, 4 μl dNTP mixture (2.5 mM each) and 50 pmole of each primer. The reactions were performed at 95°C for 5 min, followed by 30 thermal cycles of 95°C (1 min), 52°C (1 min), and 72°C (1 min) with final elongation at 72°C (5 min). These PCR products were template for nested PCR. The nested PCR consisted of preheating at 94°C (3 min) with 35 cycles each at 94°C (30 sec), 63°C (40 sec), and 72°C (1 min) and final extension at 72°C for 7 min.
Denaturing gradient gel electrophoresis (DGGE) assay
PCR products from nested PCR were resolved on 40% to 60% linear DNA denaturing gradient polyacrylamide gel (8.0% w/v) for sequence differentiation. DGGE gel was loaded with 30 μl each of samples alongside standard species-specific DGGE reference markers (Li et al., 2005, Pushalkar et al., 2011) using DCode system (BioRad, Hercules, CA). Electrophoresis was carried out for 16 hr at 58°C and 60V in 1x Tris-acetate-EDTA (TAE) buffer, pH 8.5 and stained in ethidium bromide solution (0.5 μg/ml) for 15 min. Gel images were documented using Alpha Imager 3300 system (Alpha Innotech Corporation, San Leandro, CA).
Construction of dendrogram and cluster analysis
DGGE gels were analyzed with Fingerprinting II Informatix Software (BioRad) and statistically interpreted (Li et al., 2007, Li et al., 2006, Pushalkar et al., 2011). The gels were normalized by standard DGGE markers and the background subtracted using mathematical algorithms. The similarity calculated by Dice coefficient and dendrogram constructed by Ward analysis. The Shannon diversity index was calculated by Mann-Whitney U test. Statistical analysis was performed using SPSS software v. 17.0 (SPSS inc., Chicago, IL).
Cloning and sequencing
PCR products from ten samples were ligated to pCR4-TOPO vector and transformed into E. coli DH5α cells using TOPO-TA cloning kit according to the manufacturer’s instructions (Invitrogen). From each sample 48 to 96 clones were picked. The partial sequencing of 768 clones was performed with average Phred 20 read length of 600–700 bp (Beckman Coulter Genomics, Beverly, MA) using BigDye Terminator v3.1 and M13 forward sequencing primer. Sequences were analyzed on ABI PRISM 3730xl coupled with Agencourt Clean SEQ dye terminator removal for generation of long high quality Sanger sequencing reads. The sequences were trimmed for elimination of vector sequences and adjusted for quality values.
Phylogenetic analysis
All the trimmed sequences were verified manually for vector sequences using EMBOSS pairwise alignment algorithms (Larkin et al., 2007). The resulting 748 trimmed sequences were aligned using Greengenes Nast aligner (DeSantis et al., 2006a) and chimeric sequences detected by greengenes - Bellerophon chimera check program (DeSantis et al., 2006b). The sequences ≥300 bases with similarity score S_ab score ≥0.8 (Pei et al., 2004, Vickerman et al., 2007) were assembled for phylogenetic affiliations and analyzed by Sequence Match program of Ribosomal Database Project (RDP, update 10) (Cole et al., 2009). The sequences with <0.8 S_ab score were considered unknown sequences. The resulting sequences were binned into two BRONJ libraries, with and without antibiotics and analyzed. In analysis representation, the term ‘species’ would refer to named and unnamed cultured bacterial species and the term ‘phylotypes’ would refer to uncultured species.
Species diversity and richness estimators
Clone library size was evaluated by an online program (http://www.aslo.org/lomethods/free/2004/0114a.html), and the output data were treated by the method described by (Kemp & Aller, 2004). Estimation and comparison of species richness in BRONJ tissue specimens with and without antibiotics were performed using EstimateS 8.2 (Colwell, 2009 ). Rarefaction analysis, diversity estimation and abundance model were generated by PAST version 2.01 (Hammer et al., 2001). The Shannon-Weaver index of diversity (H′) in the two sample groups was compared by the Mann-Whitney U test. Good’s coverage was calculated as [1 − (n/N)], where n is the number of species constituted by singleton and N is the total number of clones (Good, 1953).
Results
Analysis of DGGE profile from clinical samples
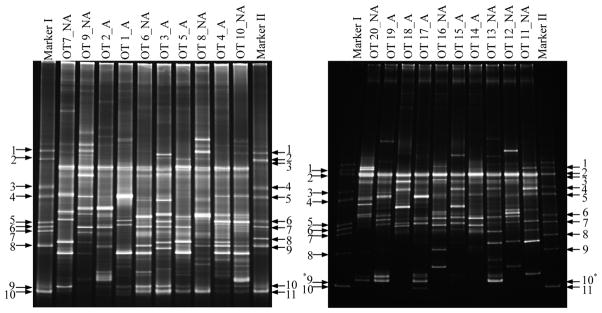
The overall diversity in bacterial community structures was assessed in 20 BRONJ tissue samples, 10 each with and without antibiotics by PCR-DGGE fingerprints. The gels were normalized using species-specific markers. The DGGE profile of 16S rDNA from BRONJ samples in both the groups showed approximately 18 to 33 bands each indicative of one or more species (Figure 1). Several high and low intensity bands were identical in both the groups but few of the bands were unique in each group. However, there were no significant differences in the total number of bands, mean 25.4±5.6 SD in group with antibiotics as compared to mean 23.4±4.5 SD in those without antibiotics.
Figure 1.
PCR-DGGE profile of bacterial 16S rDNA gene fragments of BRONJ tissue samples with and without antibiotics. Marker I & II: DGGE reference markers correspond to 16S rRNA gene fragments from quoted specific bacterial species [Marker I: 1. Fusobacterium nucleatum subsp. vincenti (ATCC 49256); 2. Fusobacterium nucleatum subsp. nucleatum (ATCC 25586); 3. Streptococcus sanguinis (ATCC 10556); 4. Streptococcus oralis (ATCC 35037); 5. Streptococcus salivarus (ATCC 7073); 6. Streptococcus mutans (UA 159); 7. Lactobacillus paracasei (ATCC 25598); 8. Porphyromonas gingivalis (ATCC 33277); 9. Actinomyces odontolyticus (ATCC 17929); 10. Actinomyces naeslundii (ATCC 12104), Marker II: F. nucleatum subsp. vincenti (ATCC 49256); 2. F. nucleatum subsp. nucleatum (ATCC 25586); 3. Bacteroides forsythus (ATCC 43037) (T.f.); 4. S. sanguinis (ATCC 10556); 5. S. oralis (ATCC 35037); 6. Veillonella parvula (ATCC 17745); 7. Prevotella intermedia (ATCC 25611); 8. Aggregatibacter actinomycemcomitans (ATCC 43717); 9. P. gingivalis (ATCC 33277); 10. A. odontolyticus (ATCC 17929); 11. A. naeslundii (ATCC 12104)]
A - BRONJ tissue samples with antibiotics; NA - BRONJ tissue samples without antibiotics
* - light bands not visible in photograph
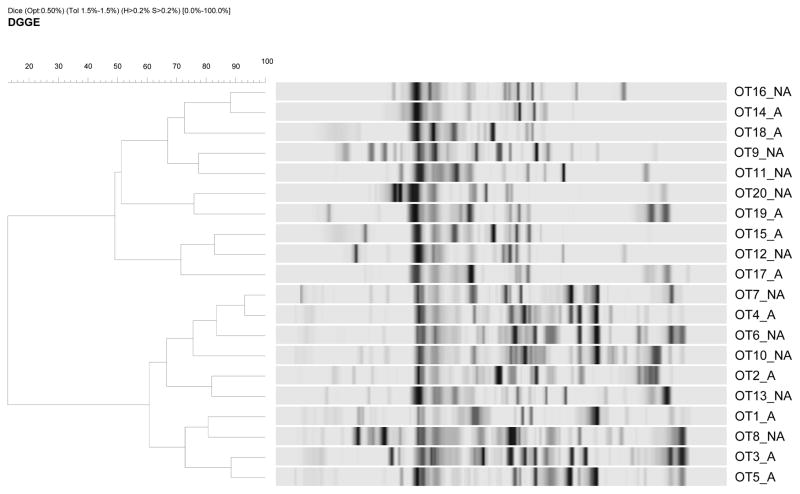
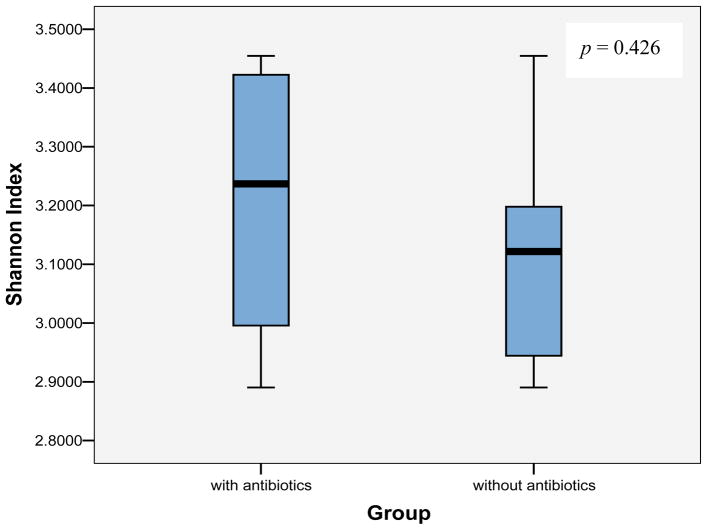
The dendrogram of cluster analysis with Dice coefficient showed clusters comprising mixed population of samples with and without antibiotics (Figure 2). The % similarity between two given samples approximately ranged from 75% to 92%. The Shannon-Weaver diversity index (H′) of groups, with and without antibiotics was mean 3.20±0.212 SD and mean 3.12±0.172 SD respectively. The Mann-Whitney U test was used to compare the Shannon diversity index of two sample groups (Figure 3). The p-value, p=0.426 was two-tailed and no significant differences were displayed in the species diversity among the BRONJ cohorts with and without antibiotics.
Figure 2.
Dendrogram of DGGE profiles of BRONJ tissue samples with and without antibiotics based on Dice coefficient depicting microbial diversity
A - BRONJ tissue samples with antibiotics; NA - BRONJ tissue samples without antibiotics
Figure 3.
Shannon index depicting bacterial diversity of BRONJ samples as in subjects with and without antibiotics
Bacterial diversity
Based on the results of the DGGE profile, we selected 10 BRONJ tissue samples, 5 each from with and without antibiotics patients for cloning and sequencing. The resulting 748 sequences from the total 768 bacterial clones were analyzed. A total of 70 (9.1%) sequences were eliminated based on sequence length cutoff limit <300 bases (5.6%) and chimeric (3.5%) sequences. About 678 (88.3%) sequences with sequence length 300–900 bases were analyzed for phylogenetic affiliations. The sequences (26%) with <0.8 S_ab score were assigned as sequences belonging to unknown species. Altogether 478 (62%) sequences were detected that belonged to 244 (32%) cultured species and 234 (30%) uncultured phylotypes. The number of observed species were 84 (69.0% Gram+, 31.0% Gram-) and 68 (75.0% Gram+, 25.0% Gram-) in BRONJ tissues with and without antibiotics respectively.
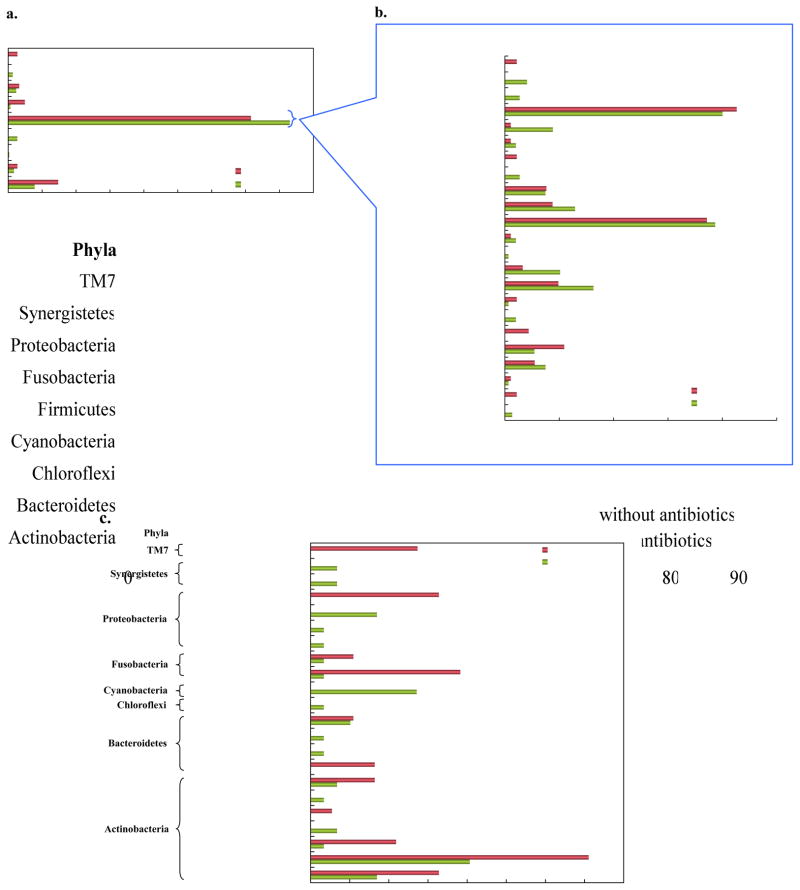
Bacterial species/phylotypes in the two groups were distributed into 9 phyla and 44 genera. The phyla represented were Actinobacteria, Bacteroidetes, Chloroflexi, Cyanobacteria, Firmicutes, Fusobacteria, Proteobacteria, Synergistetes and TM7 (Figure 4a). The phylum TM7 was uniquely present while other 2 phyla, Chloroflexi and Cyanobacteria, were absent in samples without antibiotics. The phylum Firmicutes was found elevated in subjects on antibiotics. The predominant phylum Firmicutes was represented by 22 genera (Figure 4b) with 1 unclassified genus under its cluster. Parvimonas and Streptococcus represented a larger portion of the bacterial community in both BRONJ groups. The genus, Eubacterium (5.46%) and Pseudoramibacter (3.83%) was found to be more prevalent in samples without antibiotics whereas Lactobacillus (8.14%), Peptostreptococcus (6.44%), Mogibacterium (5.08%), Solobacterium (4.41%) and Dialister (3.73%) in samples with antibiotics. Veillonella (2.03%) was detected only in antibiotic-administered group and Gemella (2.19%) only in group without antibiotics.
Figure 4.
Relative distribution of (a) phyla; (b) genera in most prevalent phylum Firmicutes; (c) genera in less abundant phyla in BRONJ tissue samples with and without antibiotics
The genera from other phyla (Figure 4c), to name a few, exclusive to antibiotic group were Olsenella (0.68%) and Pyramidobacter (0.68%) while Paludibacter (1.64%) and TM7 (2.73%) in group without antibiotic. The colonization of bacteria characterizing Atopobium (7.1%), Fusobacterium (3.83%), Actinomyces (3.28%) and Bifidobacterium (2.19%) were higher in the BRONJ group without antibiotics. Some genera usually found in environmental samples were found in either of the group viz. Desulfobulbus, Streptophyta and Thermomonas.
At species-level, Streptococcus intermedius, Lactobacillus gasseri, Mogibacterium timidum and Solobacterium moorei did show largely in patients treated with antibiotics whereas Parvimonas micra, Streptococcus anginosus and Atopobium rimae in patients not treated with antibiotics. Some unnamed cultured species and uncultured phylotypes also represented the two groups (Table 2).
Table 2.
Bacterial species/phylotypes exclusive as well as common in ONJ tissues of patients with and without prophylactic antibiotics
| Bacterial species/phylotypes | |
|---|---|
|
with antibiotics
| |
| Abiotrophia defectiva | Oribacterium sp. oral taxon 372** |
| Actinomyces cardiffensis | Propionibacterium acnes |
| Bacteroidales oral clone MCE7_20 | Pyramidobacter piscolens |
| Chloroflexi genomosp. P1 | Shuttleworthia satelles |
| Desulfobulbus sp. oral taxon 041*** | Solobacterium moorei |
| Dialister pneumosintes | Streptococcus constellatus subsp. constellatus |
| Eubacterium sp. oral clone BP1-41 | Streptococcus genomosp. C3 |
| Firmicutes oral clone AO068** | Streptococcus gordonii |
| Granulicatella adiacens | Streptococcus milleri |
| Johnsonella sp. oral taxon 166*** | Streptococcus pneumoniae |
| Lachnospiraceae oral clone MCE9_173*** | Streptococcus sp. oral taxon 071** |
| Lactobacillus fermentum | Veillonella parvula |
| Porphyromonas endodontalis | Veillonella sp. oral clone VeillA8 |
| Lactobacillus sp. | Veillonellaceae bacterium oral taxon 129** |
| Lactobacillus sp. NEQAS6172 | Veillonellaceae bacterium oral taxon 132*** |
| Olsenella uli (T) | Veillonellaceae bacterium oral taxon 150*** |
|
| |
|
without antibiotics
| |
| Actinomyces dentalis | Leptotrichia hofstadii F0254 |
| Actinomyces sp. oral taxon 525* | Parascardovia denticolens |
| Bacteroidetes bacterium oral taxon 274** | Peptostreptococcus sp. oral clone FG014 |
| Eubacterium sp. oral clone EH006 | Streptococcus sp. 99 |
| Eubacterium sp. oral taxon G32 | Thermomonas hydrothermalis (T) |
| Fusobacterium nucleatum | Thermomonas sp. FLA17 |
| Gemella morbillorum | TM7 phylum sp. oral taxon 346*** |
| Lachnospiraceae bacterium oral taxon 086*** | TM7 phylum sp. oral taxon 349*** |
| Lactobacillus rhamnosus | |
|
| |
|
common in with and without antibiotics
| |
| Atopobium rimae | Parvimonas micra |
| Bifidobacterium dentium | Peptostreptococcus stomatis |
| Bulleidia moorei | Prevotella sp. oral taxon G57 |
| Dialister invisus | Pseudoramibacter alactolyticus |
| Eubacterium infirmum | Scardovia sp. oral taxon 195* |
| Eubacterium sp. oral clone BU061 | Streptococcus anginosus |
| Eubacterium sp. oral strain A35MT | Streptococcus intermedius |
| Lactobacillus gasseri | Streptococcus mitis |
| Lactobacillus salivarius | Streptococcus mutans |
| Mogibacterium timidum | |
cultured;
unnamed cultured phylotype;
currently uncultured phylotype
Species richness and diversity
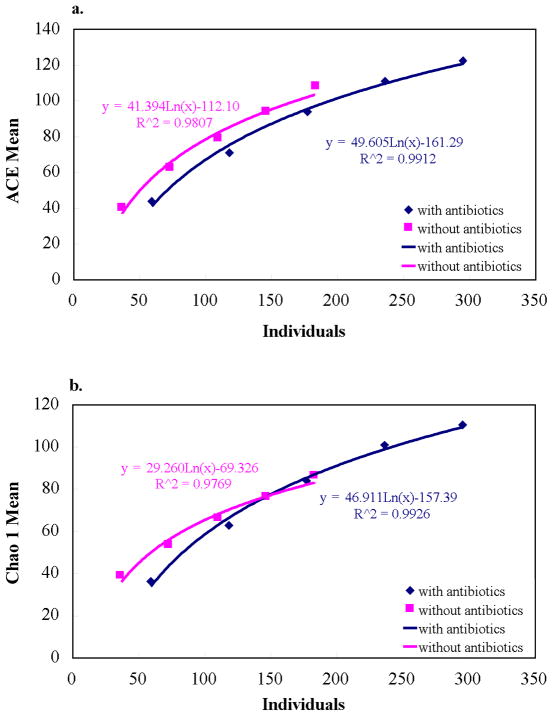
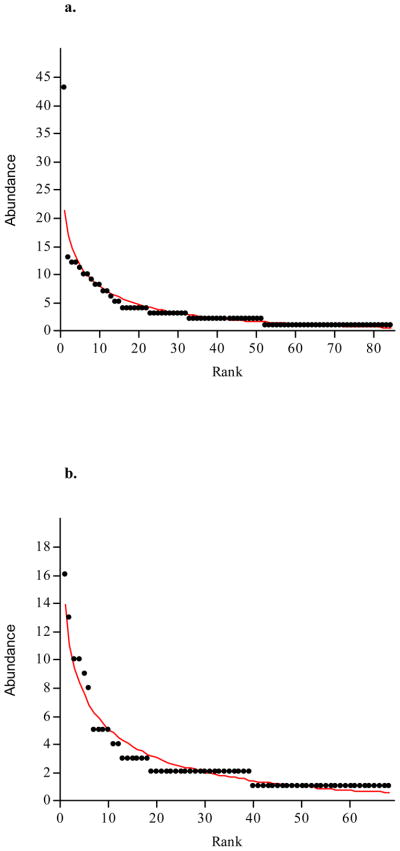
The richness estimators, Chao1 and ACE, diversity indices, evenness and rank-abundance were determined for independent and combined groups (Table 3). Chao 1 and ACE were elevated in library with antibiotics. Also, the SChao1 and SACE of the two sample groups reached an asymptote (Figure 5) suggesting that the libraries of both BRONJ tissues with and without antibiotics were large enough to yield optimal stable phylotype richness estimate. Shannon-Weaver and Simpson diversity indices revealed no significant differences among the two BRONJ libraries with and without antibiotics which was also evident from the Mann-Whitney U test of Shannon diversity (P>0.05). The evenness was higher in BRONJ group without antibiotics indicating relative species abundance. The two sample groups exhibited similar profile of abundance model with long tail representing rare species as shown in Figure 6. Good’s coverage was 89% and 84% of BRONJ tissues with and without antibiotics, respectively suggesting 11 and 16 additional species/phylotypes would be recognized if about 100 more clones would be screened.
Table 3.
Species richness and diversity indices estimators of microflora in ONJ tissues with and without antibiotics
| with antibiotics (n=5) | without antibiotics (n=5) | combined (n=10) | |
|---|---|---|---|
| No. of clones | 295 | 183 | 478 |
| Species/phylotypes (S) | 84 | 68 | 120 |
| Singletons | 33 | 29 | 45 |
| Doubletons | 19 | 21 | 26 |
| Chao1 estimator of species richness | 110.40 | 86.45 | 156.67 |
| Chao1 standard deviation | 12.11 | 9.05 | 14.38 |
| ACE estimator of species richness | 122.59 | 108.00 | 165.31 |
| Shannon’s index for diversity (H) | 3.88 | 3.83 | 4.17 |
| Simpson’s index for diversity (1-D) | 0.96 | 0.97 | 0.97 |
| Evenness (e^H/S) | 0.58 | 0.68 | 0.54 |
| Good’s estimator of coverage (%) | 88.80 | 84.20 | 90.60 |
n – number of samples; combined – with and without antibiotics
Figure 5.
Comparison of species richness between BRONJ tissue samples with and without antibiotics using estimators (a) ACE; (b) Chao1
Figure 6.
Rank-abundance curves for BRONJ tissue samples (a) with antibiotics; (b) without antibiotics
Discussion
There are many hypotheses for BRONJ pathogenesis (Allen & Burr, 2009); the manifestation of necrotic bone resulting from bisphosphonate-induced remodeling suppression that allow accumulation of nonviable osteocytes, direct cytotoxic effect of bisphosphonates on osteocytes, bisphosphonates antiangiogenic effects and role of oral bacteria. Despite infection being present in BRONJ patients, there is no clear data as to whether infection plays role in the pathophysiology (Allen & Burr, 2009). The present studies using 16S rRNA culture-independent molecular methods determined the bacterial profile of BRONJ and demonstrated that there were no significant differences in bacterial diversity (culturable and non-culturable) of BRONJ tissues from patients treated with and without antibiotics. To our knowledge, this is the first report to address the effect of antibiotics on bacterial colonization (in vivo biofilms) associated with BRONJ. Our results indicated that species/phylotypes affiliated to genera Parvimonas and Peptostreptococcus were more prevalent in antibiotic-administered group whereas Fusobacterium, Atopobium and Streptococcus predominantly existed in group without antibiotics indicating changes in the composition (relative distribution) of biofilm in two groups. S. intermedius and M. timidum, which are associated with periodontitis (Rocas & Siqueira, 2008, Rochford, 1980), were elevated in patients on antibiotics while S. anginosus and P. micra levels were higher in those without antibiotics. S. anginosus has been shown to be strongly associated with abscess formation (Gray, 2005) and P. micra is frequently related to polymicrobial infections in humans (Ota-Tsuzuki & Mayer, 2010). Each group had its unique species, for instance, S. moorei, a species that has been identified as a contributor to halitosis (Kazor et al., 2003), was solely confined to necrotic tissues with antibiotics.
Lactobacillus was found in both the groups but more prevalent in subjects on antibiotics. Actinomyces and Veillonella species were observed in the subjects treated with antibiotics unlike in those without antibiotics. However we did not observe high abundance of Actinomyces in BRONJ samples. In previous studies using histomorphometric analysis of oral mucosa and jawbones have shown that Actinomyces is associated with BRONJ (Kaplan et al., 2009). Naik & Russo, (2009) reported presence of the actinomyces-like organisms from affected bone in most of the cases studied, however, most of these assumptions are based on microscopic observations. In our study we used molecular 16S rRNA techniques to identify bacterial species/phylotypes that represented both culturables and non-culturables and did not observe high abundance of Actinomyces. However as compared to non-antibiotics group we observed elevated number of Actinomyces in our antibiotic cohort and this may be due to its fastidious nature and not due to its association with BRONJ (Kaplan, 2010). A high level of correlation has been observed between Veillonella and the three species, Lactobacilli, mutans streptococci and Actinomyces spp. which ferment carbohydrates to lactate and as a consequence, Veillonella concentrations increased due to lactate consumption (Arif et al., 2008). Our results suggested that biofilms in each cohort had a distinct combination of species/phylotypes, segregating and repopulating leading to compositional changes but maintaining functional similarity of acid production that may lead to demineralization of tooth tissues, as observed in dental caries biofilms (Arif et al., 2008, Diaz et al., 2006). It was also observed that some cultured and uncultured bacterial species/phylotypes present in one group were either reduced in numbers or lost in other group as evident from the DGGE fingerprints. The existence of uncultured microflora alongwith cultured bacterial flora indicated their interdependency on nutritional and signaling interactions or community networking (Wade, 2002). Most of the mucosal infections involves biofilms developed on natural tissues (skin, mucosa, endothelial epithelia, teeth, bones) (Lazar & Chifiriuc, 2010). The histopathologic examination have indicated that edentulous jaw contains regions of necrotic bone and microbial biofilm formation even after one year of tooth extraction and mucosal healing which may contribute to BRONJ formation (Kassolis et al., 2010). Similar observations of persist lesion for a variable period of time were reported in the case of chronic suppurative osteomyelitis (Ertas et al., 2004).
There are numerous factors that prevent collection of tissue from homogenous patient populations such as the low incidence of BRONJ, cancer biology and varying chemotherapy. Our study using limited sample size indicated that although there were no significant differences in the total number of bacteria in the two groups as distinguished by DGGE profile and phylogenetic studies, there were differences in species diversity between antibiotic and non antibiotic groups. This finding suggests an alteration in the microbial population, but not enough to reduce or eliminate infection. This may be attributed to either compromised vascular supply of necrotic tissue (Allen & Burr, 2009) or biofilm formation. Indeed, doxycycline is more effective when applied locally compared to systemic administration (Goldie, 2009). Histopathological and scanning electron microscopic studies have indicated that bacterial biofilm in BRONJ is more diverse as compared to osteomyelitis of the jaw and had co-aggregation between Actinomyces and coccal forms (Kumar et al., 2010, Sedghizadeh et al., 2008, Sedghizadeh et al., 2009). Moreover, BP effect on bone remodeling due to osteoblast and osteoclast differentiation and deficient antimicrobial response may reduce function and number of cells from the phagocytic lineage, monocytes and macrophages leading to development of local infection (Pazianas, 2011) and also affecting deeper structure of bone (Sedghizadeh et al., 2008). It is known that several species of bacteria could cause alveolar bone destruction mediated by their products (e.g. lipopolysaccharides) (Bertoldo et al., 2007, Nair et al., 1996, Pazianas, 2011). The plausible basis for BRONJ development is also the increased bacterial adhesion to the bisphosphonate covered bone (Allen & Burr, 2009, Kos, 2011, Kos & Luczak, 2009).
The finding that there were no significant differences in bacterial diversity (culturable and non-culturable) of BRONJ tissues from patients treated with and without antibiotics supports surgical treatment options (Carlson & Basile, 2009) and may explain poor success rates when using only antimicrobial therapy (Bamias et al., 2005). This was further supported by case report by Ertas et al., (2004) which highlighted that in chronic suppurative osteomyelitis the infection is localized but is persistent because the infected necrotic area remained as a sequestrum and this chronic lesion persist for a variable period up to many years with intermittent exacerbation and ultimately required surgical intervention. In another case report of chronic osteomyelitis with serious MRSA infection of the mandible demonstrated purulent discharge. The patient failed to recover despite prolonged postoperative treatment and the administration of several antibiotics. Further the treatment protocol involved a multimodal approach with parenteral and local antibiotics treatment and necessary surgical interventions (Tuzuner-Oncul et al., 2009). Conservative management including the judicious use of antibiotics may be ineffective with progressive infection and bone destruction (Hoff et al., 2008, Williamson, 2010) may needing alternative preoperative and surgical strategies in BRONJ (Hoefert & Eufinger, 2011, Montefusco et al., 2008, Pautke et al., 2011). Moreover, microbial cultures have not been helpful in directing therapy because specific pathogens have not been identified (Fantasia, 2009). Our study using 16S rDNA molecular technique reflects that the use of systemic antibiotics failed to restrict the bacterial colonization without effective healing of the lesion after the onset of BRONJ. However, this finding requires further evaluation with larger subject population.
Acknowledgments
This work was supported by R03DE019178 and R01DE020891 grants from NIDCR, and NYU Faculty Research Funds.
References
- Abraham F, Dalvi M, Farooki A, et al. Molecular analysis of bacteria associated with osteonecrosis of the jaw (abstract) J Dent Res. 2009;88:A3441. [Google Scholar]
- Allam E, Allen M, Chu TM, et al. In vivo effects of zoledronic acid on oral mucosal epithelial cells. Oral Dis. 2011;17:291–297. doi: 10.1111/j.1601-0825.2010.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67:61–70. doi: 10.1016/j.joms.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Arif N, Sheehy EC, Do T, et al. Diversity of Veillonella spp. from sound and carious sites in children. J Dent Res. 2008;87:278–282. doi: 10.1177/154405910808700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- Bertoldo F, Santini D, Lo Cascio V. Bisphosphonates and osteomyelitis of the jaw: a pathogenic puzzle. Nat Clin Pract Oncol. 2007;4:711–721. doi: 10.1038/ncponc1000. [DOI] [PubMed] [Google Scholar]
- Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxil Surg. 2009;67:85–95. doi: 10.1016/j.joms.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Cole JR, Wang Q, Cardenas E, et al. The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RE. Risks and benefits of bisphosphonates. Br J Cancer. 2008;98:1736–1740. doi: 10.1038/sj.bjc.6604382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell RK. EstimateS: statistical estimation of species richness and shared species from samples. Version 8.2. 2009. [Google Scholar]
- DeSantis TZ, Jr, Hugenholtz P, Keller K, et al. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 2006a;34:W394–399. doi: 10.1093/nar/gkl244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006b;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz PI, Chalmers NI, Rickard AH, et al. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl Environ Microbiol. 2006;72:2837–2848. doi: 10.1128/AEM.72.4.2837-2848.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel IJ, Fogelman I, Al-Nawas B, et al. Pathophysiology, risk factors and management of bisphosphonate-associated osteonecrosis of the jaw: Is there a diverse relationship of amino- and non-aminobisphosphonates? Crit Rev Oncol Hematol. 2007;64:198–207. doi: 10.1016/j.critrevonc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Dodson TB. Intravenous bisphosphonate therapy and bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:44–52. doi: 10.1016/j.joms.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertas U, Tozoglu S, Gursan N. Chronic osteomyelitis: 20 years after mandible fracture. Dent Traumatol. 2004;20:106–108. doi: 10.1111/j.1600-4469.2004.00229.x. [DOI] [PubMed] [Google Scholar]
- Estilo CL, Van Poznak CH, Wiliams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13:911–20. doi: 10.1634/theoncologist.2008-0091. [DOI] [PubMed] [Google Scholar]
- Fantasia JE. Bisphosphonates-what the dentist needs to know: practical considerations. J Oral Maxil Surg. 2009;67:53–60. doi: 10.1016/j.joms.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Fleisher KE, Doty S, Kottal S, et al. Tetracycline-guided debridement and cone beam computed tomography for the treatment of bisphosphonate-related osteonecrosis of the jaw: a technical note. J Oral Maxillofac Surg. 2008;66:2646–2653. doi: 10.1016/j.joms.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Gebara SN, Moubayed H. Risk of osteonecrosis of the jaw in cancer patients taking bisphosphonates. Am J Health Syst Pharm. 2009;66:1541–1547. doi: 10.2146/ajhp080251. [DOI] [PubMed] [Google Scholar]
- Gliklich R, Wilson J. Epidemiology of bisphosphonate-related osteonecrosis of the jaws: the utility of a national registry. J Oral Maxillofac Surg. 2009;67:71–74. doi: 10.1016/j.joms.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Goldie MP. What is new in research? Int J Dent Hyg. 2009;7:226–228. doi: 10.1111/j.1601-5037.2009.00405.x. [DOI] [PubMed] [Google Scholar]
- Good IJ. The population frequencies of species and the estimation of population parameters. Biometrika. 1953;40:237–264. [Google Scholar]
- Gray T. Streptococcus anginosus group: Clinical significance of an important group of pathogens. Clin Microbiol Newsl. 2005;27:155–159. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electronica. 2001;4:9. [Google Scholar]
- Hoefert S, Eufinger H. Relevance of a prolonged preoperative antibiotic regime in the treatment of bisphosphonate-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2011;69:362–380. doi: 10.1016/j.joms.2010.06.200. [DOI] [PubMed] [Google Scholar]
- Hoefert S, Schmitz I, Tannapfel A, et al. Importance of microcracks in etiology of bisphosphonate-related osteonecrosis of the jaw: a possible pathogenetic model of symptomatic and non-symptomatic osteonecrosis of the jaw based on scanning electron microscopy findings. Clin Oral Investig. 2010;14:271–284. doi: 10.1007/s00784-009-0300-6. [DOI] [PubMed] [Google Scholar]
- Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Min Res. 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SJ, Crean S-J, Fardy MJ, et al. A molecular analysis of the bacteria present within oral squamous cell carcinoma. J Med Microbiol. 2007;56:1651–1659. doi: 10.1099/jmm.0.46918-0. [DOI] [PubMed] [Google Scholar]
- Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol. 2005;13:589–595. doi: 10.1016/j.tim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Kamel HK. Update on osteoporosis management in long-term care: focus on bisphosphonates. J Am Med Dir Assoc. 2007;8:434–440. doi: 10.1016/j.jamda.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Kaplan I. In reply. Oral Sur Oral Med Oral Pathol Oral Radiol Endod. 2010;109:657–658. doi: 10.1016/j.tripleo.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Kaplan I, Anavi K, Anavi Y, et al. The clinical spectrum of Actinomyces-associated lesions of the oral mucosa and jawbones: correlations with histomorphometric analysis. Oral Sur Oral Med Oral Pathol Oral Radiol Endod. 2009;108:738–746. doi: 10.1016/j.tripleo.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Kassolis JD, Scheper M, Jham B, et al. Histopathologic findings in bone from edentulous alveolar ridges: a role in osteonecrosis of the jaws? Bone. 2010;47:127–130. doi: 10.1016/j.bone.2010.04.588. [DOI] [PubMed] [Google Scholar]
- Kazor CE, Mitchell PM, Lee AM, et al. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J Clin Microbiol. 2003;41:558–563. doi: 10.1128/JCM.41.2.558-563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp PF, Aller JY. Estimating prokaryotic diversity: when are 16S rDNA libraries large enough? Limnol Oceanogr-Meth. 2004;2:114–125. [Google Scholar]
- Kos M. Bisphosphonates promote jaw osteonecrosis through facilitating bacterial colonisation. Medical Hypotheses. 2011 doi: 10.1016/j.mehy.2011.04.015. (In Press, Corrected Proof) [DOI] [PubMed] [Google Scholar]
- Kos M, Luczak K. Bisphosphonates promote jaw osteonecrosis through facilitating bacterial colonisation. Biosci Hypotheses. 2009;2:34–36. doi: 10.1016/j.mehy.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Krueger CD, West PM, Sargent M, et al. Bisphosphonate-induced osteonecrosis of the jaw. Ann Pharmacother. 2007;41:276–284. doi: 10.1345/aph.1H521. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Gorur A, Schaudinn C, et al. The role of microbial biofilms in osteonecrosis of the jaw associated with bisphosphonate therapy. Curr Osteoporos Rep. 2010;8:40–48. doi: 10.1007/s11914-010-0008-1. [DOI] [PubMed] [Google Scholar]
- Kuramitsu HK, He XS, Lux R, et al. Interspecies interactions within oral microbial communities. Microbiol Mol Biol R. 2007;71:653–670. doi: 10.1128/MMBR.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrgidis A, Tzellos TG, Toulis K, et al. The facial skeleton in patients with osteoporosis: a field for disease signs and treatment complications. J Osteoporos. 2011;2011:Article ID 147689. doi: 10.4061/2011/147689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. John Wiley & sons Ltd; West Sussex, England: 1991. pp. 115–147. [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lazar V, Chifiriuc MC. Medical significance and new therapeutical strategies for biofilm associated infections. Roum Arch Microbiol Immunol. 2010;69:125–138. [PubMed] [Google Scholar]
- Lesclous P, Abi Najm S, Carrel JP, et al. Bisphosphonate-associated osteonecrosis of the jaw: a key role of inflammation? Bone. 2009;45:843–852. doi: 10.1016/j.bone.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Li Y, Ge Y, Saxena D, et al. Genetic profiling of the oral microbiota associated with severe early-childhood caries. J Clin Microbiol. 2007;45:81–87. doi: 10.1128/JCM.01622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ku CYS, Xu J, et al. Survey of oral microbial diversity using PCR-based denaturing gradient gel electrophoresis. J Dent Res. 2005;84:559–564. doi: 10.1177/154405910508400614. [DOI] [PubMed] [Google Scholar]
- Li Y, Saxena D, Barnes VM, et al. Polymerase chain reaction-based denaturing gradient gel electrophoresis in the evaluation of oral microbiota. Oral Microbiol Immun. 2006;21:333–339. doi: 10.1111/j.1399-302X.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- Migliorati CA, Schubert MM, Peterson DE, et al. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer. 2005;104:83–93. doi: 10.1002/cncr.21130. [DOI] [PubMed] [Google Scholar]
- Montefusco V, Gay F, Spina F, et al. Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leuk Lymphoma. 2008;49:2156–2162. doi: 10.1080/10428190802483778. [DOI] [PubMed] [Google Scholar]
- Naik NH, Russo TA. Bisphosphonate-related osteonecrosis of the jaw: the role of Actinomyces. Clin Infect Dis. 2009;49:1729–1732. doi: 10.1086/648075. [DOI] [PubMed] [Google Scholar]
- Nair SP, Meghji S, Wilson M, et al. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64:2371–2380. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota-Tsuzuki C, Mayer MPA. Collagenase production and hemolytic activity related to 16S rRNA variability among Parvimonas micra oral isolates. Anaerobe. 2010;16:38–42. doi: 10.1016/j.anaerobe.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Otto S, Hafner S, Grotz KA. The role of inferior alveolar nerve involvement in bisphosphonate-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2009;67:589–592. doi: 10.1016/j.joms.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Pautke C, Bauer F, Otto S, et al. Fluorescence-guided bone resection in bisphosphonate-related osteonecrosis of the jaws: first clinical results of a prospective pilot study. J Oral Maxillofac Surg. 2011;69:84–91. doi: 10.1016/j.joms.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Pazianas M. Osteonecrosis of the jaw and the role of macrophages. J Natl Cancer Inst. 2011;103:232–240. doi: 10.1093/jnci/djq516. [DOI] [PubMed] [Google Scholar]
- Pei Z, Bini EJ, Yang L, et al. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci U S A. 2004;101:4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushalkar S, Mane SP, Ji X, et al. Microbial diversity in saliva of oral squamous cell carcinoma. FEMS Immunol Med Microbiol. 2011;61:269–277. doi: 10.1111/j.1574-695X.2010.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid IR. Osteonecrosis of the jaw: who gets it, and why? Bone. 2009;44:4–10. doi: 10.1016/j.bone.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Rocas IN, Siqueira JF. Root canal microbiota of teeth with chronic apical periodontitis. J Clin Microbiol. 2008;46:3599–3606. doi: 10.1128/JCM.00431-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford JC. Pleuropulmonary infection associated with Eubacterium-Brachy, a new species of Eubacterium. J Clin Microbiol. 1980;12:722–723. doi: 10.1128/jcm.12.5.722-723.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann N Y Acad Sci. 2011;1218:38–46. doi: 10.1111/j.1749-6632.2010.05768.x. [DOI] [PubMed] [Google Scholar]
- Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws-2009 update. J Oral Maxillofac Surg. 2009;67:2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Rupf S, Merte K, Eschrich K. Quantification of bacteria in oral samples by competitive polymerase chain reaction. J Dent Res. 1999;78:850–856. doi: 10.1177/00220345990780040501. [DOI] [PubMed] [Google Scholar]
- Sedghizadeh PP, Kumar SK, Gorur A, et al. Identification of microbial biofilms in osteonecrosis of the jaws secondary to bisphosphonate therapy. J Oral Maxillofac Surg. 2008;66:767–775. doi: 10.1016/j.joms.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Sedghizadeh PP, Kumar SK, Gorur A, et al. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J Am Dent Assoc. 2009;140:1259–1265. doi: 10.14219/jada.archive.2009.0049. [DOI] [PubMed] [Google Scholar]
- Sheffield VC, Cox DR, Lerman LS, et al. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989;86:232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoot LM, Smoot JC, Smidt H, et al. DNA microarrays as salivary diagnostic tools for characterizing the oral cavity’s microbial community. Adv Dent Res. 2005;18:6–11. doi: 10.1177/154407370501800103. [DOI] [PubMed] [Google Scholar]
- Tuzuner-Oncul AM, Ungor C, Dede U, et al. Methicillin-resistant Staphylococcus aureus (MRSA) osteomyelitis of the mandible. Oral Sur Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e1–e4. doi: 10.1016/j.tripleo.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Van den Wyngaert T, Huizing MT, et al. Osteonecrosis of the jaw related to the use of bisphosphonates. Curr Opin Oncol. 2007;19:315–322. doi: 10.1097/CCO.0b013e32819f820b. [DOI] [PubMed] [Google Scholar]
- Vescovi P, Nammour S. Bisphosphonate-related osteonecrosis of the jaw (BRONJ) therapy. A critical review. Minerva Stomatol. 2010;59:181–203. 204–213. [PubMed] [Google Scholar]
- Vickerman MM, Brossard KA, Funk DB, et al. Phylogenetic analysis of bacterial and archaeal species in symptomatic and asymptomatic endodontic infections. J Med Microbiol. 2007;56:110–118. doi: 10.1099/jmm.0.46835-0. [DOI] [PubMed] [Google Scholar]
- Wade W. Unculturable bacteria-the uncharacterized organisms that cause oral infections. J R Soc Med. 2002;95:81–83. doi: 10.1258/jrsm.95.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JH, Dodson TB, Zavras AI. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a case-control study. J Oral Maxillofac Surg. 2008;66:625–631. doi: 10.1016/j.joms.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RA. Surgical management of bisphosphonate induced osteonecrosis of the jaws. Int J Oral Maxillofac Surg. 2010;39:251–255. doi: 10.1016/j.ijom.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Wong CY, Wei X, Pushalkar S, et al. Evaluating bone microbiota in bisphosphonate related osteonecrosis of the jaw (abstract) J Dent Res. 2010;89:A578. [Google Scholar]