Abstract
Preclinical studies suggest lowering dietary fat and decreasing the ratio of omega-6 to omega-3 polyunsaturated fatty acids decreases the risk of prostate cancer development and progression. We conducted a phase II randomized trial to test the effect of decreasing dietary fat combined with decreasing the dietary omega-6:omega-3 ratio on biomarkers related to prostate cancer development and progression. Patients undergoing radical prostatectomy were randomly assigned to receive a low-fat diet with 5 grams of fish oil daily (dietary omega-6:omega-3 ratio of 2:1) or a control western diet (omega-6:omega-3 ratio of 15:1) for 4–6 weeks prior to surgery. The primary endpoint was change in serum IGF-1 between arms. Secondary endpoints were serum IGFBP-1, prostate prostaglandin E-2 levels, omega-6:omega-3 fatty acid ratios, COX-2 and markers of proliferation and apoptosis. Fifty-five patients were randomized and 48 completed the trial. There was no treatment difference in the primary outcome. Positive secondary outcomes in the low-fat fish oil vs. western group were reduced benign and malignant prostate tissue omega-6:omega-3 ratios, reduced proliferation (Ki67 index), and reduced proliferation in an ex-vivo bioassay when patient sera was applied to prostate cancer cells in vitro. In summary, 4–6 weeks of a low-fat diet and fish oil capsules to achieve an omega-6:omega-3 fatty acid ratio of 2:1 had no effect on serum IGF-1 levels, though in secondary analyses the intervention resulted in decreased prostate cancer proliferation and decreased prostate tissue omega-6:omega-3 ratios. These results support further studies evaluating reduction of dietary fat with fish oil supplementation on modulating prostate cancer biology.
INTRODUCTION
Pre-clinical studies utilizing xenografts and genetically engineered mouse models demonstrated that reducing dietary fat and decreasing the omega-6 to omega-3 fatty acid ratio delays the development and progression of prostate cancer (1–5). Epidemiologic studies also found that a high-fat diet and low intake of fish and marine-derived omega-3 fatty acids were associated with increased risk of developing prostate cancer and increased risk of advanced disease (6–12), though other reports do not support this association (13–15). Other studies found increased intake of fish and marine-derived omega-3 fatty acids was associated with decreased prostate cancer mortality (16, 17). Studies have been mixed with regards to the relationship between circulating marine-derived omega-3 fatty acid levels and prostate cancer risk with one showing a negative association (18), others demonstrating a positive association with high grade prostate cancer (19, 20) and others showing no association (15, 21, 22). The main mechanisms underlying the purported anticancer effects of modulating dietary fat appear to be through reduced insulin-like growth factor (IGF) signaling (5, 23, 24) and alterations of membrane omega-6 to omega-3 fatty acid ratios leading to suppressed COX-2-dependent PGE-2 production, though other mechanisms may also be involved (1, 4, 25, 26).
The aim of the present pre-prostatectomy trial was to examine the effects of modulating dietary fat and the omega-6/omega-3 fatty acid ratio in men with prostate cancer on the IGF/IGFBP system and the COX-2/PGE-2 pathways. To obtain a dietary omega-6 to omega-3 fatty acids ratio of 2:1, we combined dietary fat reduction with fish oil capsule supplementation. Other endpoints examined in the present trial (and established in pre-clinical models) were fatty acid ratios in prostate tissue membranes and markers of angiogenesis, proliferation and apoptosis (4, 5, 24). This trial was designed to establish whether modulating dietary fat and the dietary omega-6 to omega-3 fatty acid ratio alters prostate cancer biomarkers and may therefore support the conduct of large scale prospective trials incorporating dietary fat modulation.
PATIENTS AND METHODS
Patients
Participants were recruited from the urology clinics at the Veterans Administration Greater Los Angeles Healthcare System, UCLA, and Santa Monica UCLA from 2005–2008. Participants were required to have a diagnosis of clinically localized prostate adenocarcinoma and scheduled to undergo radical prostatectomy at least 4 weeks from study entry. The diagnostic needle biopsy was required to have >5% cancer in one core or to have >1 core with cancer to increase the likelihood of having prostate cancer tissue for experimental studies. Subjects needed to be willing to stop nutritional supplements and herbal therapies (ie. lycopene, selenium, vitamin E, fish oil, saw palmetto), and medications that inhibit the COX-2 pathway (ie. aspirin, nonsteroidal anti-inflammatory agents) at least 1-wk prior to starting the intervention. Subjects were ineligible if they were on insulin or ever took 5-alpha reductase inhibitors, anti-androgens, or LHRH agonists. The study was approved by the institutional review board.
Study Design
This was a Phase II, randomized trial designed to study intermediate biologic end-points. This trial is registered with ClinicalTrial.gov (# NCT00836615). All subjects signed informed consent documents prior to study entry. Randomization (1:1) was by a permuted random block design and pre-randomization stratification was performed by study site. Study duration was four to six weeks. The western diet provided 40% Kcal from fat, 15% kcal from protein and 45% kcal from carbohydrates (15 grams of fiber/day) and the dietary omega-6:omega-3 fatty acid ratio was 15:1. The low- fat/fish oil diet provided 15% Kcal from fat, 15% kcal from protein, 70% kcal from carbohydrates (39 grams of fiber/day) and subjects took five 1.1gm fish oil capsules daily bringing the dietary ratio of omega-6:omega-3 fatty acids to 2:1. Subjects were instructed to consume 3 fish oil capsules with breakfast and 2 with dinner. The fish oil capsules were provided by Pharmavite (Mission Hills, CA). Each 1.1gm capsule contained 200mg eicosapentaenoic acid and 367mg docosahaxaenoic acid. The same lot of fish oil capsules was used throughout the study. The research dietitian designed the dietary intervention for each patient in both arms to maintain patient weight. To tightly control the dietary components in both arms, all meals were prepared by the UCLA General Clinical Research Center nutrition staff. Packaged meals were delivered to patient’s homes in coolers two days/week and 1-day/week subjects picked up the packaged meals and returned uneaten food which was weighed by the dietary staff. Compliance was determined by the research dietitian based on weekly meetings with subjects, daily food checklists completed by subjects, and measurement of uneaten foods. Body weight was measured weekly and caloric intake was adjusted when weight changes exceeded 1kg/week. Fish oil capsule compliance was measured by pill counts every 2 weeks. Participants were asked to maintain their usual physical activity level throughout the study.
At baseline all subjects received a history and physical examination and completed a 3-day food diary. At baseline and within 5-days of the radical prostatectomy all subjects had urine and fasting AM blood collected and bioelectrical impedance was performed to determine body composition.
Outcomes
The primary objective was to compare the change between the baseline and pre-surgery fasting serum IGF-1 levels between arms. Secondary endpoints measured in fresh frozen radical prostatectomy tissue and compared between arms were benign and malignant prostate tissue membrane omega-6:omega-3 fatty acid ratios and PGE2 levels. Other secondary endpoints compared between arms were prostate cancer tissue proliferation (Ki67), apoptosis (TUNEL), COX-2, and angiogenesis (CD31), urine prostaglandin E2 metabolite (PGEM) levels (a stable metabolite of prostaglandin E-2) (27), peripheral red blood cell membrane omega-6:omega-3 fatty acid ratios, serum-stimulated growth and proliferation of 22RV1 cells in an ex-vivo bioassay (24), fasting serum IGFBP-1, IGFBP-3, insulin, lipids, and PSA levels.
Fatty acids analysis
Fatty acid analysis was performed on membrane preparations of red blood cells and fresh frozen benign and malignant radical prostatectomy tissue using gas chromatography after formation of fatty acid methyl esters (28). Our method measures phospholipids and cholesteryl esters. The intra-assay coefficients were less than 6% for all fatty acids analyzed. The inter-assay coefficients were less than 10% for all fatty acids analyzed except for palmitoleic and linolenic acids for which the inter-assay coefficient was less than 12.5%.
Prostaglandin E2 and PGEM ELISAs
Prostaglandin E2 levels in extracts from fresh frozen prostate tissue specimens were measured in duplicate by EIA following the manufacturer protocol (Assay Designs Inc., Ann Arbor, MI). The intra and inter-assay coefficient of variation were 6.1 and 12.6% respectively. The total protein concentration was assessed to standardize the results. Urinary PGEM measurements (Cayman Chemical Company, Ann Arbor) relative to urinary creatinine (Assay Designs Inc.) levels were performed in triplicates with EIA assays following manufacturer protocols. PGEM EIA intra and inter assay coefficients of variation were 5.5% and 11.2% respectively. The creatinine EIA intra and inter assay coefficients of variation were 3.2% and 2.2% respectively.
Ex vivo Bioassay
The ex-vivo bioassay measuring 22RV1 proliferation in pre and post-intervention patient serum was performed twice in duplicate. 22RV1 cells were obtained from American Type Culture Collection and grown in RPMI medium without phenol red (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 100 IU penicillin, 100 ug/mL streptomycin, 10mM HEPES. 22Rv1 cells cultures were maintained at 37°C and supplemented with 5% CO2 in a humidified incubator. The mitogenic effect of human serum on 22RV1 proliferation was studied using an in-house bioassay. The cells were plated at 5×103 cells per well in 96-well plate and incubated for 24h before changing to fresh media containing 10% study subject serum or 10% FBS (control). The cell proliferation in media containing study subject serum or FBS was measured using the BrDu cell proliferation assay kit. BrdU was added for the last 4 hours of the 48 hour incubation and uptake measured per manufacturer’s instructions (Millipore, Billerica, MA). The intra-assay and inter-assay coefficients of variation were 8.4% and 10% respectively.
Serum analysis
Serum IGF-1, IGFBP-1, and IGFBP-3 levels were measured in triplicate using an in-house ELISA assay (29). The intra-assay coefficients of variation for human IGF-1, IGFBP-1 and IGF-BP3 are <10%, <10% and <6%, respectively. The inter-assay coefficients of variation for human IGF-1, IGFBP-1 and IGF-BP3 are <10%, <10% and <8%, respectively.
PSA, lipids, and insulin were measured in the UCLA clinical laboratory.
Prostate Tissue Harvesting
Following surgical removal, the prostate was immediately placed in a container surrounded by crushed ice. Within 10–15 minutes the prostate was inked and serially sectioned (typically into 5 levels for the average prostate). Levels 2 and 4 were used for research purposes and each divided into six blocks, embedded in OCT (Tissue-Tek, Sakura Finetek USA), and stored at −80C. The other sections were fixed in 10% neutral buffered formalin for whole mount embedding in paraffin blocks. Cryostat sections of the OCT blocks were evaluated by the pathologist and areas of adenocarcinoma circled. Circled areas and adjacent areas of benign tissue were macrodissected from the OCT blocks for fatty acid and prostaglandin E2 measurements.
Immunohistochemistry
Serial sections for immunohistochemical analysis were cut from paraffin embedded blocks with the largest cancer volume and with the Gleason grade corresponding to the grade on the final pathology report. Slides were stained for Ki67 and TUNEL (4), the areas of adenocarcinoma were circled by the study pathologist (JS) and digitally scanned using the Ariol SL-50 high throughput scanning system (Applied Imaging, Grand Rapids, MI) in the Translational Pathology Core Laboratory (TPCL) at UCLA. The cancerous glands within the areas of adenocarcinoma were circled by the study pathologist (JS), and the Ariol software was trained to score Ki67 or TUNEL positive cells within the circled glands. The number of Ki67 or TUNEL stained nuclei per 1,000 nuclei scored was used to calculate the percent of positive stained cells (30).
For angiogenesis, the number of CD31 stained vessels were visually counted in five 20X fields per specimen. COX2 staining was visually scored by the same pathologist for percent of cancer epithelial cells with positive staining (1=5–25%, 2=26–50%, 3=51–75% and 4=76–100%) and intensity of COX-2 staining was scored on a scale of 1 to 3 (1=low; 2=medium; 3=high intensity) (31) The COX-2 immunoreactivity score was calculated by multiplying the percent of cells staining positive by the intensity score (32). In all cases the pathologist was blinded to treatment arm.
Statistical Analysis
A sample size of 70 (35 per group) was estimated to provide 80% power to detect a 20% difference in between group changes in serum IGF-1 levels with a 2-sided alpha of 0.05. This power calculation was based on results from a prior intervention (24).
Primary Analysis
After 48 subjects fully completed the trial an interim analysis was performed to evaluate the primary outcome. We performed a conditional power analysis simulation and estimated that, with completion of enrollment to the trial, there was only a 7% chance of finding a significant difference in the mean change of IGF-1 levels between groups. Therefore, study enrollment was closed and secondary endpoint analyses were performed.
Baseline subject characteristics were compared between groups using t-tests or Fisher’s exact tests. Secondary outcomes were either measured only at surgery or operationalized as the change from baseline to surgery. These outcomes were then compared between groups using t-tests. For outcomes that had skewed distributions log(x+1) transformation was used. P-values <0.05 were considered significant. The data are presented as mean ± SD or SEM where appropriate.
Linear regression models were constructed to examine the effect of covariates on the log transformed Ki-97 outcome. Factors included race, the change in weight during the study and the biopsy or radical prostatectomy gleason grade which was separately analyzed as the gleason sum or as a categorical variable with both the primary and secondary gleason scores.
RESULTS
Baseline Characteristics
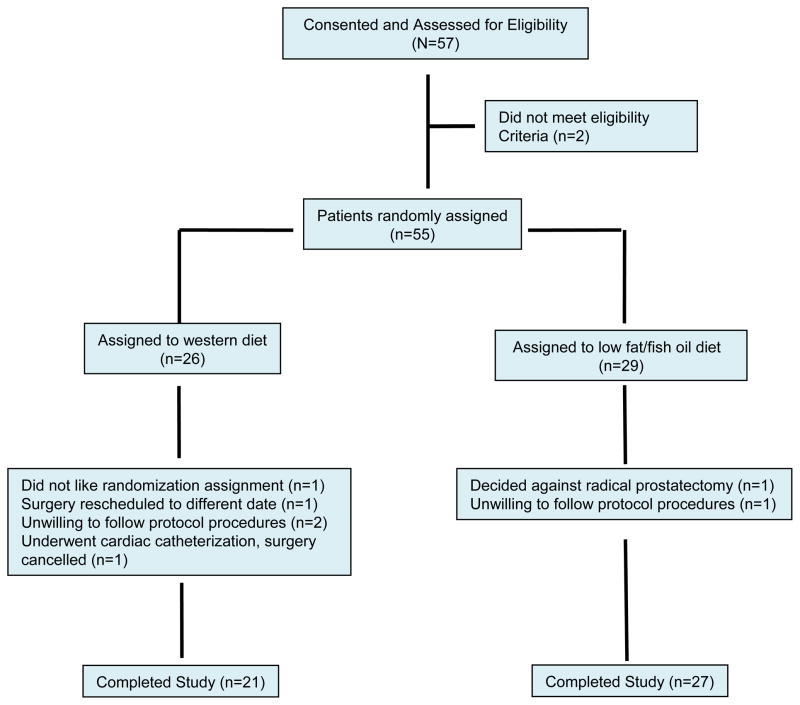
Between August 2005 and November 2008 fifty-five subjects signed consent forms and were randomized. 26 subjects were randomized to the western diet group and 29 to the low-fat/fish oil group. Of these 55 subjects, 7 withdrew from the study and 48 completed the trial (Figure 1). The baseline characteristics of these 48 subjects are shown in Table 1. The majority of patients in both groups were either overweight or obese. The median PSA in the western diet group was 6.25 ng/ml (range: 2.1–28.1 ng/ml) and the median PSA in the low-fat/fish oil group was 5.30 ng/ml (range: 1.4–22.3 ng/ml). The mean intake of fat, carbohydrates, and protein at baseline (calculated from the 3-day food records) for the group as a whole and expressed as a percentage of total energy intake was 35 ± 8%, 44 ± 10%, and 19 ± 5%, respectively, and the mean omega-6:omega-3 fatty acid ratio at baseline for the group as a whole was 9.6 ± 4 to 1.
Figure 1.
CONSORT diagram
Table 1.
Research subject baseline characteristics
| Western Diet (n=21) | Fish Oil Diet (n=27) | |
|---|---|---|
| Ethnicity | ||
| Caucasian (No.) | 10 | 21 |
| Black American (No.) | 9 | 6 |
| Hispanic (No.) | 2 | 0 |
| Age (years), mean ± SD | 60.4 ± 6.7 | 60.5 ± 6.3 |
| Weight (kg), mean ± SD | 91 ± 19.1 | 92.5 ± 13.1 |
| BMI (kg/m2), mean ± SD | 29 ± 4.2 | 29.8 ± 3.8 |
| Percent Body fat, mean +/− SD | 25.9 ± 4.7 | 29.6 ± 3.1 |
| IGF-1 (ng/ml), (mean ± SD) | 120.4 ± 47.9 | 141.8 ± 42.9 |
| IGFBP-1(ng/ml), (mean ± SD) | 15.9 ± 15.5 | 11.3 ± 13.2 |
| IGFBP- 3 (ng/ml), (mean ± SD) | 2166 ± 617 | 2181 ± 579.9 |
| PSA (ng/ml) | ||
| Mean | 7.6 | 6.9 |
| Range | 2.1–28.1 | 1.4–22.3 |
| SD | 5.6 | 4.9 |
| Median | 6.3 | 5.3 |
|
Gleason sum at diagnosis
| ||
| 6 | ||
| No. | 8 | 16 |
| % | 40 | 59.3 |
|
| ||
| 7 | ||
| No. | 11 | 9 |
| % | 55 | 33.3 |
| 3+4 | ||
| No. | 7 | 7 |
| % | 35 | 25.9 |
| 4+3 | ||
| No. | 5 | 2 |
| % | 25 | 7.4 |
|
| ||
| 8–9 | ||
| No. | 1 | 2 |
| % | 5 | 7.4 |
| No. of positive cores | ||
| Mean | 4 | 3.7 |
| Range | 1–12 | 1–10 |
| SD | 2.5 | 2.6 |
| Median | 4 | 3 |
Treatment Duration and Compliance
Subjects remained on the diet intervention prior to prostatectomy for a mean of 27.7 ± 0.5 days in the western diet group and 30.2 ± 1.9 days in the low-fat/fish oil group. Subjects in both groups were compliant with the dietary intervention with 94.8 ± 5.0% of prepared food consumed in the western diet group, and 89.5 ± 8.3% of prepared food consumed in the low-fat/fish oil group. Subjects were also compliant with consuming fish oil capsules with greater than 95% of capsules consumed based on pill counts.
Biomarker Results
The anthropometrics and changes in fasting serum biomarkers and urine PGEM levels are shown in Table 2. There were no statistically significant between group changes in serum IGF-1 levels (the primary endpoint). There were also no statistically significant changes in serum IGFBP-1, IGFBP-3, insulin, and PSA and no changes in urine PGEM levels. Triglyceride and cholesterol levels were significantly reduced in the low-fat/fish oilgroup versus the western diet group. There was a trend for increased weight loss in the low-fat/fish oil group versus the western diet group (p=0.06). There was a greater reduction in 22RV1 cell proliferation (measured by BrdU incorporation) in media containing post-intervention vs. pre-intervention patient serum in the low-fat/fish oil group versus the western diet group (−5.0 ± 1.8% vs. 0.6 ± 1.9%, p=0.039).
Table 2.
Changes in Serum and Urine Biomarkers and Anthropometrics. The data represent the mean ± SEM for each diet group
| Western Diet | Fish Oil Diet | p value | |
|---|---|---|---|
| post-intervention minus pre-intervention | post-intervention minus pre-intervention | ||
| Body weight (kg) | −1.7 ± 0.3 | −2.5 ± 0.3 | 0.06 |
| % of Body Fat | −0.5 ± 0.4 | −0.76 ± 0.3 | 0.84 |
| % of lean body mass | −0.5 ± 0.4 | −0.8 ± 0.3 | 0.58 |
| IGF-1 (ng/ml) | −0.4 ± 4.3 | 8.8 ± 6.2 | 0.25 |
| IGF BP-1 (ng/ml) | 3.3 ±1.9 | 3.8 ± 1.6 | 0.84 |
| IGF BP-3 (ng/ml) | 16.4 ± 35.3 | −62.8 ± 37.5 | 0.14 |
| Cholesterol (mg/dL) | −10.4 ± 4.4 | −31.7 ± 7.0 | 0.02 |
| LDL (mg/dL) | −4.1 ± 3.4 | −15.2 ± 7.2 | 0.22 |
| HDL (mg/dL) | −2.9 ±1.5 | −5.2 ± 1.1 | 0.24 |
| Triglycerides (mg/dL) | −13.6 ± 14.8 | −56.5 ± 12.5 | 0.03 |
| Cholesterol non HDL (mg/dL) | 3.5 ± 5.4 | − 18.1 ± 10.3 | 0.13 |
| PSA (mg/ml) | −0.09 ± 0.3 | 0.08 ± 0.4 | 0.53 |
| Insulin (mcU/ml) | −0.1 ± 1.2 | − 2.7 ± 1.2 | 0.17 |
| PGEM/Creatinine ratio | 0.3 ± 1.5 | −2.1 ± 2 | 0.36 |
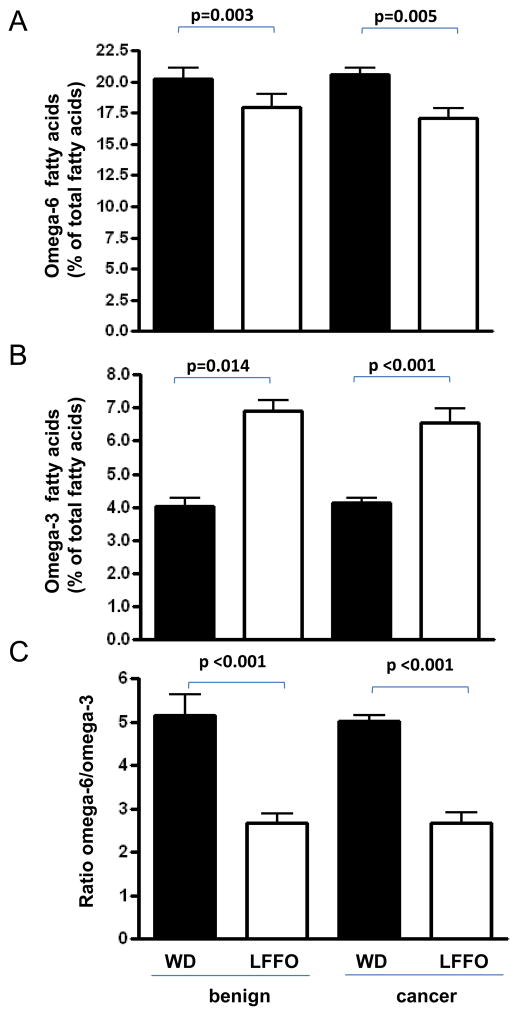
There was a significant decrease in mean levels of omega-6 fatty acids, an increase in levels of total omega-3 fatty acids, and a decrease in the omega-6:omega-3 fatty acid ratio in benign and malignant prostate tissue membranes (Figure 2 and Table 3) and in red blood cell membranes (Supplementary Table S1) from subjects consuming the low-fat/fish oil diet versus the western diet.
Figure 2.
Effect of a low-fat/fish oil diet and western diet on omega-3 and omega-6 fatty acid levels in benign and malignant prostate tissue obtained from post-intervention radical prostatectomy specimens. (A) Omega-6 fatty acid levels in benign and malignant prostate membranes are expressed as a percent of total fatty acids. (B) Omega-3 fatty acids levels in benign and malignant prostate membranes are expressed as a percent of total fatty acids. (C) Omega-6:omega-3 fatty acid ratio in benign and malignant prostate tissue membranes. In all graphs the data are presented as the mean ± SEM for each diet group. Statistical significance was assessed using unpaired t-test.
Table 3. Fatty acid levels in benign and malignant prostate tissue membranes from radical prostatectomy specimens.
The fatty acids were measured in post-intervention radical prostatectomy tissue.
| Fatty Acids | Benign Prostate Tissue | Malignant Prostate Tissue | ||||
|---|---|---|---|---|---|---|
| Western diet (n=18) (mean ± SEM) |
Fish Oil (n=19) (mean ± SEM) |
P value | Western diet (n=7) (mean ± SEM) |
Fish Oil (n=6) (mean ± SEM) |
P value | |
| Palmitic | 30.42 ± 0.87 | 30.02 ± 0.49 | 0.69 | 30.05 ± 0.38 | 31.06 ± 0.36 | 0.08 |
| Palmitoleic | 1.43 ± 0.38 | 1.33 ± 0.24 | 0.82 | 0.81 ± 0.11 | 0.75 ± 0.12 | 0.73 |
| Stearic | 18.74 ± 1.09 | 18.32 ± 0.99 | 0.78 | 20.07 ± 0.77 | 21.13 ± 0.68 | 0.33 |
| Oleic | 25.63 ± 1.78 | 26.90± 1.55 | 0.59 | 23.59 ± 1.32 | 22.47 ± 1.46 | 0.58 |
| LA (18:2, n-6) | 11.23 ± 0.75 | 10.50 ± 0.57 | 0.44 | 12.11 ± 0.46 | 9.73 ± 0.48 | 0.005 |
| α-linolenic (n-3) | 0.67 ± 0.13 | 0.57 ± 0.08 | 0.50 | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.98 |
| Eicosadienoic (n-6) | 0.72 ± 0.07 | 0.72 ± 0.13 | 0.98 | 0.82 ± 0.06 | 1.00 ± 0.17 | 0.33 |
| AA (20:4, n-6) | 7.11 ± 0.58 | 5.85 ± 0.44 | 0.09 | 8.44 ± 0.60 | 7.32 ± 0.68 | 0.24 |
| EPA (20:5, n-3) | 0.10 ± 0.02 | 0.42 ± 0.05 | <0.001 | 0.07 ± 0.01 | 0.44 ± 0.07 | <0.001 |
| Docosapentaenoic(n-3) | 0.70 ± 0.07 | 0.81 ± 0.33 | 0.33 | 0.85 ± 0.06 | 0.90 ± 0.08 | 0.58 |
| DHA (22:6, n-3) | 3.25 ± 0.30 | 4.55 ± 0.38 | 0.011 | 3.01 ± 0.13 | 5.03 ± 0.39 | <0.001 |
| Total n-6 | 18.34 ± 0.50 | 16.35 ± 0.41 | 0.003 | 20.5 ± 0.57 | 17 ± 0.86 | 0.005 |
| Total n-3 | 4.72 ± 0.38 | 6.35 ± 0.50 | 0.014 | 4.11 ± 0.15 | 6.54 ± 0.43 | <0.001 |
| n-6/n-3 | 4.48 ± 0.52 | 3.07 ± 0.42 | 0.042 | 5.02 ± 0.46 | 2.68 ± 0.24 | <0.001 |
Fatty acid content is expressed as a percent of total fatty acids. n-3= omega-3;, n-6= omega 6; LA= linoleic acid; AA= arachidonic acid; EPA= Eicosapentaenoic acid, DHA= docosahexaenoic acid
Immunohistochemistry and Tissue Prostaglandin E2 Levels
There was a significant 32.2% decrease in malignant epithelial cell proliferation (Ki67) in the low-fat/fish oil group versus the western diet group (Figure 3). After controlling for race, change in weight and gleason grade (prostate needle biopsy or radical prostatectomy) either as the sum or the primary and secondary scores, we found that the intervention effect on Ki-67 remained significant (p<0.05). There was no significant difference in mean prostaglandin E2 levels in benign or malignant prostate tissue between treatment groups and no difference in COX-2, angiogenesis (CD-31), or apoptosis (TUNEL) immunostaining (Supplementary Figure S1).
Figure 3.
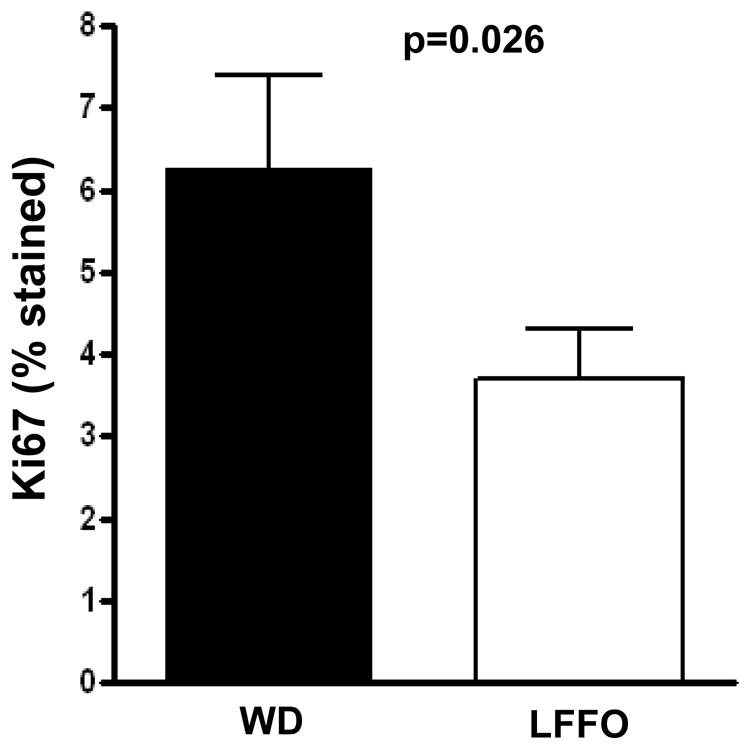
Effect of a low-fat/fish oil diet compared with a western diet on Ki67 expression in malignant epithelial prostate cells in post-intervention radical prostatectomy specimens. Ki67 immunostaining was digitally quantified using Ariol software and expressed as percent of positive cells. The data represent the mean ± SEM for each diet group. The Ki67 results had a skewed distribution and were therefore log transformed using log(x+1) for statistical analysis. Statistical significance was assessed using unpaired t-test.
Safety
Overall the diet interventions were well tolerated and all adverse events were Grade 1. In the low-fat/fish oil group, 5 subjects reported increased flatulence, 2 reported diarrhea that was self limited, and one reported eructation (burping). In the western diet group, adverse events reported were increased frequency of bowel movements in 1 subject, flatulence in 1 subject, and constipation in 1 subject.
DISCUSSION
Based on pre-clinical studies demonstrating that decreasing dietary fat and decreasing the omega-6:omega-3 fatty acid ratio inhibits carcinogenesis and prostate cancer progression (1, 4, 5, 23) we conducted a prospective randomized Phase II trial to evaluate biomarkers associated with prostate cancer development and progression. The rationale for the primary endpoint of this trial (between group change in serum IGF-1 levels) was based on prior pre-clinical studies suggesting decreased dietary fat and omega-6 fatty acid intake decreased prostate cancer development and progression through a reduction in serum IGF-1 levels (4, 24) In the present trial we found no significant change in serum IGF-1, IGFBP-1 or insulin levels. Several other dietary intervention trials incorporating dietary fat reduction also demonstrated no change in serum IGF-1 or IGFBP-1 levels (33–37). In prior clinical trials, decreased IGF-1 and increased IGFBP-1 levels were typically found in the setting of significant weight loss or reduction in serum insulin (24, 38–40). Subjects in the present study did not have significant weight loss or reduction in serum insulin which might explain the lack of effect on IGF-1 and IGFBP-1 levels.
While we found no effect on the primary outcome, we did find significant effects in some secondary analyses. Malignant epithelium proliferation, as measured by Ki67 immunostaining, was significantly reduced in the low-fat/fish oil group versus the western diet group. Ki67 immunostaining has been shown to independently predict recurrence after radical prostatectomy and prostate cancer specific survival (41–44). The mechanism through which the dietary intervention affected malignant epithelium proliferation is unknown. The fact that there were no changes in the serum IGF axis parameters and no change in tissue COX-2 and PGE-2 levels suggests the intervention targeted other pathways involved in proliferation. The finding that the low-fat/fish oil intervention reduced serum-stimulated proliferation of 22RV1 cells in an ex-vivo bioassay suggests alterations in serum growth factors may be responsible for the reduced proliferation seen in the tissue. Potential targets affected by the low-fat/fish oil intervention include eicosanoid synthesis pathways and expression of inflammatory cytokines (26, 45). Given the well-established association of Ki67 and prostate cancer progression, and the impact of the low-fat/fish oil intervention on Ki67, future trials are warranted evaluating whether altering dietary fat and the dietary omega-6:omega-3 ratio favorably alters proliferation and other clinical prostate cancer endpoints.
A novel finding in this trial was that reducing dietary fat and the omega-6:omega-3 fatty acid ratio resulted in significant changes in the fatty acid levels in benign and malignant prostate tissue membranes. Subjects in the low-fat/fish oil group had lower omega-6 levels and higher omega-3 levels in the prostate tissue membranes relative to the western diet group. The ratio of omega-6:omega-3 fatty acid levels in cell membranes is believed to play an important role in signaling pathways leading to prostate cancer development and progression (1, 4, 25, 26, 45, 46). In prior xenograft studies, decreasing the dietary omega-6:omega-3 fatty acid ratio resulted in a reduction in the omega-6:omega-3 fatty acid ratio in the xenograft membranes and decreased COX-2 and PGE-2 levels (2, 4). PGE2 is known to increase prostate cancer proliferation, invasiveness, and angiogenesis (26, 45, 47). Whereas altering the dietary omega-6:omega-3 fatty acid ratio in our trial led to alteration in the fatty acid composition of benign and malignant prostate tissue membranes, there was no significant difference in COX-2 or PGE-2 levels in prostate tissue and no difference in urinary PGEM levels, a stable metabolite of PGE-2 (27). In a 3-month trial evaluating 3 grams of fish oil per day (without modifying dietary fat) in men on expectant management, Chan et al. found no change in COX-2 expression in prostate tissue (48). Berquin et al. previously demonstrated that a high omega-3 diet delayed the development and progression of prostate cancer in a prostate-specfic PTEN-knockout mouse model (1). In their model they saw increased apoptosis in the omega-3 fed group, possibly through effects on Bad phosphorylation. In the present trial altering the omega-6:omega-3 fatty acid ratio in humans did not impact on apoptosis in malignant tissue.
One shortcoming of the present trial is the short duration of the intervention. In our experience, patients that elect to undergo radical prostatectomy generally desire their surgery within 1–2 months and it is not feasible to enroll large numbers of patients for a longer duration. A potential criticism of this trial is that dietary fat reduction alone or intake of fish oil capsules without dietary fat reduction was not tested. As such, we cannot discern whether either treatment alone would have affected proliferation or whether the combination of dietary fat and fish oil supplementation is required. Demark-Wahnefried et al. previously found no effect of dietary fat reduction on proliferation (Ki67) in men undergoing radical prostatectomy, suggesting that dietary fat reduction alone does not alter proliferation (34). However, the low-fat intervention in that study was targeted to 20% Kcal fat whereas in our trial the low-fat diet provided 15% Kcal from fat, so it remains possible that a more stringent reduction of dietary fat (as was the case in the present trial) may have led to reduced proliferation. It is unknown if consumption of the fish oil capsules without changing the dietary fat content would have affected proliferation. We elected to combine the two interventions (dietary fat reduction and fish oil supplementation) based on preclinical trials that demonstrated decreased development and progression of prostate cancer associated with reducing the ratio of omega-6:omega-3 fatty acids and reducing dietary fat intake (2, 4, 45, 49). We would not have been able to achieve an omega-6:omega-3 ratio of 2:1 without reducing fat intake since lowering dietary fat intake reduces omega-6 fatty acid intake and, when combined with the omega-3 fish oil capsules, allows for a significant reduction in the omega-6:omega-3 ratio. Another potential criticism of the present trial is that there was a difference in carbohydrate intake between the groups with 45% of energy from carbohydrates (15 grams fiber/day) in the western diet group vs. 70% of energy from carbohydrates (39 grams fiber/day) in the low-fat fish oil group, and potentially the alteration in carbohydrate and/or fiber intake may have been responsible for the change in prostate cancer proliferation. The low-fat/fish oil group also had a reduction in serum cholesterol levels relative to the western diet group, and cholesterol (through a number of mechanisms) may potentially affect prostate cancer growth (50). In addition, there was a trend for increased weight loss in the low-fat/fish oil group relative to the western group and this may potentially effect proliferation. It is likely that multiple factors play a role in nutritional effects on tumor biology including nutrient-nutrient interactions, gene-nutrient interactions, and host susceptibility factors (11, 45). The findings in the present trial, that modulation of dietary fat with fish oil intake modified benign and malignant prostate tissue fatty acid levels and affected prostate cancer proliferation, suggests that the dietary intervention has the potential to affect important aspects of tumor biology related to progression. These results are to be considered hypothesis generating, and further prospective randomized trials are warranted to evaluate alteration of quantity and quality of dietary fat on tumor biology and carcinogenesis.
In summary, 4–6 weeks of neoadjuvant dietary fat reduction with fish oil supplementation did not affect serum IGF-1 levels. In secondary analyses, we found this intervention resulted in a decrease in omega-6:omega-3 fatty acid ratios in benign and malignant prostate tissue and a decrease in malignant epithelial cell proliferation as measured by Ki67 immunostaining. Validation of these results with proliferation as the primary outcome will support the performance of long-term dietary intervention trials with clinical progression endpoints.
Supplementary Material
Effect of a low-fat/fish oil diet compared to a western diet on (A) apoptosis, (B) angiogenesis, (C) COX2 expression in radical prostatectomy tissue, and on PGE2 levels in (D) benign and (E) malignant prostate tissue extract. In all graphs the data are presented as the mean ± SEM for each diet group. Statistical significance was assessed using unpaired t-test.
Acknowledgments
Supported by National Cancer Institute (NCI) Grant Number P50CA92131, the UCLA General Clinical Research Center with NCI grant number M01-RR000865, and grant 2P30DK063491, and Wendy and Ken Ruby, President and Secretary of the Ruby Family Foundation.
Grant support: Supported by National Cancer Institute (NCI) Grant Number P50CA92131, the UCLA General Clinical Research Center with NCI grant number M01-RR000865, and grant 2P30DK063491.
We acknowledge Edward Shneyvas from Pharmavite LLC for his intellectual input and providing the fish oil capsules for this trial. We thank Clara Magyar from the Translational Pathology Core Laboratory at UCLA for her expert training with the Ariol SL-50 system.
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest to declare.
References
- 1.Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117:1866–75. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karmali RA, Reichel P, Cohen LA, Terano T, Hirai A, Tamura Y, et al. The effects of dietary omega-3 fatty acids on the DU-145 transplantable human prostatic tumor. Anticancer Res. 1987;7:1173–9. [PubMed] [Google Scholar]
- 3.Rose DP, Cohen LA. Effects of dietary menhaden oil and retinyl acetate on the growth of DU 145 human prostatic adenocarcinoma cells transplanted into athymic nude mice. Carcinogenesis. 1988;9:603–5. doi: 10.1093/carcin/9.4.603. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi N, Barnard RJ, Henning SM, Elashoff D, Reddy ST, Cohen P, et al. Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin Cancer Res. 2006;12:4662–70. doi: 10.1158/1078-0432.CCR-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngo TH, Barnard RJ, Cohen P, Freedland S, Tran C, deGregorio F, et al. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003;9:2734–43. [PubMed] [Google Scholar]
- 6.Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764–6. doi: 10.1016/S0140-6736(00)04889-3. [DOI] [PubMed] [Google Scholar]
- 7.Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC, et al. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:64–7. [PubMed] [Google Scholar]
- 8.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–16. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CC, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–9. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 10.Whittemore AS, Kolonel LN, Wu AH, John EM, Gallagher RP, Howe GR, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst. 1995;87:652–61. doi: 10.1093/jnci/87.9.652. [DOI] [PubMed] [Google Scholar]
- 11.Fradet Y, Meyer F, Bairati I, Shadmani R, Moore L. Dietary fat and prostate cancer progression and survival. Eur Urol. 1999;35:388–91. doi: 10.1159/000019913. [DOI] [PubMed] [Google Scholar]
- 12.Lophatananon A, Archer J, Easton D, Pocock R, Dearnaley D, Guy M, et al. Dietary fat and early-onset prostate cancer risk. Br J Nutr. 2010;103:1375–80. doi: 10.1017/S0007114509993291. [DOI] [PubMed] [Google Scholar]
- 13.Schuurman AG, van den Brandt PA, Dorant E, Brants HA, Goldbohm RA. Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study. Cancer. 1999;86:1019–27. [PubMed] [Google Scholar]
- 14.Crowe FL, Key TJ, Appleby PN, Travis RC, Overvad K, Jakobsen MU, et al. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;87:1405–13. doi: 10.1093/ajcn/87.5.1405. [DOI] [PubMed] [Google Scholar]
- 15.Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci EL, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994;86:281–6. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- 16.Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta-analysis. Am J Clin Nutr. 2010;92:1223–33. doi: 10.3945/ajcn.2010.29530. [DOI] [PubMed] [Google Scholar]
- 17.Chavarro JE, Stampfer MJ, Hall MN, Sesso HD, Ma J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am J Clin Nutr. 2008;88:1297–303. doi: 10.3945/ajcn.2008.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999;81:1238–42. doi: 10.1038/sj.bjc.6690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brasky TM, Till C, White E, Neuhouser ML, Song X, Goodman P, et al. Serum Phospholipid Fatty Acids and Prostate Cancer Risk: Results From the Prostate Cancer Prevention Trial. Am J Epidemiol. 2011 doi: 10.1093/aje/kwr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowe FL, Allen NE, Appleby PN, Overvad K, Aardestrup IV, Johnsen NF, et al. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;88:1353–63. doi: 10.3945/ajcn.2008.26369. [DOI] [PubMed] [Google Scholar]
- 21.Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer. 1997;71:545–51. doi: 10.1002/(sici)1097-0215(19970516)71:4<545::aid-ijc7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Wilkens LR, Henning SM, Le Marchand L, Gao K, Goodman MT, et al. Circulating fatty acids and prostate cancer risk in a nested case-control study: the Multiethnic Cohort. Cancer Causes Control. 2009;20:211–23. doi: 10.1007/s10552-008-9236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi N, Barnard RJ, Said J, Hong-Gonzalez J, Corman DM, Ku M, et al. Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-Myc transgenic mouse model. Cancer Res. 2008;68:3066–73. doi: 10.1158/0008-5472.CAN-07-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States) Cancer Causes Control. 2002;13:929–35. doi: 10.1023/a:1021911517010. [DOI] [PubMed] [Google Scholar]
- 25.Reese AC, Fradet V, Witte JS. Omega-3 fatty acids, genetic variants in COX-2 and prostate cancer. J Nutrigenet Nutrigenomics. 2009;2:149–58. doi: 10.1159/000235565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–45. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 27.Demers LM, Brennecke SP, Mountford LA, Brunt JD, Turnbull AC. Development and validation of a radioimmunoassay for prostaglandin E2 metabolite levels in plasma. J Clin Endocrinol Metab. 1983;57:101–6. doi: 10.1210/jcem-57-1-101. [DOI] [PubMed] [Google Scholar]
- 28.Aronson WJ, Glaspy JA, Reddy ST, Reese D, Heber D, Bagga D. Modulation of omega-3/omega-6 polyunsaturated ratios with dietary fish oils in men with prostate cancer. Urology. 2001;58:283–8. doi: 10.1016/s0090-4295(01)01116-5. [DOI] [PubMed] [Google Scholar]
- 29.Moran A, Jacobs DR, Jr, Steinberger J, Cohen P, Hong CP, Prineas R, et al. Association between the insulin resistance of puberty and the insulin-like growth factor-I/growth hormone axis. J Clin Endocrinol Metab. 2002;87:4817–20. doi: 10.1210/jc.2002-020517. [DOI] [PubMed] [Google Scholar]
- 30.Ong CW, Kim LG, Kong HH, Low LY, Wang TT, Supriya S, et al. Computer-assisted pathological immunohistochemistry scoring is more time-effective than conventional scoring, but provides no analytical advantage. Histopathology. 2010;56:523–9. doi: 10.1111/j.1365-2559.2010.03496.x. [DOI] [PubMed] [Google Scholar]
- 31.Rao DS, Gui D, Koski ME, Popoviciu LM, Wang H, Reiter RE, et al. An inverse relation between COX-2 and E-cadherin expression correlates with aggressive histologic features in prostate cancer. Appl Immunohistochem Mol Morphol. 2006;14:375–83. doi: 10.1097/01.pai.0000210417.61117.6c. [DOI] [PubMed] [Google Scholar]
- 32.Fux R, Schwab M, Thon KP, Gleiter CH, Fritz P. Cyclooxygenase-2 expression in human colorectal cancer is unrelated to overall patient survival. Clin Cancer Res. 2005;11:4754–60. doi: 10.1158/1078-0432.CCR-04-2586. [DOI] [PubMed] [Google Scholar]
- 33.Aronson WJ, Barnard RJ, Freedland SJ, Henning S, Elashoff D, Jardack PM, et al. Growth inhibitory effect of low fat diet on prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J Urol. 2010;183:345–50. doi: 10.1016/j.juro.2009.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demark-Wahnefried W, Polascik TJ, George SL, Switzer BR, Madden JF, Ruffin MTt, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomarkers Prev. 2008;17:3577–87. doi: 10.1158/1055-9965.EPI-08-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flood A, Mai V, Pfeiffer R, Kahle L, Remaley AT, Rosen CJ, et al. The effects of a high-fruit and -vegetable, high-fiber, low-fat dietary intervention on serum concentrations of insulin, glucose, IGF-I and IGFBP-3. Eur J Clin Nutr. 2008;62:186–96. doi: 10.1038/sj.ejcn.1602726. [DOI] [PubMed] [Google Scholar]
- 36.Al-Delaimy WK, Natarajan L, Rock CL, Sun S, Flatt SW, Pierce JP. Insulin-like growth factor I, insulin-like growth factor I binding protein 1, insulin, glucose, and leptin serum levels are not influenced by a reduced-fat, high-fiber diet intervention. Cancer Epidemiol Biomarkers Prev. 2006;15:1238–9. doi: 10.1158/1055-9965.EPI-06-0160. [DOI] [PubMed] [Google Scholar]
- 37.Gann PH, Kazer R, Chatterton R, Gapstur S, Thedford K, Helenowski I, et al. Sequential, randomized trial of a low-fat, high-fiber diet and soy supplementation: effects on circulating IGF-I and its binding proteins in premenopausal women. Int J Cancer. 2005;116:297–303. doi: 10.1002/ijc.21042. [DOI] [PubMed] [Google Scholar]
- 38.Kiddy DS, Hamilton-Fairley D, Seppala M, Koistinen R, James VH, Reed MJ, et al. Diet-induced changes in sex hormone binding globulin and free testosterone in women with normal or polycystic ovaries: correlation with serum insulin and insulin-like growth factor-I. Clin Endocrinol (Oxf) 1989;31:757–63. doi: 10.1111/j.1365-2265.1989.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 39.Kaaks R, Bellati C, Venturelli E, Rinaldi S, Secreto G, Biessy C, et al. Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: the Diet and Androgens (DIANA) Randomised Trial. Eur J Clin Nutr. 2003;57:1079–88. doi: 10.1038/sj.ejcn.1601647. [DOI] [PubMed] [Google Scholar]
- 40.Tymchuk CN, Barnard RJ, Heber D, Aronson WJ. Evidence of an inhibitory effect of diet and exercise on prostate cancer cell growth. J Urol. 2001;166:1185–9. [PubMed] [Google Scholar]
- 41.Bubendorf L, Sauter G, Moch H, Schmid HP, Gasser TC, Jordan P, et al. Ki67 labelling index: an independent predictor of progression in prostate cancer treated by radical prostatectomy. J Pathol. 1996;178:437–41. doi: 10.1002/(SICI)1096-9896(199604)178:4<437::AID-PATH484>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Bubendorf L, Tapia C, Gasser TC, Casella R, Grunder B, Moch H, et al. Ki67 labeling index in core needle biopsies independently predicts tumor-specific survival in prostate cancer. Hum Pathol. 1998;29:949–54. doi: 10.1016/s0046-8177(98)90199-x. [DOI] [PubMed] [Google Scholar]
- 43.Veltri RW, Isharwal S, Miller MC, Epstein JI, Mangold LA, Humphreys E, et al. Long-term assessment of prostate cancer progression free survival: evaluation of pathological parameters, nuclear shape and molecular biomarkers of pathogenesis. Prostate. 2008;68:1806–15. doi: 10.1002/pros.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berney DM, Gopalan A, Kudahetti S, Fisher G, Ambroisine L, Foster CS, et al. Ki-67 and outcome in clinically localised prostate cancer: analysis of conservatively treated prostate cancer patients from the TransAtlantic Prostate Group study. Br J Cancer. 2009;100:888–93. doi: 10.1038/sj.bjc.6604951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–77. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fradet V, Cheng I, Casey G, Witte JS. Dietary omega-3 fatty acids, cyclooxygenase-2 genetic variation, and aggressive prostate cancer risk. Clin Cancer Res. 2009;15:2559–66. doi: 10.1158/1078-0432.CCR-08-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain S, Chakraborty G, Raja R, Kale S, Kundu GC. Prostaglandin E2 regulates tumor angiogenesis in prostate cancer. Cancer Res. 2008;68:7750–9. doi: 10.1158/0008-5472.CAN-07-6689. [DOI] [PubMed] [Google Scholar]
- 48.Chan JM, Weinberg V, Magbanua MJ, Sosa E, Simko J, Shinohara K, et al. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer Causes Control. 2011;22:141–50. doi: 10.1007/s10552-010-9684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelavkar UP, Hutzley J, Dhir R, Kim P, Allen KG, McHugh K. Prostate tumor growth and recurrence can be modulated by the omega-6:omega-3 ratio in diet: athymic mouse xenograft model simulating radical prostatectomy. Neoplasia. 2006;8:112–24. doi: 10.1593/neo.05637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of a low-fat/fish oil diet compared to a western diet on (A) apoptosis, (B) angiogenesis, (C) COX2 expression in radical prostatectomy tissue, and on PGE2 levels in (D) benign and (E) malignant prostate tissue extract. In all graphs the data are presented as the mean ± SEM for each diet group. Statistical significance was assessed using unpaired t-test.