Abstract
Preclinical and clinical studies suggest that 5-lipoxygenase (5-LOX), like cyclo-oxygenase-2 (COX-2), is a potential target for colon cancer inhibition and, in part, contributes to cardiovascular side effects associated with COX-2 inhibitors. Experiments were designed to assess the chemopreventive effects of a novel dual 5-LOX/COX inhibitor, Licofelone, in APCMin/+ mouse intestinal tumorigenesis. Six week-old male and female APCMin/+ mice (n=10 per group) were fed control AIN-76A diet or diets containing 150 or 300 ppm licofelone for 14 weeks (~100 days), and intestinal tumors were evaluated for tumor multiplicity and size. Licofelone significantly inhibited total intestinal tumor multiplicity and size in a dose-dependent manner (p<0.0001; mean tumors for 0, 150 and 300 ppm: 48.8, 17, and 8, respectively, in male mice; and 34.3, 8.8, and 5.5, respectively, in female mice). Licofelone at high-dose showed >83% (p<0.0001) tumor inhibition in both genders of mice. 150 and 300 ppm licofelone resulted 86%–97% inhibition of polyps>2-mm. 150 and 300 ppm licofelone caused >72% and 100% inhibition of colonic tumors, respectively. Importantly, in mice fed licofelone, tumors showed significantly reduced PCNA expression (70%, P<0.0001), increased TUNEL positive cells (75%, p<0.0001), and there was dose-dependent suppression of serum triglycerids (71–83%, p<0.0001), decreased inflammatory cytokines; and decreased COX and 5-LOX activities (57–64%, p<0.0001). Also, compared with 300 ppm celecoxib, 300 ppm licofelone provided better efficacy in suppressing tumor growth. These observations demonstrate that a novel dual 5-LOX/COX inhibitor dramatically suppresses small intestinal and colonic tumor formation in APCMin/+ mice.
Keywords: colon cancer, chemoprevention, LOX/COX inhibitor, Licofelone, APCMin/+
Introduction
Colon cancer is the third leading cause of cancer mortality for men and women in the USA (1). Globally, 1.1 million cases and 600,000 deaths are reported every year from colorectal cancers. Drug development has led to discovery of potential Chemopreventive agents that are effective at the preclinical and clinical levels (2–7). Agents that target COX-2, such as celecoxib, are noteworthy because of their clinical efficacy in the prevention of polyp formation, and for contributing to cardiovascular risk (7). Recent five-year efficacy and safety analysis of adenoma prevention with celecoxib suggests a significant interaction between celecoxib treatment and cardiovascular and thrombotic events for those reporting a baseline history of atherosclerotic heart disease (7). Overall, targeting COX-2 for colon cancer prevention is still valid; but use of higher doses of COX-2 inhibitors in individuals at high-risk for colon cancer and more so, in those at high risk for atherosclerotic events suggests a need for new approaches for colon cancer prevention and treatment.
Several lines of evidence show that 5-lipoxygenase (5-LOX) and its molecular partner, 5-LOX activating protein (FLAP), are significantly over-expressed in colonic tumors harvested from preclinical and clinical samples (8–13). It is evident from recent studies that the 5-LOX metabolite leukotriene (LT) B4 (LTB4) and the cysteinyl leukotrienes (cys-LT) LTC4, LTD4, and LTE4 may contribute to the development and progression of colon cancer (14, 15). Similarly to COX-2, the expression and activity of 5-LOX have been found to be up-regulated during colon carcinogenesis (16) and closely related to tumor size, depth and vessel invasion (14). Thus, 5-LOX has been implicated in both inflammation and carcinogenesis (9–13). Hence, 5-LOX has been considered a potential target for colon cancer inhibition (17, 18).
Some effects of COX-2 inhibition include shunting of arachidonic acid (AA) into the 5-LOX pathway. There is evidence linking leukotrienes to cardiovascular disease (19) with increased urinary levels of LTE4 in patients with myocardial infarction and coronary artery disease (19). In this context, it is noteworthy that studies by Duffield-Lillico et al. demonstrate higher urinary levels of prostaglandin (PG)E metabolites and LTE4 in smokers. Smokers on celecoxib, however, showed shunting of arachidonic acid in to 5-LOX pathways with increased levels of LTE4 (19). In addition, the 5-LOX pathway is abundantly expressed in arterial walls of patients with various stages of atherosclerosis (20) and 5-LOX also has been linked to atherosclerosis in some mouse models (21). Furthermore, two human genetic studies have correlated polymorphisms of the 5-LOX pathway with relative risk for myocardial infarction, stroke, and atherosclerosis (22,23). In fact, therapies are being developed to target the 5-LOX pathway to reduce the risk of myocardial infarction (24,25). The striking interrelationship of their biological functions suggest that molecules that are able to block both COX-2 and 5-LOX pathways may provide a promising approach to colon cancer prevention.
To date, several dual COX/LOX inhibitors have been designed and several compounds currently are undergoing clinical development as anti-inflammatory drugs (26). However, no data is available concerning their potential use as anticancer agents in colon cancer. It is important to evaluate the possible anti-tumorigenic effects of the dual COX/5-LOX inhibitor licofelone {[6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1H-pyrrolizin-5-yl] acetic acid} in APCMin/+ mouse intestinal tumorigenesis. Among dual COX/LOX inhibitors, licofelone is the molecule in the most advanced phase of clinical trials as an anti-inflammatory drug (27), and its safety and efficacy, in comparison with the non-steroidal anti-inflammatory drugs (NSAIDs) naproxen and rofecoxib, have been reviewed (28,29). In the following experiments we show that licofelone is able to inhibit dramatically both small intestinal and colon tumorigenesis in APCMin/+ mice. Licofelone, as well as similar dual COX/5-LOX inhibitors, may represent a novel class of chemopreventive agents for colon cancer prevention and treatment.
Materials and Methods
Chemicals
Licofelone (Merckle–ratiopharm, GmbH, Ulm, Germany) (Fig. 1A) was kindly provided by the National Cancer Institute chemopreventive drug repository (Rockville, MD). Primary antibodies (monoclonal/polyclonal) to COX-2, 5-LOX, and proliferating cell nuclear antigen (PCNA), were from Santa Cruz Biotechnology (Santa Cruz, CA); horseradish peroxidase-conjugated secondary antibodies were from Santa Cruz Biotechnology. Multi-Analyte ELISArray™ kit was procured from SA Biosciences (Frederick, MD).
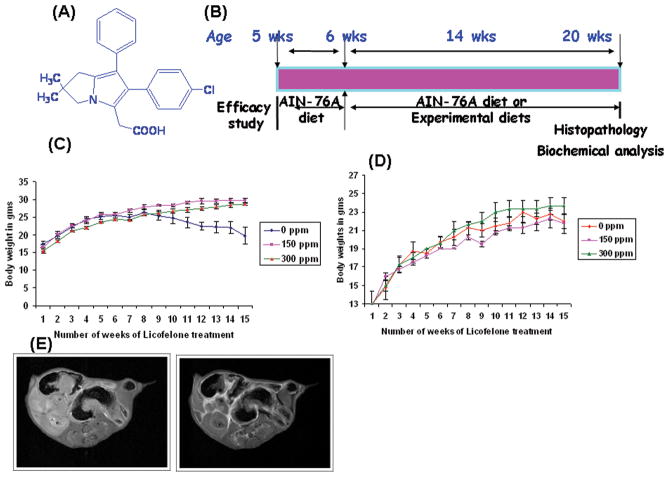
Figure 1.
A. Chemical structure of licofelone,(2-[6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1H-pyrrolizin-5-yl]acetic acid)
B. Experimental design for the evaluation of the chemopreventive efficacy of licofelone in APCmin/+ mice. Groups (10 mince/group) of mice were fed diets containing 0, 150 or 300 ppm licofelone from 6 weeks of age to the end of the experiment. The study was terminated after 100 days of exposure to the experimental diets. (See Materials and Methods for more details).
C & D. Changes in body weights over time for male (C) or female (D) mice fed control diets and/or experimental diets containing 150 or 300 ppm licofelone. Statistically significant differences in body weight gain were observed between licofelone-treated and control groups. Data are presented as Means ± SEM. Statistical Analysis was carried with ANOVA. Licofelone-treated animals were found to gain weight by the end of the study.
E. MRI of the small intestine in APCMin/+ mice. Small intestines of the treated and control animals were scanned for polyps by MRI. Comparing images from pre-and post-magnivest injection, polyps were identified as bright from accumulating magnivest. Left and right panels show polyp images in control and treated mice respectively.
Breeding and genotyping of APCMin/+ mice
All animal experiments were performed in accordance with the institutional guidelines of the American Council on Animal Care and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Oklahoma Health Sciences Center (OUHSC). Male APCMin/+ (C57BL/6J) and female wild-type littermate mice were purchased initially from The Jackson Laboratory (Bar Harbor, ME) as founders, and our own breeding colony was established in the OUHSC rodent barrier facility and genotyped by the polymerase chain reaction (PCR) method using primers (IMR0033 5′-GCC ATC CCT TCA CGT TAG-3′, IMR0034 5′-TTC CAC TTT GGC ATA AGG C-3′, IMR0758 5′-TTC TGA GAA AGA CAG AAG TTA-3′) according to vendor’s instructions. All mice were housed 3 per cage in ventilated cages under standardized conditions (21°C, 60% relative humidity, 12h light/12 h dark cycle, 20 air changes/hr). All mice were allowed ad libitum access to the respective diets and automated tap water purified by reverse osmosis.
Experimental Diets
All ingredients for the semi-purified diets were purchased from Bioserv (Frenchtown, NJ) and stored at 4 °C before diet preparation. Diets were based on the modified American Institute of Nutrition (AIN)-76A diet with slight modification. Licofelone was premixed with a small quantity of diet and then blended into bulk diet using a Hobart mixer. Both control and experimental diets were prepared weekly and stored in a cold room. Agent content in the experimental diets was determined periodically in multiple samples taken from the top, middle, and bottom portions of individual diet preparations to verify uniform distribution. In this study, we used 0 ppm, 150 ppm or 300 ppm licofelone in the AIN-76A diet.
Efficacy studies in APCMin/+ mice
Both male and female APCMin/+ mice were used in the efficacy study. The experimental protocol is summarized in Fig. 1B. Five week-old mice were randomized so that average body weights in each group were equal (10 APCMin/+ mice in each group) and mice were fed AIN-76A diet for one week. At 6 weeks of age, mice were fed control or experimental diets containing 0 ppm, 150 or 300 ppm licofelone until termination of the study. Body weight, food and water consumption were monitored regularly and mice were evaluated weekly for signs of weight loss or lethargy that might indicate intestinal obstruction or anemia. After 100 days (~14 weeks) of exposure to licofelone diet, all mice were euthanized by CO2 asphyxiation, blood was collected immediately by heart puncture, and serum was separated by centrifugation and stored at −80°C until further analysis. This point in time was chosen to minimize the risk of mortality caused by severe progressive anemia, rectal prolapse, or intestinal obstruction, which usually occurs among Min mice at >20 weeks of age. After necropsy, the entire intestinal tract was harvested, flushed with 0.9% NaCl and opened longitudinally from the esophagus to the distal rectum. The tissue was flattened on filter paper to expose the tumors and briefly frozen on dry ice to aid visual scoring of tumors. The number, location, and size of visible tumors in the entire intestine were determined under a dissection microscope (X5). All tumors were scored and subdivided by location (duodenal, jejunal and ileum and colorectal) and size (>2 mm, 1–2 or <1 mm in diameter). This procedure was completed by two individuals who were blinded to the experimental group and the genetic status of the mice. Colonic and other small intestinal tumors that required further histopathologic evaluation to identify adenoma, adenocarcinoma, and enlarged lymph nodes were fixed in 10% neutral-buffered formalin, embedded in paraffin blocks, and processed by routine H&E staining. In addition, multiple samples of tumors from the small intestines were harvested and stored in liquid nitrogen for analysis of COX-2 and 5-LOX activities and expression levels.
Small intestinal and colon tumor count by Magnetic Resonance Imaging(MRI)
At the end of the study, before euthanasia, MRI imaging was performed using Tesla to assess the polyp count in live mice. Briefly, mice were anasthetized using 1.5% isoflurane at 0.7 L/min oxygen. A tail vein catheter was installed and the mouse was placed in a MR probe in a supine position. A 2D dataset was acquired (1 mm thick transverse slices covering the rectum, colon and small intestine, giving an in-plane resolution of 120 × 120 um2, for an acquisition time of 14 min) as well as a 3D dataset (volume acquired 30 × 18 × 36 mm3, pixel resolution of 120 × 200 × 555 um3, for an acquisition time of 24 min). After the scans were acquired, magnivist (gadopentate dimeglumine) was injected into the mice at a dose of 0.17 mmol/Kg. Magnivist contrast agent is paramagnetic and decreases T1 and T2 relaxation times. In T1 weighted datasets, tissues that accumulate magnivest appear bright in the case of polyps.
Serum Triglycerides, Packed cell volume
Triglycerides were determined in the non-hemolysed serum with InfinityTM Triglycerides liquid stable reagent (Thermo scientific) as per the manufacturer’s instructions. For packed cell volume/Hematocrit measurement, blood was sampled by cardiac puncture with a 21 gauge needle attached to a 1 ml syringe and dispensed into a plastic microfuge tube while on ice. Micro-hematocrit tubes containing ammonium heparin were then placed in the microfuge tube and centrifuged in a hematocrit centrifuge for 5 min.
Apoptosis
Intestinal tumor tissues from licofelone-treated and -untreated mice were fixed in 10% formalin for 24 h and then embedded in paraffin. Sections of 5 μ thickness were cut and mounted on slides, rehydrated, and stained using the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) method as previously described. TUNEL positive cells were visualized by chromogenic staining with DAB and slides were counterstained with methyl green. Stained apoptotic epithelial cells (a minimum of 10 microscopic fields per section) were counted manually in a single-blind fashion.
Analysis of COX-1 and -2 and 5-LOX activity using Radio HPLC
Frozen intestinal polyps of male mice fed with 150 ppm of licofelone and/or control diet were homogenized using an ice-cold homogenizing buffer. Briefly, 150 μL of the reaction mixture containing 12 μmol/L [14C]arachidonic acid (AA; 420,000 dpm), 1 mmol/L epinephrine, 1 mmol/L glutathione in 50 mmol/L phosphate buffer (pH 7.4), and 30 mg of protein from intestinal polyps were used for each assay. Similarly, 5-LOX activity was carried out in cytosolic fractions of tumor samples. After incubation at 37 °C for 20 minutes, the reaction was terminated by adding 40 μL of 0.2 mol/L HCl. The COX-generated metabolites of AA were extracted with ethyl acetate (3 × 0.5 mL). The combined extracts were evaporated to dryness under N2, dissolved in 1 mL of acetonitrile and 10 μL were injected into a reverse phase HPLC system (Shimadzu Scientific Instruments, USA) equipped with a Phenomenix C18 column (300 × 3.90 mm; pore size 10 μ). The [14C]-PGs, [14C]-TxB2, [14C]-PGE2 and 5-[14C]-(S)-HETE were eluted with a gradient solvent system containing solvent A: Acetonitrile:Water:Acetic acid (35:65:0.1%) and solvent B: Acetonitrile:Water:acetic acid (65:30:0.1%). The eluted metabolites were monitored and quantitated by an IN/US Systems β-RAM radio HPLC detector.
Cell proliferation, COX-2 and 5-LOX expression
To evaluate the effect of licofelone, we assessed proliferating cell nuclear antigen (PCNA) expression in intestinal tumor tissue sections along with COX-2 and 5-LOX expressions levels by immunohistochemistry (IHC). Briefly, for PCNA, COX-2 and 5-LOX IHC staining, paraffin sections were deparaffinized in xylene and rehydrated through graded ethanol solutions to phosphate-buffered saline (PBS). Antigen retrieval was carried out by heating sections in 0.01 M Citrate buffer (pH 6.0) for 30 minutes in a boiling water bath. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 in PBS for 5 minutes. Nonspecific binding sites were blocked using Protein Block for 20 minutes. Sections were then incubated overnight at 4 °C with 1:300 dilutions of monoclonal/polyclonal primary antibodies against PCNA, COX-2 and 5-LOX (Santa Cruz Biotechnology, CA). After several washes with PBS, the slides were incubated with secondary antibodies for PCNA, COX-2 and 5-LOX for two hours. The color reaction was developed with DAB. Non-immune rabbit immunoglobulins were substituted for primary antibodies as negative controls. In addition, we have used blocking peptide to prove specificity for immunostaining in normal crypts and SI tumors for COX-2 expression and we used a negative control without primary antibody for further confirmation (Supplemental Fig 2). PCNA-positive cell scoring in the polyps was performed by two investigators blinded to the identity of the samples (light microscopy at 400 X magnification). Cells with a brown nucleus were considered positive. The proliferation index was determined by dividing the number of positive cells per polyp (upper, middle, and lower) and multiplying by 100.
COX-2, PGES-2, 5-LOX and FLAP mRNA expression analysis by Real time and/or RT-PCR
Total RNA from tumor samples was extracted using RNA™ Kit for isolation of total cellular RNA (Ambion) as per the manufacturer’s instructions. Equal quantities of DNA-free RNA were used for reverse transcription reactions for making cDNA using SuperScript™ reverse transcriptase (Invitrogen). The real time PCR was carried out in a 25 μL reaction volume using 3 μL of a 1:10 cDNA dilution containing SYBR Green master mix (BioRad) and primers for COX-2 (forward primer: 5′-GCATTCGCCCAGCACTT-3′ and reverse primer: 5′-AGACCAGGCACCGACCAAAGA-3′), PGES-2 (forward primer: 5′-AAGACATGTCCCTTCTGC-3′’ and reverse primer: 5′-CCAAGATGGGCACTTTCC-3′) and 5-LOX (forward primer: 5′-GGACCTCAGCATGTGGTATG-3′ and reverse primer: 5′-GCTGGGTCAGGGGTACTTTA-3′). Glyceraldehyde phosphate dehydrogenase (GAPDH) used as an internal control also was amplified using forward primer: 5′-CCTCGTCCCGTAGACAAAATG-3′, reverse primer: 5′-TGAAGGGGTCGTTGATGGC-3′. The cDNA samples were amplified at 95°C for 13′, 95°C for 30 seconds, 58°C for 30 seconds for a total of 40 cycles. All PCRs were done in an iCycler iQ real-time PCR detection system. The fluorescence threshold values (Ct) were calculated using the manufacturer’s software. Relative mRNA levels were assessed by standardization to GAPDH. Results were expressed as a fold difference in gene expression.
Reverse Transcription-PCR was performed for COX-2, PGES-2, 5-LOX and FLAP using the following conditions. COX-2 denaturation at 94°C for 2′, followed by 35 cycles at 94°C for 30 seconds, 52°C for 30 seconds, and 72°C for 1′. Oligonucleotide primer sequences used were as follows: 5′-CCTGTGCCTGATGATTGC-3′ sense, 5′-CGGTGAAACTCTGGCTAG-3′ anti-sense. PGES-2 denaturation at 94°C for 2′, followed by 35 cycles at 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 45 seconds. Oligonucleotide primer sequences used were as follows: 5′-GACCTCTCCTTTATTACAAATACAATA-3′ sense, 5′-TCTGTGGGTAAAGGGTCT-3′ anti-sense. 5-LOX denaturation was at 94°C for 3′, followed by 35 cycles at 94°C for 30 seconds, 60°C for 20 seconds, and 72°C for 45 seconds. Oligonucleotide primer sequences used were as follows: 5′-GGACCTCAGCATGTGGTATG-3′ sense, 5′-GCTGGGTCAGGGGTACTTTA-3′ anti-sense. FLAP denaturation was at 94°C for 2′, followed by 35 cycles at 94°C for 30 seconds, 50°C for 12 seconds, and 72°C for 1′. Oligonucleotide primer sequences used were as follows: 5′-GCCGGACTGATGTACCTGTT-3′ sense, 5′-GGTGAGCGTCCTTCTCTGTC-3′ anti-sense. LTB4 receptor denaturation was at 94°C for 3′, followed by 35 cycles at 94°C for 30 seconds, 60°C for 20 seconds, and 72°C for 45 seconds. Oligonucleotide primer sequences used were as follows: 5′-TACGCCAGCGTCCTGCTT-3′ sense, 5′-GCTGCTCAGGAAGGCGAG-3′ anti-sense. PCR was done using the Taq polymerase master mix (Qiagen Inc., CA). The PCR products were visualized and photographed under UV illumination.
Inflammatory Cytokines and Prostaglandin E2 assays
Determination of inflammatory cytokine levels in serum and PGE2 in SI polyps were evaluated by ELISA - SA Biosciences and Cayman chemical respectively as per manufacturers’ instructions. The mouse Inflammatory Cytokines and Chemokines Multi-Analyte ELISArray Kit analyzes a panel of 12 pro-inflammatory cytokines using ELISA protocol all at once under uniform conditions in serum. The cytokines and chemokines represented in this array are Interleukin (IL)-1A, IL-1B, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17A, Interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and granulocyte-macrophage colony stimulating factor (GM-CSF). Results are expressed as ng/ml of serum. Determinations were carried out in triplicate from each sample. SI polyps were solubilized with homogenization buffer, and total protein was measured and analyzed for PGE2 content using commercially available ELISA kits (Cayman Chemical). The data were expressed as ng of PGE2 produced/mg protein.
Statistical analyses
All results were expressed as means ±SE and statistical significance was analyzed by one-way ANOVA or Student’s ‘t’-test. Differences were considered significant at the p< 0.05 level.
Results
General observations
APCMin/+ mice fed the control diet showed significantly lower body weight gain than the mice fed the experimental diets containing either 150 or 300 ppm licofelone (Fig. 1C&D) in both genders. No significant body weight change or noticeable signs of toxicity were observed in treatment groups. The body weight loss in control group mice is mainly due to the increased small intestinal tumor burden and impairment of food absorption and anemia. Our studies have shown that licofelone administered to male wild-type mice (up to 600 ppm in diet)for six weeks has not caused any observable toxicity or significant body weight loss (data not shown). Thus doses applied in the efficacy studies were expected to benontoxic.
Dietary Licofelone dramatically suppresses intestinal polyposis and colon tumors in APCMin/+ mice
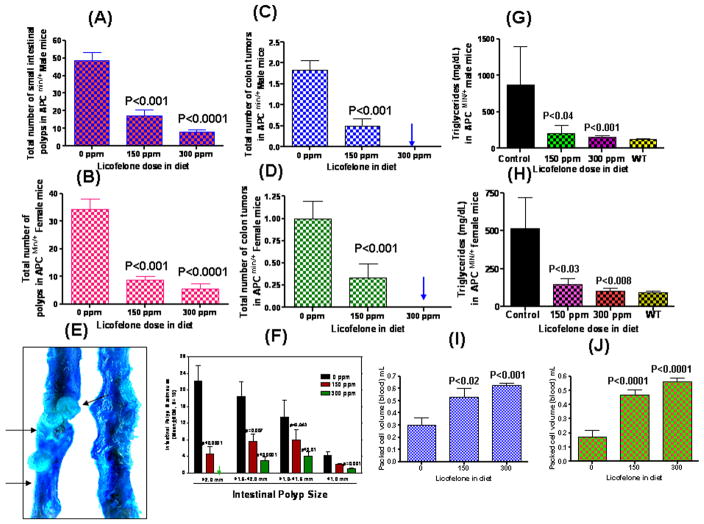
APCMin/+ mice spontaneously develop intestinal tumors, mostly in the small intestine with fewer tumors in the colon. All histopathologically classified tumors in the small intestine, as well as those in the colon, were adenomas (adenomatous polyps), with no evidence of local invasion of the lamina propria. Fig. 2A&B summarizes the chemopreventive effect of dietary licofelone administered at 150 or 300 ppm on tumor multiplicity in the small intestine. Male and female APCMin/+ mice fed with control diet developed an average of 48.8 ± 4.3 and 34.3 ± 3.8 intestinal polyps, respectively (Fig. 2A&B). Licofelone administration at 150 and 300 ppm for 14 weeks significantly reduced total intestinal tumor multiplicity and size dose-dependently in both male and female mice (means ± SEM tumors for 150 and 300 ppm; 17 ± 3.7 and 8 ± 1.4, respectively, in male mice; and 8.8 ± 1.4 and 5.5 ± 1.8, respectively, in female mice) (Fig. 2A&B). Importantly, high-dose licofelone showed ~83% (P<0.0001) intestinal tumor inhibition in both male and female mice. The mean number of colon tumors in male and female mice was 1.83 and 1.0, respectively in control diet fed mice; whereas mice fed 150 ppm licofelone showed colon tumor inhibition of 72% (male) and 67% (female), respectively. It is noteworthy that both male and female mice fed with 300 ppm licofelone showed 100% inhibition of colon tumors (Fig. 2C&D). Interestingly, the number of large-sized polyps (>2 mm) was dramatically reduced with licofelone treatments (Fig. 2E&F). Mice fed 150 ppm licofelone had 90% (male) and 86% (female) fewer polyps with sizes of >2-mm. Mice fed 300 ppm licofelone showed >97% suppression of polyps of >2 -mm size in both genders compared with control mice.
Figure 2.
(A). Inhibition of total small intestinal polyp formation in maleAPCMin/+ mice by licofelone. Data values are means ± SEM of ten animals per treatment group. Control and treated groups are significantly different from one another (P < 0.001 or P < 0.0001).
(B). Inhibition of total small intestinal polyp formation in female APCMin/+ mice by licofelone. Data values are means ± SE of ten animals per treatment. Control and treated groups are significantly different from one another (P < 0.001 or P < 0.0001).
(C). Average number of colon tumors per mouse in control and treated male APCmin/+ mice. A siginificant (P < 0.001) inhibition of colon tumors was observed with low dose licofelone and 100% inhibition was observed with high dose treatment.
(D). Average number of colon tumors per mouse in control and treated APCmin/+ female mice. A siginificant (P < 0.001) inhibition of colon tumors was observed with low dose licofelone and 100% inhibition was observed with high dose treatment.
(E). Photograph showing large polyps in control APCmin/+ mice compared with complete suppression of polyps in small intestine of licofelone treated mice.
(F). Tumor sizes in the small intestine of APCMin/+ mice. Intestines were divided into sections, examined under a stereomicroscope, and the size of polyps was determined. Data values are given as means ± SE of ten animals per treatment. Tumors >2-mm diameter were completely suppressed in high dose licofelone-treated mice.
(G&H). Inverse correlation between tumor number and triglyceride levels was observed with control and licofelone-treated groups of male APCmin/+ mice. In licofelone-treated groups, the triglyceride levels were almost equal to the levels observed in wild-type mice. The decrease in triglyceride levels was significantly different from control mice versus treated male mice (P < 0.001 to P < 0.0001); albeit lesser in female mice (P < 0.04 to P < 0.001) (I&J). Packed blood cell volumes (PCV) of male or female APCMin/+ mice fed either control or licofelone diets. A significant dose-dependent increase in PCV was observed in control mice vs treated mice
MRI of mice for polyp count in small intestine and colon
At the termination of the study, live mice were assessed for total small intestinal polyps and colon tumors by magnetic resonance imaging. Images are collected pre- and post-injection of magnivest and polyps are identified as bright areas accumulating magnivest (Fig. 1E). This method helps in localizing hidden polyps within the crypts that cannot be evaluated under dissection microscope. However, the polyp count was almost identical when compared to that evaluated under dissection microscope after necropsy.
Licofelone decreases serum triglycerides and increases packed cell volume (PCV)
A decrease in serum triglycerides (TGs) was observed in mice fed licofelone compared to control mice. Compared with wild type C57BL/6J mice, control APCMin/+ mice showed significant increases in serum TGs whereas triglyceride levels of APCmin/+ mice fed high dose licofelone were comparable with those of wild type mice. Both male and female mice fed with licofelone diets showed dose-dependent suppression of serum TGs (76.5% P<0.001 or 71.5% P<0.001 with 150 ppm and 82.4% P<0.0001 or 79.7% P<0.0001 with 300 ppm licofelone, respectively) (Fig. 1G&H). Also, we observed a dose-dependent increase in packed cell volume (blood cells) in licofelone fed male and female APCMin/+ mice (Fig. 1I&J).
Administration of licofelone inhibits intestinal tumor COX and 5-LOX activities
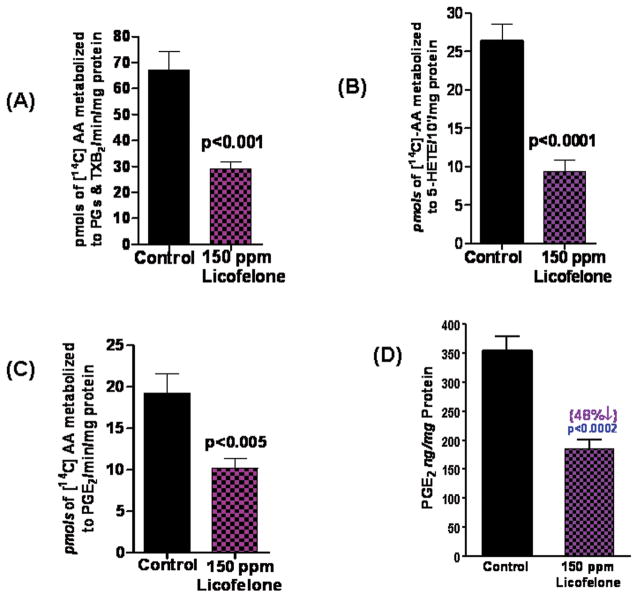
Based on radio-HPLC analysis, a significant decrease was seen in total COX and 5-LOX metabolites in intestinal polyps of mice fed a low-dose licofelone diet. As shown in Fig. 3A–C, mean total PG and thromboxane B2 (TXB2) levels in polyps from control mice vs. mice fed 150 ppm locofelone were 69.2 vs. 28.9 pmoles/min; PGE2 was 19.3 vs. 10.2 pmoles/min and 5-HETE was 26.4 vs. 9.3 pmoles/min. The total COX and 5-LOX activities were significantly reduced in intestinal polyps from low-dose licofelone-treated mice compared with polyps from control mice by 57% (P<0.001) and 64.5% (P<0.0001), respectively (Fig. 3A–C). As shown in Fig. 3D, mean total PGE2 levels in SI polyps from control mice vs. those from mice fed 150 ppm licofelone were 355 vs. 185 ng/mg protein as measured by ELISA
Figure 3.
(A). Effects of a diet with 150 ppm licofelone on COX activity in intestinal polyps from APCmin/+ mice as assessed with the Radio-HPLC method. Values are means ± SEM, N=6/treatment group. A significant (P < 0.001) inhibition of arachidonic acid metabolites (PGs and TXB2) was observed in polyps from licofelone-treated mice compared with polyps from control mice.
(B). Effects of a diet with 150 ppm licofelone on 5-LOX activity in intestinal polyps from APCMin/+ mice as assessed with the Radio-HPLC method. Values are means ± SEM, N=6/treatment group. A significant (P < 0.0001) inhibition of the 5-LOX metabolite 5-HETE was observed in polyps l from icofelone-treated mice compared with polyps from control mice.
(C). Effects of 150 ppm licofelone diet on PGE2 generation in intestinal polyps from APCmin/+ mice as assessed with the Radio-HPLC method. Values are means ± SEM, N=6/treatment group. A significant (P < 0.001) decrease in the arachidonic acid metabolite PGE2 was observed in polyps from treated mice compared with those from control mice.
(D). Effects of 150 ppm licofelone diet on PGE2 levels in intestinal polyps from APCmin/+ mice as determined with ELISA. Values are means ± SEM, N=6/treatment group. A significant (P < 0.001) decrease of the arachidonic acid metabolite PGE2 was observed in polyps from treated mice compared with those from control mice.
Effect of dietary Licofelone on intestinal polyp proliferative and apoptotic index
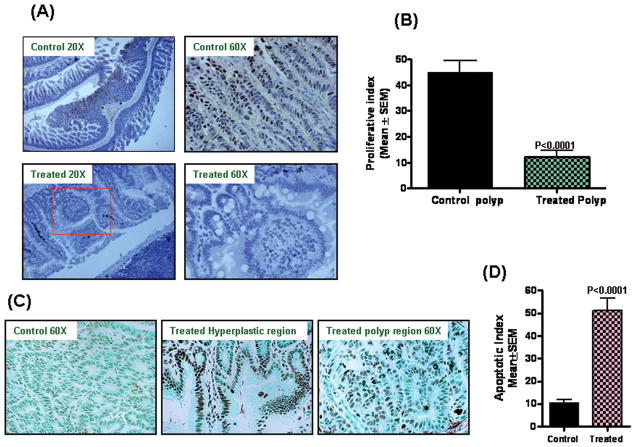
Fig. 4A&B summarize the effects of licofelone on tumor cell proliferation as measured by PCNA overexpression. Qualitative microscopic examination of PCNA-stained sections showed a substantial decrease in PCNA-positive cells in tumors from male mice exposed to Licofelone compared with tumors from the mice fed the control diet. Licofelone at 300 ppm significantly suppressed proliferation in the intestinal polyps as compared with control (Fig. 4A). The quantification of PCNA staining showed 45.1 ± 4.4 (mean ± SEM) PCNA-positive cells in control polyps, as compared with 12.2 ±2.4 (mean ± SEM) PCNA-positive cells in polyps from licofelone treated mice, accounting for a decrease in the proliferation index of ~70% (P< 0.0001). Fig. 4C& D summarize the effects of licofelone on tumor cell apoptosis. Qualitative microscopic examination of TUNEL-stained cells showed a substantial increase in TUNEL-positive cells in the polyps of mice treated with 300 ppm Licofelone. The quantification of tunnel-positive cells in polyps from control diet-fed mice showed 10.62 ± 1.34 (Mean ± SEM), as compared with 51.2 ±5.36 (mean ± SEM) -positive cells in S1 polyps from licofelone treated mice, accounting for an increase in the apoptotic index by >75% (P<0.0001). Interestingly, hyperproliferative regions of the small intestines from mice fed 300 ppm licofelone showed a robust increase in apoptosis rather than polyps.
Figure 4.
A. Immunohistochemical staining for PCNA in intestinal tumors from APCMin/+ mice fed control diet or treated with licofelone.
B. Significant difference was observed in proliferative index between licofelone-treated and control group polyps.
C. TUNEL assay was performed for apoptotic cells in intestinal tumors from APCMin/+ mice fed control diet or treated with licofelone. A significant induction of apoptosis was observed in hyperplastic regions and polyps of treated mice compared with untreated mice.
D. A significant difference was observed in apoptotic index between licofelone-treated and control groups. Polyps from treated showed ~75% induction of apoptosis compared with polyps from control mice.
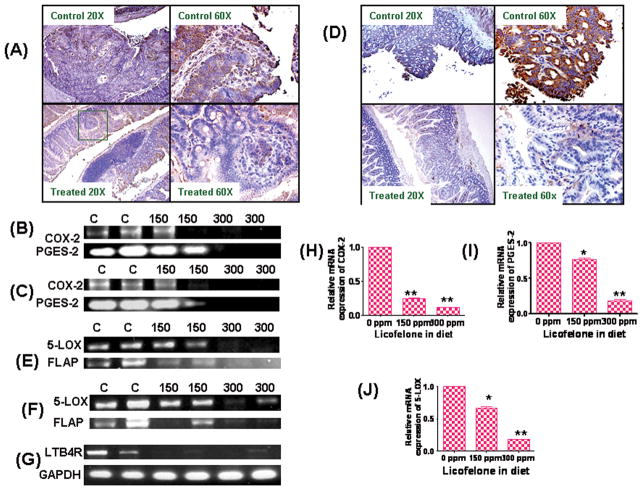
Modulation of COX-2, 5-LOX, PGES-2, FLAP, and LTB4 receptor expressions in SI polyps
Expression levels of COX-2, 5-LOX, PGES-2, FLAP and LTB4 receptor, which are important arachidonic acid pathway indicators of proliferation and tumorigenesis, were analyzed in intestinal polyps by immunohistochemistry, real time and RT-PCR (Fig. 5A-J). Dietary administration of 300 ppm licofelone resulted in significant decreases in COX-2 and 5-LOX protein expression in intestinal polyps as compared with polyps from mice fed the control diet (Fig. 5A&D). Also, dose dependent decreases of COX-2, 5-LOX, prostaglandin E synthase-2 (PGES-2), FLAP and LTB4 receptor mRNA expression levels were observed in tumors from mice fed licofelone (Fig. 5B, C, E–J).
Figure 5.
A. Immunohistochemical staining for COX-2 expression in intestinal tumors from APCMin/+ mice fed control diet or treated with licofelone.
B. Modulatory effects of licofelone on COX-2 and PGES-2 mRNA expression in intestinal polyps of treated and untreated male APCMin/+ mice. A significant dose-dependent suppression of COX-2 mRNA was observed upon licofelone treatment in APCMin/+ mice.
C. Modulatory effects of licofelone on COX-2 and PGES-2 mRNA expression in intestinal polyps of treated and untreated female APCMin/+ mice. A significant dose-dependent suppression of COX-2 mRNA was observed in licofelone treated APC min/+ mice.
D. Immunohistochemical staining for 5-LOX expression in intestinal tumors from APCMin/+ mice fed control diet or treated with licofelone.
E. Modulatory effects of licofelone on 5-LOX and FLAP mRNA expression in intestinal polyps of treated and untreated male APCMin/+ mice. A significant dose dependent suppression of 5-LOX and FLAP mRNA was observed in licofelone treated APCMin/+ mice.
F. Modulatory effects of licofelone on 5-LOX and FLAP mRNA expression in intestinal polyps of treated and untreated female APCMin/+ mice. A significant dose-dependent suppression of 5-LOX and FLAP mRNA was observed in licofelone treated APCMin/+ mice.
G. Modulatory effects of licofelone on LTB4 receptor mRNA expression in intestinal polyps of treated and untreated APCMin/+ mice. A significant dose-dependent suppression of LTB4 receptor mRNA was observed in licofelone treated polyps. GAPDH was used as loading control.
H. Real time PCR analysis for COX-2 mRNA expression in intestinal polyps of treated and untreated male APCMin/+ mice. A significant dose-dependent suppression of COX-2 mRNA was observed upon licofelone treatment in APCMin/+ mice.
I. Real time PCR analysis for PGES-2 mRNA expression in intestinal polyps of treated and untreated male APCMin/+ mice. A significant dose-dependent suppression of PGES-2 mRNA was observed upon licofelone treatment in APCMin/+ mice.
J. Real time PCR analysis for 5-LOX mRNA expression in intestinal polyps of treated and untreated male APCMin/+ mice. A significant dose-dependent suppression of 5-LOX mRNA was observed upon licofelone treatment in APCMin/+ mice.
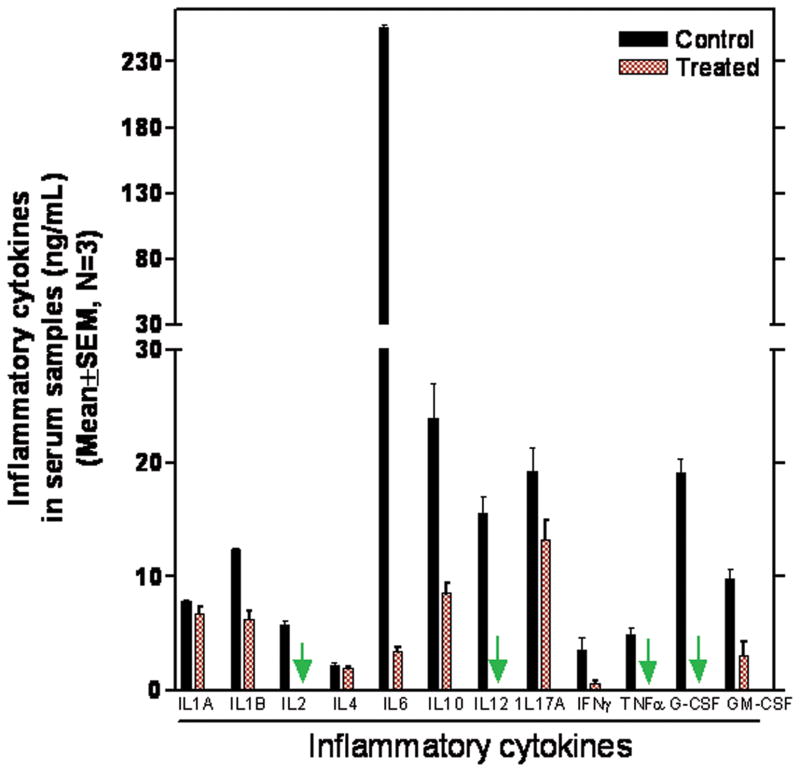
Modulation of inflammatory cytokines
Levels of the cytokines IL-1B, IL-2, IL-6, IL-10, IL-12, IFN-γ, G-CSF, and GM-CSF that either induce COX-2 or act as proinflammatory cytokines are significantly decreased in the serum of mice fed the licofelone diet(Fig. 6). IL-6 was reduced by >97% and TNF-α was completely inhibited. More importantly, no significant difference was observed in the expression levels of the anti-inflammatory cytokine IL-4 (Fig. 6).
Figure 6.
Effect of licofelone on Inflammatory cytokines in serum samples from treated and untreated APCMin/+ mice as analyzed by ELISA.
Discussion
Epidemiological and clinical studies, as well as experimental colon carcinogenesis, have clearly demonstrated that NSAIDs are effective chemopreventive agents for colorectal cancer.. NSAIDs and COX-2 selective inhibitors have been tested widely for colorectal cancer prevention. However, the gastrointestinal and cardiovascular toxicities exhibited by these agents have prompted the search for novel approaches/agents with similar or higher efficacies but devoid of unwanted side-effects. As rationalized in the introduction, targeting COX and 5-LOX pathways may provide better efficacy without unwanted cardiovascular and gastrointestinal side effects. To date, no systematic studies have been carried out to establish the potential usefulness of dual COX-5-LOX inhibitors for colorectal cancer prevention. The present study results clearly show that the novel 5-LOX-COX inhibitor licofelone suppresses small intestinal and colon carcinogenesis in both male and female APCMin/+ mice. We demonstrated that Licofelone dramatically prevents small intestinal and colon tumor formation in APCMin/+ mice in a dose-dependent manner without any gastrointestinal or other toxicities. A diet with 150 ppm and 300 ppm licofelone resulted in 70 % and 100%, respectively, colon tumor suppression in both genders of mice, suggesting the potential usefulness of licofelone as a colon cancer chemopreventive agent.
Intestinal tumor counts and size assessed under dissection microscope were comparable to those analyzed by MRI imaging of live mice. Both methods revealed a remarkable inhibition of polyp size (>2-mm) with 150 ppm licofelone and complete elimination of polyps of >2-mm with 300 ppm licofelone in the feed. The efficacy achieved with Licofelone is comparable or more effective than several NSAIDs and COX-2 selective inhibitors, celecoxib and rofecoxib (30–35). For example, in our previous studies we have shown that 300 ppm celecoxib suppressed small intestinal and colonic tumor formation by 69% and 75%, respectively, compared to that in mice fed control diet. Supplementary data presented in Figure S1, on the effect of 300 ppm of celecoxib in the diet, show inhibition of 66% for formation of SI polyps and 69% for formation of colonic polyps in male APCMin/+ mice, similar to our previous reports. Dietary administration of 300 ppm celecoxib suppressed PGE2 levels in intestinal polyps by 52% as assessed with Radio HPLC analysis and by 55% as determined with the EIA method (data not shown). Also, studies by Dr. Dubois’ group(36) showed that a 78% inhibition of small intestinal tumors was associated with a 60% reduction of tumor PGE2 levels in APCmin/+ mice exposed to 1000 ppm celecoxib in the diet.. In comparison, 300 ppm of licofelone suppressed small intestinal and colon tumors by 83% and 100%, respectively, in APCMin/+ mice. Moreover, our results indicate a 55% or 48% reduction in the intestinal tumor PGE2 levels in the in the mice treated with 150 ppm licofelone as measured by HPLC or ELISA respectively (Fig 3C, D). Collectively, these results support the better efficacy of licofelone when compared with the COX-2 selective inhibitor celecoxib. Consistent with the current results, we previously reported that licofelone administered in male F344 rats inhibits significantly the formation of azoxymethane-induced aberrant colonic crypt foci in a dose-dependent manner (8). In addition, Ye et al. (16) showed that the 5-LOX inhibitor AA861 and the COX-2 inhibitor celecoxib combined together provide better efficacy in cigarette smoke-induced colon tumor xenografts. Similar observations were made by Cianchi et al. (37) using the 5-LOX inhibitor MK866 in combination with celecoxib in colon tumor xenograft assays. Taken together, these improved efficacy results clearly validate the potential usefulness of licofelone for human colon cancer chemoprevention clinical trials.
The aberrant AA metabolism through COX-2 and its relevance to colorectal cancer is well-established (38–39). Increased production of PGE2 was found in the intestinal adenomas of FAP patients and in colon cancer tissues (38). Previous reports suggested that increased PGE2 levels generated by COX-2 up-regulation could accelerate the intestinal polyposis (39). The potential of 5-LOX as a target for colon cancer prevention has been somewhat less extensively studied. However, recent studies have shown the involvement of 5-LOX metabolites, particularly LTs, in inflammation, gastrointestinal ulceration and colon carcinogenesis (40). In this regard, we have shown previously that chemically-induced rat colon tumors abundantly produce 5-LOX metabolites and that suppression of these metabolites by naturally-occurring anti-inflammatory agents such as curcumin and caffeic acid esters in part is associated with colon tumor inhibition (41,42). In the present study, we found that not only was licofelone able to block the activity of the AA-metabolizing enzymes 5-LOX and COX, but also that it inhibited the expression of the 5-LOX activating protein FLAP and the LTB4 receptor in a dose-dependent manner in intestinal polyps (Fig 3B and Fig 5E, F, G). Previous studies have implicated over-expression of LTB4 receptors in colonic tumors and use of the LTB4 receptor antagonist LY29311 suppressed growth of colon cancer cell lines (15). In the 5-LOX-mediated pathway, 5-HETE is a key intermediate in the generation LTB4. It is noteworthy that in the present study we observed a significant decrease in LTB4 receptor expression and reduction in 5-HETE levels in intestinal polyps of mice treated with licofelone. These results further support the dual COX-LOX inhibitory function of licofelone and enhanced chemopreventive effects.
The present results further corroborate the anti-carcinogenic effects of licofelone against HCA-7 colon cancer and prostate cancer cells (30, 43). Licofelone was observed to induce apoptosis in HCA-7 cells and reduce COX-2 and 5-LOX activities in prostate cancer cells (30, 43). We have observed a significant decrease in PCNA and a robust increase in apoptotic index in intestinal polyps from licofelone-treated mice compared with polyps from untreated mice. Interestingly, hyperproliferation regions of intestinal crypts showed a marked increase in apoptotic cells in the mice fed licofelone but not in the intestinal crypts from the mice fed the control diet. This results suggests that licofelone induces apoptosis at an early stage and inhibits colonocytes in the hyperproliferative regions from transforming further into polyps. The mechanisms through which licofelone inhibits cell proliferation and induces apoptosis have been studied in in vitro models (30). However, the exact mechanisms by which licofelone suppresses tumor cell proliferation and induces apoptosis needs further study.
Recent studies suggest that hyperlipidemia may promote colon polyp development in FAP patients and in rodent models of FAP. Thus, anti-hyperlipidemic agents may be beneficial for colon cancer prevention and treatment (44). Our study shows that licofelone reduced the serum triglycerides by 71–83 %, which is very well correlated with polyp inhibition. The triglyceride levels in serum samples from high dose-treated mice are comparable to the triglyceride levels in the serum of wild type mice in both genders.
Licofelone treatment also led to significant decreases in most proinflammatory cytokines (Fig. 6). The levels of circulating IL-6, IL-8, M-CSF, and the IL-1 receptor antagonist significantly increase with the clinical stage of colorectal cancer (CRC) (45, 46). Also, increased levels of IL-6, TNF receptor type I (RI), soluble IL-2 receptor α and TNF-α were observed with increasing tumor grade and bowel wall invasion (45). In the present study, we observed>96% decrease in IL-6 and almost complete inhibition of IL-2, IL-12, TNF-α and G-CSF in licofelone-treated serum samples compared with serum samples from untreated mice (Fig. 6).
Previously, no tumor inhibition studies had been reported in vivo using the dual COX-LOX inhibitor licofelone. Compared with nonsteroidal anti-inflammatory drugs (e.g., celecoxib and sulindac) and other agents studied previously in the APCmin/+ model, the efficacy of licofelone observed in this study is dramatic and comparable or even higher (31–35). Overall, our results demonstrate that licofelone, a dual 5-LOX/COX inhibitor, suppresses SI and colonic tumor formation in APCMin/+ mice dose-dependently with high efficacy and devoid of unwanted side effects. These findings support further development of licofelone for colon cancer prevention and treatment.
Supplementary Material
Supplemental Figure 1: Inhibition of total small intestinal polyp formation and colonic tumors in APCMin/+ male mice by Celecoxib. Celecoxib at 300 ppm reduced the SI polyps by ~66% and colon tumors by ~69%. Data values are means ± SE of ten animals per treatment. Control and treated groups are significantly different from one another (P < 0.014 or P < 0.0001).
Supplemental Figure 2: Immunohistochemical staining for COX-2 expression in normal intestinal crypts and tumors from APCMin/+ mice fed control diet. A) Normal crypt without primary antibody B) Normal crypt showing COX-2 expression C) SI tumor without primary antibody D) SI tumors showing COX-2 expression.
Acknowledgments
The authors would like to thank University of Oklahoma Health Sciences Center Rodent Barrier Facility staff. The authors also thank Ms. Ashley Duff for helping in preparation of this manuscript and Dr. Julie Sando for editing. This work was supported in part by National Cancer Institute CN-NO1-53300. The author Altaf Mohammed would like to dedicate this manuscript to the late Dr. M. Shashidhar Reddy who passed away due to cancer at the age of 26 in 2007.
References
- 1.American Cancer Society. Cancer Facts and Figures 2011. Atlanta, GA: 2011. [Google Scholar]
- 2.Reddy BS, Rao CV. Novel approaches for colon cancer prevention by cyclooxygenase-2 inhibitors. J Environ Pathol Toxicol Oncol. 2002;21:155–64. [PubMed] [Google Scholar]
- 3.Reddy BS, Hirose Y, Lubet R, Steele V, Kellof G, Paulson S, et al. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res. 2000;60:293–7. [PubMed] [Google Scholar]
- 4.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–12. [PubMed] [Google Scholar]
- 5.Swamy MV, Herzog CR, Rao CV. Celecoxib inhibition of COX-2 in colon cancer cell lines increases the nuclear localization of functionally active p53. Cancer Res. 2000;63:5239–42. [PubMed] [Google Scholar]
- 6.Rao CV, Wang CQ, Simi B, Rodriguez JG, Cooma I, El-Bayoumy K, et al. Chemoprevention of colon cancer by a glutathione conjugate of 1,4-phenylenebis(methylene) selenocyanate, a novel organoselenium compound with low toxicity. Cancer Res. 2001;61:3647–52. [PubMed] [Google Scholar]
- 7.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Breazna A, Kim KM, et al. Five-Year Efficacy and Safety Analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev Res. 2009;2(4):285–7. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao CV, Swamy MV, Choi C, Janakiram NB, Patlolla JM, Steele VE. Chemoprevention of colon carcinogenesis by licofelone, a novel dual 5-LOX/COX inhibitor in F-344rats. AACR Meeting Abstracts; 2007. p. 11. [Google Scholar]
- 9.Peters-Golden M, Henderson WR. Leukotrienes. N Engl J Med. 2007;357:1841–54. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 10.Capra V, Thompson MD, Sala A, Cole DE, Folco G, Rovati GE. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med Res Rev. 2007;27:469–27. doi: 10.1002/med.20071. [DOI] [PubMed] [Google Scholar]
- 11.Stanke-Labesque F, Pofelski J, Moreau-Gaudry A, Bessard G, Bonaz B. Urinary leukotriene E4 excretion: a biomarker of inflammatory bowel disease activity. Inflamm Bowel Dis. 2008;14:769–74. doi: 10.1002/ibd.20403. [DOI] [PubMed] [Google Scholar]
- 12.Avis IM, Jett M, Boyle T, Vos MD, Moody T, Treston AM, Martinez A, Mulshine JL. Growth control of lung cancer by interruption of 5-lipoxygenase-mediated growth factor signaling. J Clin Invest. 1996;97:806–13. doi: 10.1172/JCI118480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Z, Sood S, Li N, Ramji D, Yang P, Newman RA, Yang CS, Chen X. Involvement of the 5-lipoxygenase/leukotriene A4 hydrolase pathway in 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch, and inhibition of carcinogenesis by its inhibitors. Carcinogenesis. 2006;27:1902–8. doi: 10.1093/carcin/bgl039. [DOI] [PubMed] [Google Scholar]
- 14.Soumaoro LT, Lida S, Uetake H, Ishiguro M, Takagi Y, Higuchi T, Yasuno M, Enomoto M, Sugihara K. Expression of 5-lipoxygenase in human colorectal cancer. World J Gastroenterol. 2006;12:6355–60. doi: 10.3748/wjg.v12.i39.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci. 2007;103:24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- 16.Ye YN, Wu WKK, Shin VY, Bruce IC, Wong BCY, Cho CH. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26:827–34. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- 17.Shureiqi I, Lippman SM. Lipoxigenase modulation to reverse carcinogenesis. Cancer Res. 2001;61:6307–12. [PubMed] [Google Scholar]
- 18.Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, et al. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol Biomarkers Prev. 1999;8:467–83. [PubMed] [Google Scholar]
- 19.Duffield-Lillico AJ, Boyle JO, Zhou XK, Ghosh A, Butala GS, Subbaramaiah K, et al. Levels of Prostaglandin E Metabolite and Leukotriene E4 Are Increased in the Urine of Smokers: Evidence that Celecoxib Shunts Arachidonic Acid into the 5-Lipoxygenase Pathway. Cancer Prev Res. 2009;2(4):322–29. doi: 10.1158/1940-6207.CAPR-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K. Expanding expression of the 5-lipoxygenase pathway with the arterial wall during human atherogenesis. Proc Natl Acad Sci U S A. 2003;100:1238–43. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehrabian M, Allayee H, Wong J, Shih W, Wang XP, Shaposhnik Z, et al. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–6. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- 22.Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 23.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–9. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 24.Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov. 2005;4:664–72. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 25.Hakonarson H, Thorvaldsson S, Helgadottir A, Gudbjartsson D, Zink F, Andresdottir M, et al. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction. JAMA. 2005;293:2245–56. doi: 10.1001/jama.293.18.2245. [DOI] [PubMed] [Google Scholar]
- 26.Leone S, Ottani A, Bertolini A. Dual acting anti-inflammatory drugs. Curr Top Med Chem. 2007;7:265–75. doi: 10.2174/156802607779941341. [DOI] [PubMed] [Google Scholar]
- 27.Cicero AF, Derosa G, Gaddi A. Combined lipoxygenase/cyclo-oxygenase inhibition in the elderly: the example of licofelone. Drugs Aging. 2005;22(5):393–403. doi: 10.2165/00002512-200522050-00004. [DOI] [PubMed] [Google Scholar]
- 28.Moreau M, Daminet S, Martel-Pelletier J, Fernandes J, Pelletier JP. Superiority of the gastroduodenal safety profile of licofelone over rofecoxib, a COX-2 selective inhibitor, in dogs. J Vet Pharmacol Ther. 2005;28:81–86. doi: 10.1111/j.1365-2885.2004.00640.x. [DOI] [PubMed] [Google Scholar]
- 29.Bias P, Buchner A, Klesser B, Laufer S. The gastrointestinal tolerability of the LOX/COX inhibitor, licofelone, is similar to placebo and superior to naproxen therapy in healthy volunteers: results from a randomized, controlled trial. Am J Gastroenterol. 2004;99:611–18. doi: 10.1111/j.1572-0241.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- 30.Tavolari S, Bonafè M, Marini M, Ferreri C, Bartolini G, Brighenti E, et al. Licofelone, a dual COX/5-LOX inhibitor, induces apoptosis in HCA-7 colon cancer cells through the mitochondrial pathway independently from its ability to affect the arachidonic acid cascade. Carcinogenesis. 2008;29(2):371–80. doi: 10.1093/carcin/bgm265. [DOI] [PubMed] [Google Scholar]
- 31.Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res. 2006;66:7370–7. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- 32.Jacoby RF, Marshall DJ, Newton MA, Novakovic K, Tutsch K, Cole CE, et al. Chemoprevention of spontaneous intestinal adenomas in APCMin mouse model by the nonsteroidal anti-inflammatory drug piroxicam. Cancer Res. 1996;56:710–4. [PubMed] [Google Scholar]
- 33.Rao CV, Reddy BS. NSAIDs and chemoprevention. Curr Cancer Drug Targets. 2004;4:29–42. doi: 10.2174/1568009043481632. [DOI] [PubMed] [Google Scholar]
- 34.Rao CV, Cooma I, Rodriguez JG, Simi B, El-Bayoumy K, Reddy BS. Chemoprevention of familial adenomatous polyposis development in the APC(min) mouse model by 1,4-phenylene bis(methylene)selenocyanate. Carcinogenesis. 2000;21:617–21. doi: 10.1093/carcin/21.4.617. [DOI] [PubMed] [Google Scholar]
- 35.Orner GA, Dashwood WM, Blum CA, Díaz GD, Li Q, Dashwood RH. Suppression of tumorigenesis in the Apc(min) mouse: down-regulation of β-catenin signaling by a combination of tea plus sulindac. Carcinogenesis. 2003;24:263–7. doi: 10.1093/carcin/24.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchanan FG, Holla V, Katkuri S, Matta P, Dubios RN. Targetting cyclooxygenase-2 and the epidermal growth factor receptor for the prevention and treatment of intestinal cancer. Cancer Res. 2007;67(19):9380–8. doi: 10.1158/0008-5472.CAN-07-0710. [DOI] [PubMed] [Google Scholar]
- 37.Cianchi F, Cortesini C, Magnelli L, Fanti E, Papucci L, Schiavone N, et al. Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol Cancer Ther. 2006;5(11):2716–26. doi: 10.1158/1535-7163.MCT-06-0318. [DOI] [PubMed] [Google Scholar]
- 38.Yang VW, Shields JM, Hamilton SR, Spannhake EW, Hubbard WC, Hylind LM, et al. Size dependent increase in prostanoid levels in adenomas of patients with familial adenomatous polyposis. Cancer Res. 1998;58:1750–3. [PubMed] [Google Scholar]
- 39.Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(D716) knockout mice. Nat Med. 2001;7:1048–51. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 40.Jupp J, Hillier K, Elliott DH, Fine DR, Bateman AC, Johnson PA, et al. Colonic expression of leukotriene-pathway enzymes in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:537–46. doi: 10.1002/ibd.20094. [DOI] [PubMed] [Google Scholar]
- 41.Rao CV. Regulation of COX and LOX by curcumin. Adv Exp Med Biol. 2007;595:213–26. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 42.Reddy BS. Chemoprevention of colon cancer by select phytochemicals. Functional foods for disease prevention. 1998;3:23–33. [Google Scholar]
- 43.Narayanan NK, Nargi D, Attur M, Abramson SB, Narayanan BA. Anticancer effects of licofelone (ML-3000) in prostate cancer cells. Anticancer Res. 2007;27:2393–402. [PubMed] [Google Scholar]
- 44.Mutoh M, Takahashi M, Wakabayashi K. Roles of prostanoids in colon carcinogenesis and their potential targeting for cancer chemoprevention. Curr Pharm Des. 2006;12:2375–82. doi: 10.2174/138161206777698972. [DOI] [PubMed] [Google Scholar]
- 45.Kaminska J, Nowacki MP, Kowalska M, Rysinska A, Chwalinski M, Fuksiewicz M, et al. Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: soluble tumor necrosis factor receptor Type I - An independent prognostic factor. Tumor biology. 2005;26:186–94. doi: 10.1159/000086951. [DOI] [PubMed] [Google Scholar]
- 46.Ueda T, Shimada E, Urakawa T. Serum levels of cytokines in patients with colorectal cancer: Possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol. 1994;29:423–29.s. doi: 10.1007/BF02361238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Inhibition of total small intestinal polyp formation and colonic tumors in APCMin/+ male mice by Celecoxib. Celecoxib at 300 ppm reduced the SI polyps by ~66% and colon tumors by ~69%. Data values are means ± SE of ten animals per treatment. Control and treated groups are significantly different from one another (P < 0.014 or P < 0.0001).
Supplemental Figure 2: Immunohistochemical staining for COX-2 expression in normal intestinal crypts and tumors from APCMin/+ mice fed control diet. A) Normal crypt without primary antibody B) Normal crypt showing COX-2 expression C) SI tumor without primary antibody D) SI tumors showing COX-2 expression.