Abstract
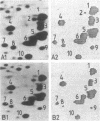
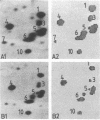
Rates of protein turnover have been measured in three normal and three Duchenne muscular dystrophy (DMD) skin fibroblast cell lines. Cell populations were analyzed at identical states with regard to cell number, state of topoinhibition, and cumulative population doublings (CPD). Net protein synthesis measured by the incorporation of [35S]methionine in an 18-hr pulse was reduced by an average of 34%; degradation of total cellular protein measured after an 18-hr pulse with [35S]methionine and a 24-hr chase was enhanced by an average of 50% in DMD fibroblasts. Two-dimensional gel electrophoresis analyses revealed that the breakdown of the majority of [35S]methionine polypeptides was markedly increased in DMD fibroblasts. Quantitative determinations of the differential degradation rates of 10 selected proteins in the tropomyosin region of two-dimensional gels were undertaken by scintillation counting and computer analyses. In three series of experiments, the degradation of the 10 proteins in DMD fibroblasts was enhanced by individual polypeptides between 12.0% and 151.2% as measured by scintillation counting or between 0.8% and 128% as determined by computer analyses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Tomas F. M., Stern L. M. Increased turnover of muscle contractile proteins in Duchenne muscular dystrophy as assessed by 3-methylhistidine and creatinine excretion. Clin Sci (Lond) 1979 Apr;56(4):347–352. doi: 10.1042/cs0560347. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boulé M., Vanasse M., Brakier-Gingras L. Decrease in the rate of protein synthesis by polysomes from cultured fibroblasts of patients and carriers with Duchenne muscular dystrophy. Can J Neurol Sci. 1979 Aug;6(3):355–358. doi: 10.1017/s0317167100024008. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Up-dated catalogue of HeLa cell proteins: percentages and characteristics of the major cell polypeptides labeled with a mixture of 16 14C-labeled amino acids. Clin Chem. 1982 Apr;28(4 Pt 2):766–781. [PubMed] [Google Scholar]
- Ettienne E. M., Swartz K., Singer R. H. Increased turnover of proteins from the sarcoplasmic reticulum of dystrophic chicken muscle cells in tissue culture. J Biol Chem. 1981 Jun 25;256(12):6408–6412. [PubMed] [Google Scholar]
- Fingerman E., Campisi J., Pardee A. B. Defective Ca2+ metabolism in Duchenne muscular dystrophy: effects on cellular and viral growth. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7617–7621. doi: 10.1073/pnas.81.23.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman B. B., Papa L., Davis M. H., Gruenstein E. Decreased lysosomal dipeptidyl aminopeptidase I activity in cultured human skin fibroblasts in Duchenne's muscular dystrophy. J Clin Invest. 1980 Jun;65(6):1398–1406. doi: 10.1172/JCI109804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Baracos V., Rodemann P., Waxman L., Dinarello C. Control of protein degradation in muscle by prostaglandins, Ca2+, and leukocytic pyrogen (interleukin 1). Fed Proc. 1984 Apr;43(5):1301–1306. [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Witkowski J. A. Reduced adhesiveness between skin fibroblasts from patients with Duchenne muscular dystrophy. J Neurol Sci. 1979 Nov;43(3):465–470. doi: 10.1016/0022-510x(79)90025-x. [DOI] [PubMed] [Google Scholar]
- Kameyama T., Etlinger J. D. Calcium-dependent regulation of protein synthesis and degradation in muscle. Nature. 1979 May 24;279(5711):344–346. doi: 10.1038/279344a0. [DOI] [PubMed] [Google Scholar]
- Kent C. Increased rate of cell-substratum detachment of fibroblasts from patients with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 1983 May;80(10):3086–3090. doi: 10.1073/pnas.80.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liechti-Gallati S., Moser H., Siegrist H. P., Wiesmann U., Herschkowitz N. N. Abnormal growth kinetics and 5'-nucleotidase activities in cultured skin fibroblasts from patients with Duchenne muscular dystrophy. Pediatr Res. 1981 Nov;15(11):1411–1414. doi: 10.1203/00006450-198111000-00004. [DOI] [PubMed] [Google Scholar]
- Lucy J. A. Is there a membrane defect in muscle and other cells? Br Med Bull. 1980 May;36(2):187–192. doi: 10.1093/oxfordjournals.bmb.a071636. [DOI] [PubMed] [Google Scholar]
- Mariash C. N., Seelig S., Oppenheimer J. H. A rapid, inexpensive, quantitative technique for the analysis of two-dimensional electrophoretograms. Anal Biochem. 1982 Apr;121(2):388–394. doi: 10.1016/0003-2697(82)90498-5. [DOI] [PubMed] [Google Scholar]
- McElligott M. A., Dice J. F. Intracellular protein degradation in cultures of dystrophic muscle cells and fibroblasts. Exp Cell Res. 1984 Feb;150(2):442–451. doi: 10.1016/0014-4827(84)90588-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pato C. N., Davis M. H., Doughty M. J., Bryant S. H., Gruenstein E. Increased membrane permeability to chloride in Duchenne muscular dystrophy fibroblasts and its relationship to muscle function. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4732–4736. doi: 10.1073/pnas.80.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzey J., Witkowski J., Jones G. Monensin-induced inhibition of cell spreading in normal and dystrophic human fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4960–4964. doi: 10.1073/pnas.81.15.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodemann H. P., Bayreuther K. Abnormal collagen metabolism in cultured skin fibroblasts from patients with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5130–5134. doi: 10.1073/pnas.81.16.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Rodemann H. P., Waxman L., Goldberg A. L. The stimulation of protein degradation in muscle by Ca2+ is mediated by prostaglandin E2 and does not require the calcium-activated protease. J Biol Chem. 1982 Aug 10;257(15):8716–8723. [PubMed] [Google Scholar]
- Rourke A. W. Myosin in developing normal and dystrophic chicken pectoralis. I. Synthesis and degradation. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):343–351. doi: 10.1002/jcp.1040860406. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Biochemistry of muscle membranes in Duchenne muscular dystrophy. Muscle Nerve. 1980 Jan-Feb;3(1):3–20. doi: 10.1002/mus.880030103. [DOI] [PubMed] [Google Scholar]
- Statham H. E., Witkowski J. A., Dubowitz V. Protein degradation in skin fibroblasts from patients with Duchenne muscular dystrophy. Biochem J. 1980 Oct 15;192(1):257–262. doi: 10.1042/bj1920257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt P. R., Cox D. M. Duchenne's muscular dystrophy: studies in cultured fibroblasts. Lancet. 1977 Jan 22;1(8004):172–174. doi: 10.1016/s0140-6736(77)91768-8. [DOI] [PubMed] [Google Scholar]