Abstract
Background
Compressive force across the growth plate may cause retardation and even arrest of physeal growth. The purpose of this study was to investigate histologic changes, metabolic changes in terms of glycosaminoglycan (GAG) concentration, and contrast-enhanced micro-computed tomography (CEMCT) findings of physeal cartilage in a rabbit model of physeal damage caused by excessive compression.
Methods
Compressive forces were applied via external fixators for two weeks to the growth plates of distal femurs and proximal tibiae of right hind-legs in 8-week-old rabbits. Left hind-legs remained intact and were used as controls. Forty-four bone specimens containing growth plates of distal femurs or proximal tibiae were harvested one week (n = 12) and four weeks (n = 32) after surgery, and examined for histologic findings (H&E staining) and GAGs quantification in physeal cartilage. After incubation in an ionic contrast material for 48 hours, specimens were scanned by CEMCT, and the pixel values of physeal cartilage were measured.
Results
CEMCT showed a thin, highly attenuated line parallel to the growth plate in compressed specimens harvested at four weeks after surgery, which was found to be transversely connected trabecular bone. In these specimens, GAG content in physeal cartilage was significantly lower, and CEMCT pixel values of physeal cartilage were significantly higher than in the specimens from the contralateral control side.
Conclusions
Excessive compressive force applied to growth plates produces altered histologic features and metabolic function in terms of decreased GAG content in physeal cartilage, changes that can be demonstrated by CEMCT.
Keywords: Contrast-enhanced micro-CT, Growth plate, Growth arrest, Rabbit, Glycosaminoglycan
Plain radiography is the standard imaging modality for assessing limb shortening and malalignment caused by physeal growth disturbance. Computed tomography (CT) and magnetic resonance imaging (MRI) have been used to assess the location and extent of physeal growth arrest. However, these diagnostic tools have focused mainly on the established complications of growth disturbance and on the bone bridge, the latter of which becomes evident usually more than six months after injury.1) Currently, no diagnostic tools are available to assess altered physeal function before its clinical and radiological manifestations.
Contrast-enhanced micro-computed tomography (CEMCT) is based on the interaction between negatively charged glycosaminoglycans (GAGs) in the extracellular matrix of cartilage and ionic contrast material. This imaging tool has been applied successfully to the research of degenerative arthritis given that decreased GAG content in degenerative articular cartilage allows easier entrance of negatively charged ionic contrast material into the cartilage matrix than in normal articular cartilage, and hence, increases X-ray attenuation.2-4)
Because growth plates are highly differentiated cartilage tissue in which active metabolism of chondrocytes occurs, we postulated that GAG content in physeal cartilage might change when physeal chondrocytes are damaged by excessive compressive stress, and that this change could be detected by CEMCT. The purpose of this study was to investigate histologic changes, metabolic changes in terms of the GAG concentration, and CEMCT findings of physeal cartilage in a rabbit model of physeal damage caused by static compression.
METHODS
The study had Institutional Animal Care and Use Committee approval. Eight-week-old male New Zealand white rabbits (n = 11) were anesthetized with a subcutaneous injection of ketamine (50 mg/kg) and xylazine (20 mg/kg). After sterile skin preparation, two pins each were transfixed in the distal femur and in the proximal tibia of the right hind-leg under fluoroscopic guidance. The pins were connected to a set of custom-made external fixators applied at both sides of the leg with the knee extended (Fig. 1). Immediate compression was applied across the knee joint as tight as possible and maintained for two weeks, and then the external fixators were removed. Left hind-legs remained intact and served as controls. Postoperatively, animals were given 30 mg/kg of Cefazolin and housed in individual cages with no restrictions on diet or exercise.
Fig. 1.
Two custom-made external fixators were used to apply compressive force to the growth plate of the distal femur and proximal tibia across the knee joint in the right hind-leg.
Animals were euthanized one (n = 3) and four weeks (n = 8) after surgery by injecting excessive amount of anesthetics. After removing soft tissues, 44 bone specimens containing epiphysis, physis, and metaphysis were obtained from distal femurs (n = 22) and proximal tibiae (n = 22). Each specimen was cut into two pieces in the coronal plane; the anterior half was used for GAGs quantification, and the posterior half was washed twice in phosphate- buffered saline containing 1% protease inhibitors (Protease Inhibitor Cocktail, Sigma-Aldrich Co., St. Louis, MO, USA) and then incubated in an ionic contrast agent (Telebrix 30-meglumine ioxitalamate, Guerbet, Aulnaysous- Bois, France) containing 1% protease inhibitors for 48 hours at 37.5℃. Specimens were then CEMCT scanned and examined histologically (H&E staining).
CEMCT Imaging
Bone specimens were scanned using a micro-CT unit (Sky-Scan 1076; SkyScan, Aartselaar, Belgium). Samples were rotated through 360° at a rotation step of 0.5°. The X-ray settings were standardized to 75 kV and 100 µA with an exposure time of 0.3 sec/frame, and a 0.5-mm-thick aluminum filter and 100% beam-hardening correction were used to minimize beam-hardening artifacts. A cone-beam algorithm was used to reconstruct 8-bit crosssectional images, with each pixel representing a 17.6 mm3 voxel. A stack of two-dimensional sections was reconstructed for each sample and stored in bitmap format with indexed gray levels ranging from 0 (black) to 255 (white).
Six representative CT images per hind-leg (3 from the femur and 3 from the tibia) were selected. Pixel values were measured from five regions of interest that were set on physeal cartilage in each CT image using imaging software (Sante DICOM viewer, Santesoft, Athens, Greece). Measured pixel values were normalized versus image background pixel values: Normalized pixel value (%) = pixel value of physeal cartilage / pixel value of background image × 100.
GAGs Quantification
Physeal cartilage was removed from specimens by manual dissection and weighed. A 1,9-dimethylmethylene blue (DMMB) colorimetric assay5) was used to quantify sulfated GAGs. In brief, physeal cartilage explants were incubated overnight at 65℃ in 20 µL/mL of papain digest solution (5 mM EDTA, 0.1 M sodium formate and 5 mM cysteine; pH 3.0). Using a multi-pipettor, 250 µL of DMMB solution was then dispensed into the wells of a 96-well plate containing 50 µL of papain digested sample per well. A linear calibration curve was generated using chondroitin-6 sulfate (Sigma-Aldrich Co.) at concentrations of 0-500 µg/mL. The samples were read on a microplate reader at 530 nm and 590 nm.
Data Analysis
Normalized pixel values of physeal cartilages in compressed and contralateral control sides were compared using the Student t-test. GAG levels per mg of cartilage were compared using the Mann-Whitney U-test between compressed and control sides. The p-values of < 0.05 were considered significant.
RESULTS
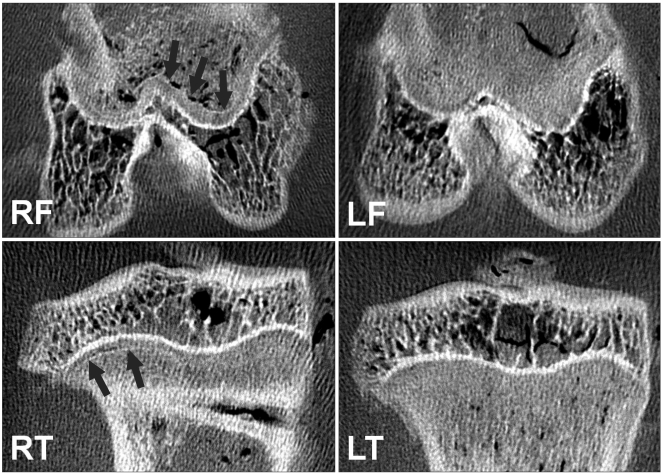
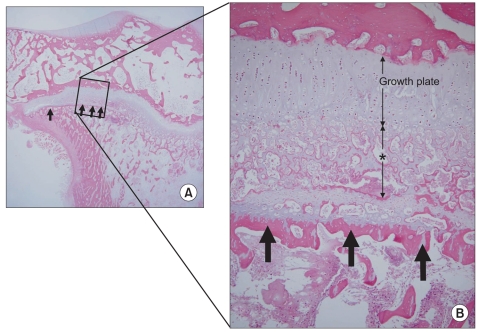
CEMCT visualized a thin, highly attenuated line parallel to the growth plate in compressed specimens harvested four weeks after surgery (Fig. 2). Histologic observation revealed that the highly attenuated line in CEMCT was transversely connected trabecular bone, which corresponded with the growth arrest line of Harris.6) Newly formed trabecular bones between the growth plate and the Harris growth arrest line were interconnected with each other, whereas all trabecular bones near the growth plate in the control side were oriented in a uniform longitudinal manner (Fig. 3). Disorganized columnar architecture and decreased cellularity in proliferative and hypertrophic zones were observed in 13 compressed specimens harvested four weeks after surgery (81%, 13/16), but not in compressed specimens harvested one week after surgery or in control specimens.
Fig. 2.
Contrast-enhanced micro-computed tomography images showed that a thin, highly attenuated line parallel to the growth plate (arrows) was formed in compressed specimens (RF and RT) harvested at four weeks after surgery. RF: right femur, RT: right tibia, LF: left femur, LT: left tibia.
Fig. 3.
A histologic section of a compressed specimen harvested at four weeks after surgery showed newly formed trabecular bones that were interconnected haphazardly (asterisk) and transversely connected trabecular bone (arrows), the latter of which corresponded with the highly attenuated line observed in the contrast-enhanced micro-computed tomography (A: × 10, B: H&E, × 100).
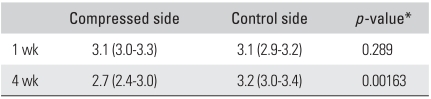
Glucosaminoglycans levels per mg of physeal cartilage in specimens harvested one week after surgery were similar in compressed and control sides (p = 0.289). However, at four4 weeks after surgery, GAG levels were significantly lower in compressed specimens (p = 0.00163) than in controls (Table 1).
Table 1.
Sulfated Glycosaminoglycan Levels per mg of Physeal Cartilage (µg/mg) in Rabbits Harvested One and Four Weeks after Surgery
Values are presented as median (range).
*Mann-Whitney U-test.
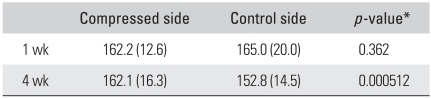
Normalized pixel values of physeal cartilages measured on CEMCT images were similar in compressed and control sides at one week after surgery (p = 0.362). However, they were significantly higher in compressed specimens harvested at four weeks after surgery than in corresponding controls (p = 0.00512) (Table 2).
Table 2.
Normalized Pixel Numbers (%) of Physeal Cartilage in Rabbits Harvested One and Four Weeks after Surgery
Values are presented as mean (standard deviation).
*t-test.
DISCUSSION
We found that GAG levels significantly decreased and pixel numbers that were normalized by background pixel numbers significantly increased in the compressed physeal cartilage as compared to the contralateral normal side. We postulated that decreased GAG concentration represented altered function of the damaged physeal chondrocytes and that increased pixel numbers (X-ray attenuation) were associated with easier entrance of contrast material into the cartilage matrix by decreased negative ionic compulsion in the damaged cartilage. Histologic observation demonstrated damaged physeal cartilage in the compressed specimens. However, we could not delineate the doseresponse relationship to further support the relationship between the two parameters regarding the degree of physeal dysfunction, because we did not use an animal model with controlled compression and therefore we could not investigate changes in these parameters or physeal growth disturbance by the applied compression forces.
The growth arrest line was first described by Harris, although he thought it was a calcium deposit.6) In a histologic study using cadavers and amputees, Ogden7) demonstrated that the growth arrest line was a transversely interconnected trabecular bone formed by the retardation or cessation of growth, and that longitudinally arranged new trabucular bones between the growth plate and the growth arrest line were thought to be formed by resumed normal physeal growth. In general, growth arrest lines parallel to the growth plate were considered as a sign of normal growth while tilting, angulation, or disruption suggested a physeal abnormality.8)
Our histologic observations confirmed that the growth arrest line was a transversely interconnected trabecular bone associated with growth disturbance. However, compressed specimens in the present study showed that the newly formed trabecular bones were arranged haphazardly instead of the normal longitudinal arrangement, suggesting persistent physeal growth disturbance. Decreased GAG contents in these specimens also suggested altered metabolism in the physeal cartilage. We believe that these findings corresponded with the clinical observation in which the presence of a growth arrest line parallel to the growth plate does not always mean a normal resumption of physeal growth and that some of the physes close earlier than other normal physes.
Micro-CT has been used to evaluate subchondral bone plate morphology, trabecular patterns of the epiphysis, and osteophyte formation in a small animal model of osteoarthritis;4) however, its application to cartilage tissue has been limited due to poor soft tissue contrast. A technique of equilibrium partitioning of anionic contrast agent (Hexabrix 320) before CT scanning3) and a similar technique using gadolinium as a contrast material,2) substantively overcame this poor soft tissue contrast issue and enabled quantitative measurements of GAGs in diseased articular cartilage in vitro, ex vivo, and in vivo.2-4) Recently this technique was used to monitor cartilage repair.9) The present study applied this technique to the assessment of growth arrest and associated physeal cartilage dysfunction. The applicability of CEMCT in the assessment of physeal dysfunction has depended on whether GAG content in physeal cartilage changes by compressive stress or not. While aggrecan loss in the articular cartilage of patients with osteoarthritis has been widely studied to elucidate the abnormal underlying metabolism, such as, the proteolytic cleavage of core protein, the inhibition of biosynthesis and/or the inhibition of proteoglycan retention due to hyaluronan deficits,10,11) only one previous study has investigated proteoglycan expression in physeal cartilage after mechanical compression.12) In this previous study, which was conducted using a rat model, proteoglycan expression assessed by Safranin-O staining and aggrecan mRNA production in physeal cartilage was found not to be altered by mechanical stress which contradicted our results. In this previous study, the authors applied controlled compression (0.2 MPa) that was sufficient to retard, but not to cease physeal growth in rat vertebra, whereas histologic evidence of growth cessation and physeal cartilage damage were observed in our uncontrolled maximal compression model. Therefore, it is likely that the two studies differ substantially in terms of the load effect. Bonnel et al.13) showed that distal femoral growth rates in rabbits decreased in proportion to compressive force, and that forces greater than 30 N caused cell damage and halted physeal growth.
Our findings indicate that CEMCT appears to be suitable for the assessment of physeal damage that is sufficient to result in growth cessation, but further studies encompassing CEMCT imaging and GAG quantification with different compressive stresses on physeal cartilage are necessary to determine whether CEMCT can detect mild and reversible physeal damage as well.
Current treatment options for physeal damage have focused mainly on the management of disabilities associated with growth disturbances such as limb shortening, angular deformity, and joint incongruity. However, surgical complications have not been uncommon during multiple limb lengthening procedures in young children, and incongruent joints have barely been restored by surgery. Although interposition of fat and other inert or biological materials after resection of a focal bone bridge can be performed before deformity worsens, this procedure has been reserved only for some selected patients with a small bone bridge and substantial growth remaining,14) and surgical outcomes often have been unpredictable.15) In this current situation, new therapeutic approaches may target early intervention before clinical and radiological manifestations of growth disturbance.
An early resection of damaged physeal cartilage before bone bridge formation has been reported.16) However, this strategy may be limited by the risk of overtreatment in the absence of a reliable method of assessing physeal function before surgery. We believe that early assessment of physeal growth arrest would be necessary for selecting patients who require a closer follow-up and when considering new potential early treatments designed to prevent complications of growth disturbances.
Certain limitations of this study require consideration. First, statistical power may not have been strong due to the small number of animals used. Estimation of an adequate number of animals was difficult because, to our knowledge, it was the first attempt to investigate abnormal physeal function following compression injury using CEMCT, and there have been no similar reports in literature. We believe controlled physeal compression in a larger number of animals may delineate the relationship between compressive stress and abnormal physeal metabolism, and further determine the role of CEMCT in physeal growth arrest. Second, changes in X-ray attenuation represented by pixel numbers were not readily differentiated by the naked eye. Third, early detection of physeal dysfunction and prediction of prognosis have not yet been clinically feasible. In fact, the current technique doesn't seem to be applicable even to an in vivo animal study presumably because of limited blood supply to growth plates and high concentration-related toxicity of the contrast material. Only ex vivo animal experiments seem to be feasible for the evaluation of physeal cartilage function at the present time. However, we expect that this type of research using CEMCT may give some implications to clinical practice because this type of research may reveal a favorable or unfavorable effect of some drugs or surgical interventions on physeal cartilage function in an animal model of physeal dysfunction.
We conclude that excessive compressive force applied to growth plates produced altered histologic features and metabolic function in terms of decreased GAG contents in the physeal cartilage, and that these changes could be demonstrated by CEMCT.
ACKNOWLEDGEMENTS
This study was supported by a Seoul National University Hospital Research Fund (Grant No. 04-2008-061).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Futami T, Foster BK, Morris LL, LeQuesne GW. Magnetic resonance imaging of growth plate injuries: the efficacy and indications for surgical procedures. Arch Orthop Trauma Surg. 2000;120(7-8):390–396. doi: 10.1007/pl00013768. [DOI] [PubMed] [Google Scholar]
- 2.Cockman MD, Blanton CA, Chmielewski PA, et al. Quantitative imaging of proteoglycan in cartilage using a gadolinium probe and microCT. Osteoarthritis Cartilage. 2006;14(3):210–214. doi: 10.1016/j.joca.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc Natl Acad Sci U S A. 2006;103(51):19255–19260. doi: 10.1073/pnas.0606406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piscaer TM, van Osch GJ, Verhaar JA, Weinans H. Imaging of experimental osteoarthritis in small animal models. Biorheology. 2008;45(3-4):355–364. [PubMed] [Google Scholar]
- 5.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 6.Harris HA. Bone growth in health and disease. London: Oxford University Press; 1933. [Google Scholar]
- 7.Ogden JA. The evaluation and treatment of partial physeal arrest. J Bone Joint Surg Am. 1987;69(8):1297–1302. [PubMed] [Google Scholar]
- 8.Shapiro F. Pediatric orthopedic deformities: basic science, diagnosis, and treatment. San Diego: Academic Press; 2001. p. 555. [Google Scholar]
- 9.Moyer HR, Wang Y, Farooque T, et al. A new animal model for assessing cartilage repair and regeneration at a nonarticular site. Tissue Eng Part A. 2010;16(7):2321–2330. doi: 10.1089/ten.TEA.2009.0245. [DOI] [PubMed] [Google Scholar]
- 10.Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12(2):69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 11.Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cell Mater. 2006;12:92–101. doi: 10.22203/ecm.v012a11. [DOI] [PubMed] [Google Scholar]
- 12.Cancel M, Grimard G, Thuillard-Crisinel D, Moldovan F, Villemure I. Effects of in vivo static compressive loading on aggrecan and type II and X collagens in the rat growth plate extracellular matrix. Bone. 2009;44(2):306–315. doi: 10.1016/j.bone.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Bonnel F, Peruchon E, Baldet P, Dimeglio A, Rabischong P. Effects of compression on growth plates in the rabbit. Acta Orthop Scand. 1983;54(5):730–733. doi: 10.3109/17453678308996619. [DOI] [PubMed] [Google Scholar]
- 14.Langenskiold A. Traumatic premature closure of the distal tibial epiphyseal plate. Acta Orthop Scand. 1967;38(4):520–531. doi: 10.3109/17453676708989658. [DOI] [PubMed] [Google Scholar]
- 15.Hasler CC, Foster BK. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res. 2002;(405):242–249. doi: 10.1097/00003086-200212000-00031. [DOI] [PubMed] [Google Scholar]
- 16.Foster BK, John B, Hasler C. Free fat interpositional graft in acute physeal injuries: the anticipatory Langenskiold procedure. J Pediatr Orthop. 2000;20(3):282–285. [PubMed] [Google Scholar]