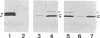Abstract
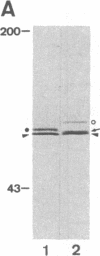
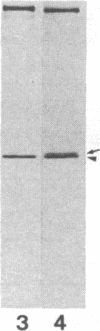
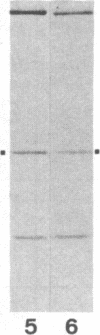
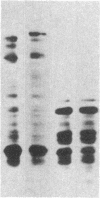
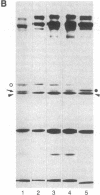
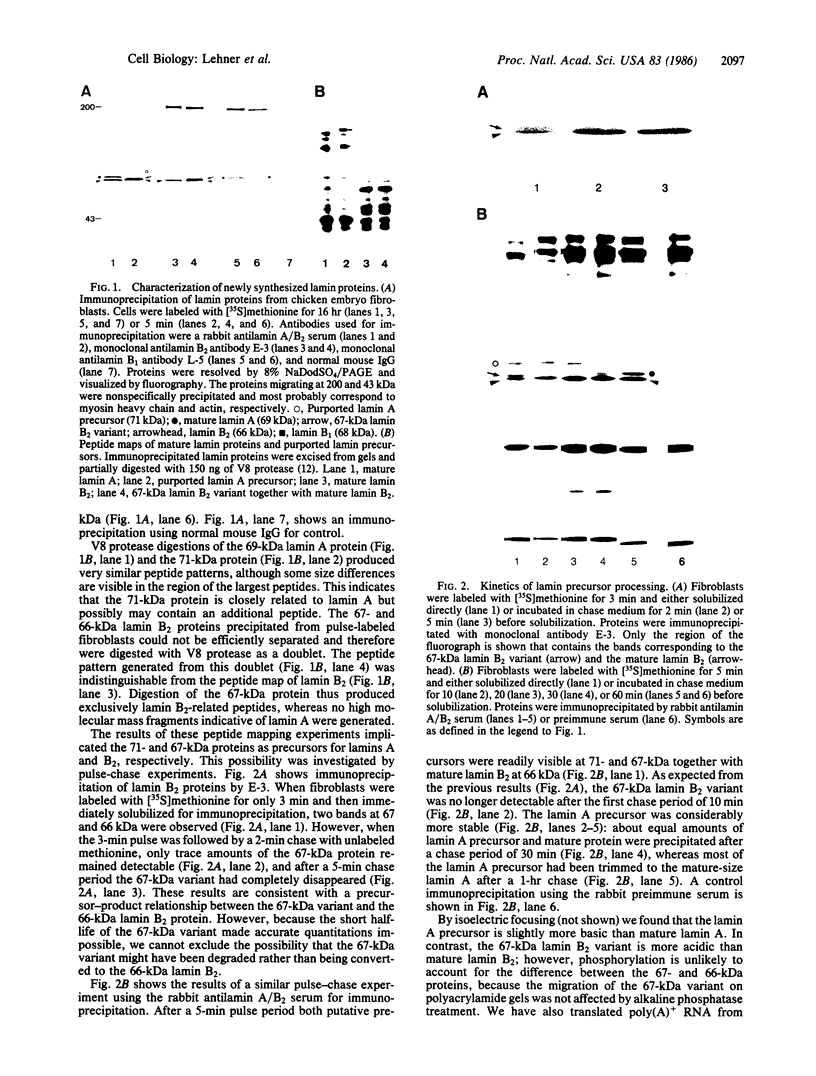
Utilizing antibodies against lamins A, B1, and B2, we have studied the biogenesis of the nuclear lamina in chicken embryo fibroblasts. (Lamins B1 and B2 have been identified recently as structurally distinct "lamin B" proteins.) We demonstrate that, unique among the nuclear proteins studied to date, lamin A is synthesized as a higher molecular mass precursor. A short-lived higher molecular mass variant (t 1/2 approximately equal to 3 min) accompanying the mature-size protein was also detected in the case of lamin B2 biosynthesis, but no precursor was found for lamin B1. By combining pulse-chase experiments with subcellular fractionation, we provide evidence that synthesis of lamin proteins occurs on free polysomes; subsequently, the newly synthesized proteins become rapidly associated with a crude nuclear fraction. The lamin A precursor is processed within the nucleus with a half-time of about 30 min. Concomitantly, lamin proteins acquire a characteristic resistance to detergent extraction, suggesting their insertion into a submembraneous protein network. The described biogenetic pathway involving precursor synthesis and processing is very unusual for nuclear proteins; it may have interesting implications for the mechanisms of transport and assembly of poorly soluble nuclear proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agutter P. S., Richardson J. C. Nuclear non-chromatin proteinaceous structures: their role in the organization and function of the interphase nucleus. J Cell Sci. 1980 Aug;44:395–435. doi: 10.1242/jcs.44.1.395. [DOI] [PubMed] [Google Scholar]
- Blobel G. Control of intracellular protein traffic. Methods Enzymol. 1983;96:663–682. doi: 10.1016/s0076-6879(83)96056-1. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dabauvalle M. C., Franke W. W. Karyophilic proteins: polypeptides synthesized in vitro accumulate in the nucleus on microinjection into the cytoplasm of amphibian oocytes. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5302–5306. doi: 10.1073/pnas.79.17.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M., Longthorne R. F., Gurdon J. B. Intracellular migration of nuclear proteins in Xenopus oocytes. Nature. 1978 Mar 16;272(5650):254–256. doi: 10.1038/272254a0. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M. Nucleocytoplasmic segregation of proteins and RNAs. Cell. 1983 Apr;32(4):1021–1025. doi: 10.1016/0092-8674(83)90285-4. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Krohne G., Jarasch E. D. The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol. 1981 Dec;91(3 Pt 2):39s–50s. doi: 10.1083/jcb.91.3.39s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blobel G. Nuclear lamina and the structural organization of the nuclear envelope. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):967–978. doi: 10.1101/sqb.1982.046.01.090. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerace L., Comeau C., Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. doi: 10.1242/jcs.1984.supplement_1.10. [DOI] [PubMed] [Google Scholar]
- Hancock R., Boulikas T. Functional organization in the nucleus. Int Rev Cytol. 1982;79:165–214. doi: 10.1016/s0074-7696(08)61674-5. [DOI] [PubMed] [Google Scholar]
- Laliberté J. F., Dagenais A., Filion M., Bibor-Hardy V., Simard R., Royal A. Identification of distinct messenger RNAs for nuclear lamin C and a putative precursor of nuclear lamin A. J Cell Biol. 1984 Mar;98(3):980–985. doi: 10.1083/jcb.98.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Moon R. T. Assembly and topogenesis of the spectrin-based membrane skeleton in erythroid development. Cell. 1984 Jun;37(2):354–356. doi: 10.1016/0092-8674(84)90364-7. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Laemmli U. K. Non-histone proteins and long-range organization of HeLa interphase DNA. J Mol Biol. 1982 Apr 5;156(2):325–344. doi: 10.1016/0022-2836(82)90332-1. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., Eppenberger H. M., Fakan S., Nigg E. A. Nuclear substructure antigens. Monoclonal antibodies against components of nuclear matrix preparations. Exp Cell Res. 1986 Jan;162(1):205–219. doi: 10.1016/0014-4827(86)90439-8. [DOI] [PubMed] [Google Scholar]
- Miake-Lye R., Kirschner M. W. Induction of early mitotic events in a cell-free system. Cell. 1985 May;41(1):165–175. doi: 10.1016/0092-8674(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Ottaviano Y., Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985 Jan 10;260(1):624–632. [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Shelton K. R., Higgins L. L., Cochran D. L., Ruffolo J. J., Jr, Egle P. M. Nuclear lamins of erythrocyte and liver. J Biol Chem. 1980 Nov 25;255(22):10978–10983. [PubMed] [Google Scholar]
- Stick R., Hausen P. Changes in the nuclear lamina composition during early development of Xenopus laevis. Cell. 1985 May;41(1):191–200. doi: 10.1016/0092-8674(85)90073-x. [DOI] [PubMed] [Google Scholar]
- Stick R., Schwarz H. Disappearance and reformation of the nuclear lamina structure during specific stages of meiosis in oocytes. Cell. 1983 Jul;33(3):949–958. doi: 10.1016/0092-8674(83)90038-7. [DOI] [PubMed] [Google Scholar]
- Woods C. M., Lazarides E. Degradation of unassembled alpha- and beta-spectrin by distinct intracellular pathways: regulation of spectrin topogenesis by beta-spectrin degradation. Cell. 1985 Apr;40(4):959–969. doi: 10.1016/0092-8674(85)90356-3. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Warner J. R. Cytoplasmic synthesis of nuclear proteins. Kinetics of accumulation of radioactive proteins in various cell fractions after brief pulses. J Cell Biol. 1971 Dec;51(3):643–652. doi: 10.1083/jcb.51.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]