Abstract
Intravenous immunoglobulin (IVIg) is used for the treatment of an increasing number of autoimmune diseases. Clinical observations on IVIg-treated patients have revealed a modulation of T cell populations and functions in these patients. In vitro studies aimed at understanding the mechanisms underlying the effects of IVIg on T cells led to the conclusion that IVIg directly affected lectin-activated T cell functions. However, more recent studies have suggested the absence of a direct effect of IVIg on T cells. In the present work, we revisited the effect of IVIg on T cells using lectin-stimulated human T cells and showed that IVIg inhibited T cell functions only when added simultaneously with the activating lectin. Further, we showed that IVIg depleted from lectin-reactive IgG was no longer inhibitory, suggesting that the effect of IVIg on T cells was the consequence of lectin neutralization, possibly by interaction with glycans present in F(ab′)2 portion of IgG molecules. Our results challenge the previously widely accepted notion that IVIg exerts its anti-inflammatory effects by acting directly on T cells and suggest that effects of IVIg observed in treated patients are rather a consequence of the recently reported inhibitory effect of IVIg on antigen presentation.
Keywords: glycan, IVIg, lectin, phytohaemagglutinin, T cell activation
Introduction
Intravenous immunoglobulin (IVIg) is a therapeutic preparation of human IgG derived from the plasma of thousands of healthy donors and was used initially as replacement therapy in patients with primary or secondary immunodeficiencies. In the early 1980s, infusion of high doses of IVIg was shown to produce therapeutic effects in patients suffering from immune thrombocytopenia (ITP) by attenuating platelet clearance [1]. IVIg is now used commonly to treat a diversity of autoimmune and inflammatory diseases [2–4]. However, the mechanisms by which IVIg produces anti-inflammatory effects in such a diversity of diseases are still not well defined and require further investigation [5,6].
T cells are important players in normal immunity, but also play a critical role in the development and persistence of autoimmunity [7–9]. Clinical studies conducted on IVIg-treated patients have revealed significant modulations of T cell functions in these patients. More precisely, these studies have shown that IVIg influences the ratio of T helper type 1 (Th1)/Th2 cells, induces Th0 or Th2 responses and modifies the cytokine expression profile in patients suffering from a diversity of inflammatory disorders [10–14]. To understand the mechanisms by which IVIg modulates T cell populations and their pattern of cytokine secretion, several groups of investigators have performed in vitro assays in which purified T cells were activated with mitogenic lectins in the presence of IVIg. Conclusions derived from these studies indicated that IVIg has a direct effect on activated T cell functions, leading to inhibition of proliferation and cytokine secretion or induction of T cell apoptosis. However, a direct effect of IVIg on T cells was questioned following reports from Achiron et al. [15] and Skanse-Saphir et al. [16]. In addition, work from our laboratory has established recently that IVIg has no direct effect on antigen-activated T cells, but rather modulates the functions of antigen-specific T cells by reducing the activity of antigen-presenting cells (APCs) [17]. These recent observations on the absence of a direct effect of IVIg on T cells prompted us to re-examine the mechanisms by which IVIg interfered with the functions of T cells activated with lectins. Our results reveal that the inhibition of cytokine secretion or proliferation reported previously for lectin-activated T cells in the presence of IVIg is due to a direct interaction of IVIg with lectins, leading to neutralization of their mitogenic or stimulatory potential, rather than a direct effect of IVIg on T cells.
Materials and methods
Cells and reagents
The human Jurkat T cell line (clone D1·1) was obtained from American Type Culture Collection (Rockville, MD, USA) and was mycoplasma free. Jurkat T cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) ultra-low IgG, 1 mm sodium pyruvate (all from Invitrogen Canada Inc., Burlington, Canada), 100 units/ml of penicillin and 100 µg/ml of streptomycin (Sigma-Aldrich Canada Ltd, Toronto, Canada). IVIg (10%; Gamunex) and human serum albumin (HSA) were obtained from Talecris Biotherapeutics Ltd (Mississauga, Canada). IVIg and HSA were dialysed against RPMI-1640 medium, sterile-filtered and stored at −80°C until use. Phytohaemagglutin-L (PHA), concanavalin A (Con A) and pokeweed mitogen (PWM) were all purchased from Sigma-Aldrich. PHA conjugated to AlexaFluor 488 (PHA-AF488) was obtained from Invitrogen Canada Inc. Human peripheral blood mononuclear cells (PBMC) were isolated by density centrifugation over Ficoll-Paque (GE Healthcare Bio-Sciences, Inc., Baie d'Urfé, Canada) starting with whole blood collected from healthy volunteers after informed consent.
T cell activation assay
Jurkat T cells (5 × 105) or PBMC were seeded in 1 ml of culture medium in 24-well microplates (Corning, Fisher Scientific Company, Ottawa, Canada) and cultured at 37°C in a humidified atmosphere containing 10% CO2, in the presence of 0·5 µg/ml of PHA unless indicated otherwise, with or without IVIg or the immunoglobulin fraction tested at the indicated concentrations. In some experiments, cells were activated with 20 µg/ml of Con A instead of PHA. After 24 h of culture, T cell function was assessed by measuring the amount of human interleukin (IL)-2 secreted in the culture supernatants by enzyme-linked immunosorbent assay (ELISA), using matched antibody pairs (R&D systems, Minneapolis, MN, USA).
Determination of lectin-reactive IgG by ELISA
The presence of lectin-reactive IgG was evaluated in a standard ELISA using PHA, Con A or PWM diluted to 20 µg/ml in carbonate buffer (100 mm, pH 9·6) and bound to the wells of 96-well microplates (Immulon 2 HB; Fisher Scientific Company). The lectin-bound human IgG were detected using a goat anti-human lambda chain-specific horseradish peroxidase (HRP) conjugate (Jackson Immunoresearch Laboratories, West Grove, PA, USA) and tetramethylbenzidine as HRP substrate (ScyTek Laboratories, Logan, UT, USA). The reaction was stopped by addition of H2SO4 (1 m) and the optical densities (OD) were read using a microplate reader at 450 nm (SpectraMax Plus384; Molecular Devices, Chicago, IL, USA).
Depletion of lectin-reactive IgG from IVIg
To deplete IVIg from PHA-reactive IgG, PHA was conjugated to N-hydroxysuccinimide (NHS)-activated Sepharose in a HiTrap column (GE Healthcare), following the manufacturer's instructions. IVIg was depleted from Con A-reactive IgG using a Con A-Sepharose 4B column (GE Healthcare). Dialysed IVIg was loaded onto each of the columns and a volume of flow-through corresponding to the sample loading volume was recovered. The flow-through fractions contained IVIg depleted from PHA-reactive IgG (IVIg–PHA) or IVIg depleted from Con A-reactive IgG (IVIg–Con A), respectively, as confirmed by ELISA.
Preparation of F(ab′)2 fragments of IVIg
F(ab′)2 fragments of IVIg were obtained by digestion with immobilized pepsin (Fisher Scientific Company). The immobilized pepsin was first equilibrated in IVIg buffer (glycine 0·2 m, pH 4·25) prior to addition of IVIg. After overnight incubation at 37°C, digestion was stopped by addition of Tris-HCl 1 m, pH 9·0 and the crude digest was recovered after centrifugation. Undigested IgG and Fc fragments were removed by chromatography on protein A-Sepharose (GE Healthcare) followed by chromatography on an anti-human IgG-Sepharose column prepared using the mouse monoclonal C5-1 anti-human IgG antibody [18] conjugated to NHS-activated Sepharose (GE Healthcare). The purity of the F(ab′)2 preparation was >95%, as assessed by gel filtration chromatography and sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) under non-reducing conditions. The F(ab')2 fragments were dialysed against RPMI-1640 medium, sterile-filtered and stored at −80°C until use.
IVIg deglycosylation
Fc-deglycosylated IVIg was obtained by digestion with peptide:N-glycosidase F (PNGase F) (New England Biolabs, Pickering, Canada). Briefly, IVIg was diluted to 100 µg/ml with phosphate-buffered saline (PBS) (pH 8·6) and 10 µg of diluted IVIg were incubated with 50 000 units of PNGase F for 48 h at 37°C. The efficiency of the deglycosylation was assessed by SDS-PAGE under reducing conditions.
Statistical analysis
All statistical analyses were performed using GraphPad InStat software (GraphPad Software, La Jolla, CA, USA), using the appropriate parametric [t-test, (one-way analysis of variance (anova)] or non-parametric (Mann–Whitney U-, Kruskal–Wallis) tests and post-tests. Values of P < 0·05 were considered to indicate statistical significance.
Results
Presence of PHA-specific IgG in IVIg
PHA-activated Jurkat T cells are known to secrete significant levels of IL-2. To measure the effect of IVIg on IL-2 secretion, Jurkat T cells were incubated in the presence of PHA (0·5 µg/ml) and increasing concentrations of IVIg (0–20 mg/ml). After 24 h of culture, IL-2 secretion was measured by ELISA. The results showed a dose-dependent inhibition of IL-2 secretion with an almost complete inhibition in the presence of 20 mg/ml of IVIg (Fig. 1a). The inhibitory effect was specific to IVIg because addition of similar concentrations of HSA did not decrease IL-2 secretion compared to the control condition in which no protein was added (data not shown). Unstimulated Jurkat T cells were also used as control and did not secrete detectable levels of IL-2 (data not shown). These results are in agreement with those reported in previously published studies on IVIg using T cells and PHA. However, additional experiments performed with Jurkat T cells activated with a higher dose of PHA (4 µg/ml) in the presence of IVIg (10 mg/ml) did not result in a significant inhibition of IL-2 secretion (data not shown). We therefore postulated that the inhibitory activity of IVIg on IL-2 secretion was dependent upon the concentration of PHA used to activate cells. The effect of different concentrations of PHA on the inhibition of IL-2 secretion in the presence of 10 mg/ml of IVIg was thus determined. Results showed an inverse relationship between the dose of PHA used and the extent of inhibition of IL-2 secretion in the presence of IVIg. Indeed, IL-2 secretion was reduced by only 20% when high concentrations of PHA were used, compared to >90% inhibition when cells were activated with 0·25 µg/ml PHA (Fig. 1b). These results suggested that IVIg did not act on PHA-activated Jurkat T cells, but rather interfered with PHA, either by binding and neutralization or competition for PHA receptors on the T cell surface, therefore preventing Jurkat T cell activation and the subsequent IL-2 secretion.
Fig. 1.
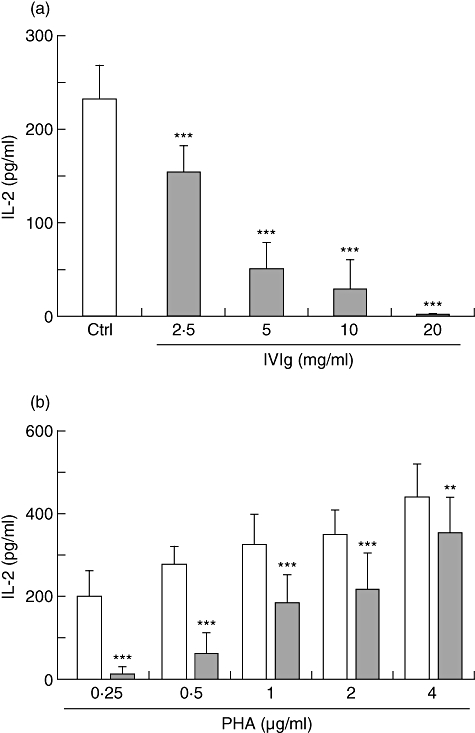
Combined effect of intravenous immunoglobulin (IVIg) and phytohaemagglutinin (PHA) concentrations on Jurkat T cell activation. (a) Jurkat T cells were activated with 0·5 µg/ml of PHA in the presence of increasing concentrations of IVIg (0–20 mg/ml) and cultured for 24 h prior to evaluation of interleukin (IL)-2 secretion by enzyme-linked immunosorbent assay (ELISA). Results shown are the mean [± standard deviation (s.d.)] of three independent experiments. (b) Jurkat T cells were activated with increasing concentrations of PHA (0·25–4 µg/ml) in the presence or not of IVIg (10 mg/ml), for 24 h prior to IL-2 determination. Results shown are the mean (± s.d.) of four independent experiments. **P < 0·01; ***P < 0·001 [one-way analysis of variance (anova)] followed by Bonferroni's post-test).
To study this hypothesis, we first determined whether IVIg interferes with the binding of PHA on the surface of Jurkat T cells. This was performed using fluorescent PHA (PHA-AF488) to allow detection of binding by flow cytometry. Jurkat T cells were incubated for 30 min with PHA-AF488 in the presence of increasing concentrations of IVIg followed by determination of the mean fluorescence intensity (MFI) of the cells. Results obtained showed a decreased binding of PHA-AF488 with increasing concentrations of IVIg (Fig. 2), indicating that IVIg prevents binding of PHA to Jurkat T cells. In contrast, similar concentrations of HSA (used as control protein) did not inhibit PHA-AF488 binding to Jurkat T cells. To gain more insight into the mechanism of inhibition of PHA binding, Jurkat T cells were first incubated with IVIg (10 mg/ml) for 4 h, followed by washing and stimulation with PHA (0·5 µg/ml), in the presence or not of freshly added IVIg. After an additional incubation of 24 h, the IL-2 concentration in the supernatants was determined by ELISA. IVIg-treated Jurkat T cells responded to PHA stimulation by secreting levels of IL-2 comparable to those observed using untreated Jurkat T cells stimulated with PHA (Fig. 3). This result indicated that PHA receptors were not blocked by IVIg. In contrast, IL-2 secretion by IVIg-treated or untreated Jurkat T cells was inhibited when IVIg was added during PHA stimulation (Fig. 3). This indicated that IVIg needs to be present at the same time as PHA to perform its inhibitory effect, suggesting that IVIg directly binds and neutralizes PHA, therefore preventing cell activation and the subsequent IL-2 secretion. Direct binding of IVIg to PHA was confirmed by ELISA, using PHA as capture antigen (see Fig. 4a).
Fig. 2.
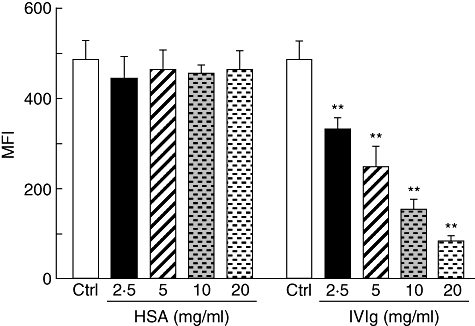
Intravenous immunoglobulin (IVIg) interferes with the binding of phytohaemagglutinin (PHA) to Jurkat T cells. Jurkat T cells were incubated with AF488-PHA in the presence of increasing concentrations of human serum albumin (HSA) or IVIg, for 30 min at 37°C, followed by fluorescence determination by flow cytometry. The mean [± standard deviation (s.d.)] of mean fluorescence intensity (MFI) from three independent experiments are shown. **P < 0·01 [one-way analysis of variance (anova) followed by Dunnett's post-test].
Fig. 3.
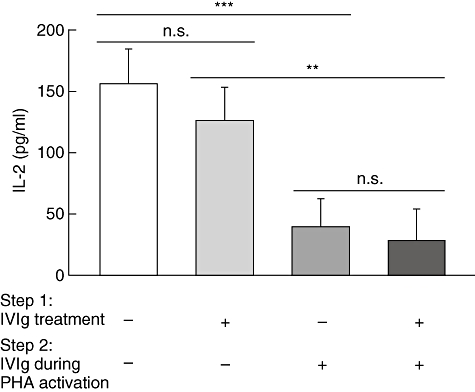
Intravenous immunoglobulin (IVIg)-treated Jurkat T cells are responsive to phytohaemagglutinin (PHA) activation. Jurkat T cells were incubated for 4 h in the presence of IVIg (10 mg/ml) (step 1), followed by three washes and PHA activation (0·5 µg/ml) in the presence or absence of 10 mg/ml of IVIg (step 2). Cells were incubated to 24 h prior to interleukin (IL)-2 determination by enzyme-linked immunosorbent assay (ELISA). Results [± standard deviation (s.d.)] are representative of five independent experiments. n.s.: not significant, **P < 0·01; ***P < 0·001 (Kruskal–Wallis with Dunn's post-test).
Fig. 4.
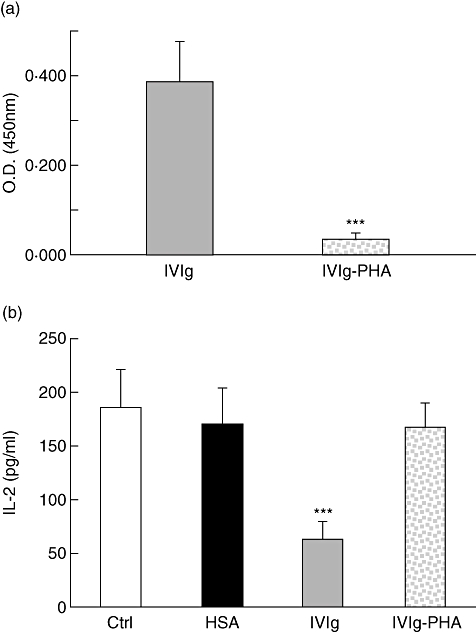
Depletion of phytohaemagglutinin (PHA)-reactive IgG from intravenous immunoglobulin (IVIg) abolishes its inhibitory activity. (a) The presence of PHA-reactive IgG in IVIg before and after passage on a PHA-Sepharose column was evaluated by enzyme-linked immunosorbent assay (ELISA) using PHA as capture antigen and 50 µg/ml of each fraction. ***P < 0·001 (unpaired t-test, Welch corrected). (b) Jurkat T cells were incubated in the presence of PHA (0·5 µg/ml) and 5 mg/ml of human serum albumin (HSA), IVIg or IVIg–PHA for 24 h, prior to interleukin (IL)-2 determination by ELISA. ***P < 0·001 (Kruskal–Wallis with Dunn's post-test). Results [± standard deviation (s.d.)] are representative of at least four independent experiments.
Inhibitory potential of IVIg depleted from PHA-reactive IgG
To confirm that IVIg inhibited IL-2 secretion by neutralizing PHA, we depleted the IVIg preparation from PHA-reactive IgG using a PHA-Sepharose affinity column. The flow-through recovered from the column after loading IVIg was considered as the PHA-reactive IgG-depleted IVIg fraction (IVIg–PHA). The extent of depletion was evaluated by ELISA (Fig. 4a) and showed > 85% depletion as evaluated by the reduction of OD in the IVIg–PHA fraction (OD of 0·322 and 0·043 for IVIg and IVIg–PHA, respectively). The effect of IVIg or IVIg–PHA (both at 5 mg/ml) on Jurkat T cell stimulation by PHA was next studied. Results obtained showed a 78% inhibition of IL-2 secretion in the presence of IVIg, while IVIg–PHA or a similar concentration of HSA did not inhibit IL-2 secretion significantly (Fig. 4b). This observation indicated that PHA-reactive IgG play a major role in the IVIg-mediated inhibition of IL-2 production by Jurkat T cells.
F(ab')2-dependent interaction between PHA and IVIg
To determine whether interaction between IVIg and PHA occurred following a classical antigen/antibody-type interaction, F(ab′)2 fragments of IVIg were prepared and their reactivity with PHA was evaluated by ELISA (Fig. 5a). Results obtained showed that equimolar amounts of purified F(ab′)2 fragments bound to PHA as effectively as IVIg, indicating that IVIg interacts with PHA via its Fab region. In contrast, Fc fragments of IVIg showed no reactivity with PHA in ELISA (data not shown), indicating that PHA does not interact with glycans present in the Fc region (Asn297) of IgG. The effect of F(ab′)2 fragments of IVIg on PHA-mediated Jurkat T cell activation was compared to that of IVIg, using equimolar amounts of proteins. As shown in Fig. 5b, the F(ab')2 fragments inhibited IL-2 secretion as efficiently as IVIg, confirming that IVIg binds and neutralizes PHA via its F(ab')2 region.
Fig. 5.
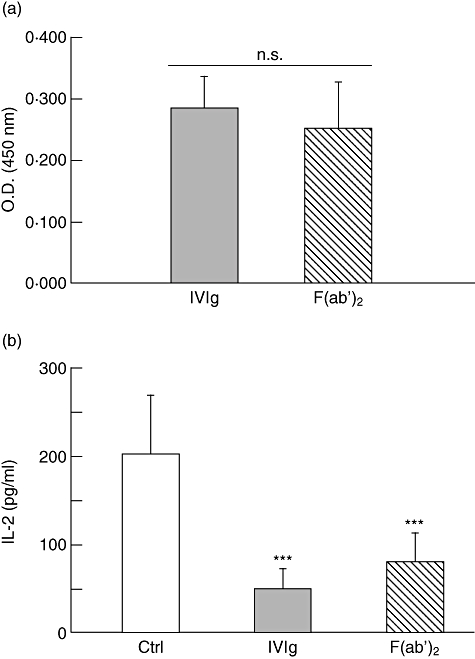
F(ab')2 fragments are as efficient as intravenous immunoglobulin (IVIg) to interact with phytohaemagglutinin (PHA) and inhibit Jurkat T cell activation. (a) The PHA reactivity of equimolar amounts of F(ab')2 fragments (33 µg/ml) was compared to that of IVIg (50 µg/ml) in enzyme-linked immunosorbent assay (ELISA). Results [± standard deviation (s.d.)] are representative of four separate experiments. n.s.: not significant (Mann–Whitney U-test). (b) Jurkat T cells were incubated in the presence of PHA and equimolar amounts of IVIg (5 mg/ml) or F(ab')2 fragments (3·3 mg/ml) for 24 h prior to evaluation of interleukin (IL)-2 secretion by ELISA. Results shown are the mean (± s.d.) of four independent experiments. ***P < 0·001 (Kruskal–Wallis with Dunn's post-test).
Interference of IVIg with PHA activation of primary T cells
We next determined whether IVIg also interferes with PHA activation of primary T cells. It is known that purified primary T cells cannot be activated by PHA alone, but also require essential signals from accessory cells [19–21]. We therefore used PBMC isolated from healthy volunteers to evaluate the effect of IVIg, IVIg–PHA and F(ab')2 fragments on primary T cell activation by PHA. IL-2 secretion was used as a measure of the extent of primary T cell activation. The results obtained showed a significant reduction in IL-2 secretion when IVIg and F(ab')2 fragments were added to the PHA-supplemented PBMC culture, while no significant reduction was observed in cultures performed in the presence of IVIg–PHA (Fig. 6). These results therefore confirm the results obtained previously using Jurkat T cells.
Fig. 6.
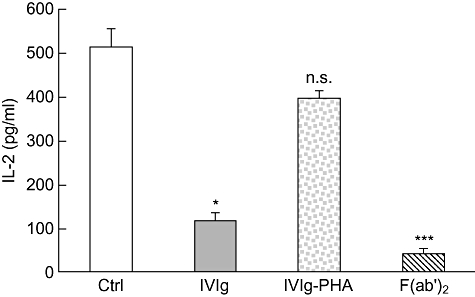
Intravenous immunoglobulin (IVIg) interferes with phytohaemagglutinin (PHA) during primary T cell activation. Peripheral blood mononuclear cells (PBMC) were activated with 0·5 µg/ml of PHA in the presence of IVIg, IVIg–PHA (5 mg/ml) or F(ab')2 (3·33 mg/ml) and cultured for 24 h prior to evaluation of interleukin (IL)-2 secretion by enzyme-linked immunosorbent assay (ELISA). Results shown are the mean [± standard deviation (s.d.)] of three independent experiments. *P < 0·05; ***P < 0·001; n.s.: not significant (Kruskal–Wallis with Dunn's post test).
Interference of IVIg with other lectins
Several groups of investigators reported that IVIg inhibited the functions of lymphocytes stimulated with Con A or PWM. To determine whether this inhibitory effect could also be the consequence of lectin neutralization rather than a direct effect of IVIg on activated cells, the presence of Con A- and PWM-reactive IgG in IVIg was evaluated by ELISA. We also tested the effect of removing Fc-associated glycans on the binding of IVIg to lectins. This was performed by digestion of IVIg with PNGase F and assay of the Fc-deglycosylated IVIg in ELISA. The removal of glycans from the Fc region of IgG was confirmed by SDS-PAGE under reducing conditions, as illustrated by the shift in molecular weight observed in the IgG heavy chains (Fig. 7a). Results showed that IVIg bound to PHA, Con A and PWM, but that removal of Fc-associated glycans reduced the binding to Con A and PWM (Fig. 7b). These results are consistent with the fact that lectins possess different glycan recognition patterns. The presence of Con A- and PWM-reactive IgG in IVIg further supports the idea that IVIg neutralizes the activity of lectins rather than inhibits the functions of activated cells. Indeed, removal of Con A-reactive IgG from IVIg by affinity chromatography (IVIg–Con A fraction) led to depletion of about 90% of Con A reactivity in ELISA (OD of 0·976 and 0·096 for IVIg and IVIg–Con A, respectively) (Fig. 7c). Similarly, Jurkat T cell activation by Con A (20 µg/ml) was inhibited strongly in the presence of IVIg while the IVIg–Con A had no significant effect on cell activation and the subsequent IL-2 secretion (Fig. 7d).
Fig. 7.
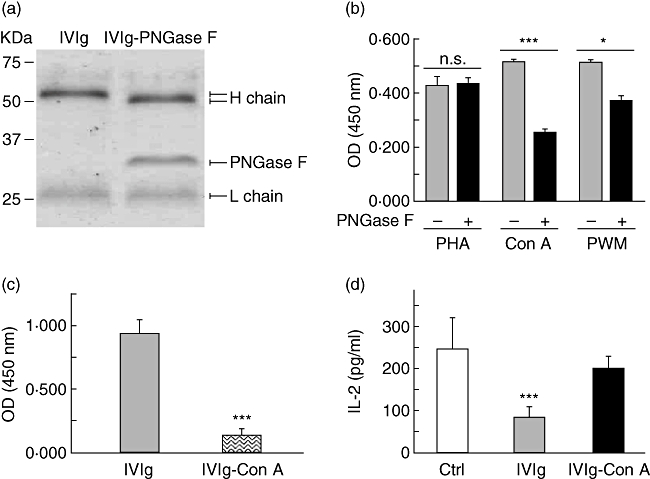
Intravenous immunoglobulin (IVIg) contains IgG reactive with lectins other than phytohaemagglutinin (PHA) and neutralizes their stimulatory activity. (a) Molecular weight analysis of IVIg and peptide:N-glycosidase F (PNGase F)-treated IVIg by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% gel. (b) The reactivity of IVIg or Fc-deglycosylated IVIg (20 µg/ml) with PHA, concanavalin A (Con A) and pokeweed mitogen (PWM) was assessed in enzyme-linked immunosorbent assay (ELISA). Results [± standard deviation (s.d.)] are representative of at least two independent experiments. *P < 0·05; ***P < 0·001; n.s.: not significant (unpaired t-test). (c) The presence of Con A-reactive IgG in IVIg before and after passage on a Con A-sepharose column was evaluated by ELISA. Results (mean ± s.d.) are representative of three independent experiments. ***P < 0·001 (unpaired t-test). (d) Jurkat T cells were incubated in the presence of Con A (20 µg/ml) and 5 mg/ml of IVIg or IVIg–Con A for 24 h, prior to interleukin (IL)-2 determination by ELISA. Mean (± s.d.) of two independent experiments. ***P < 0·001 (Kruskal–Wallis with Dunn's post test).
Discussion
In the present work, we show that IVIg interacts with and neutralizes the stimulatory effect of lectins such as PHA, Con A and PWM. These lectins have been used in numerous studies to determine the effect of IVIg on lymphocyte functions, leading to the conclusion that IVIg had a direct effect on activated T or B cells (inhibition of proliferation, cytokine secretion, expression of activation markers such as CD25 or CD69, or immunoglobulin secretion) [22–29]. In the light of the results presented here, we believe that these conclusions should be revisited. Indeed, we demonstrate clearly that IVIg preparations contain IgG fractions reacting with PHA, Con A and PWM. Furthermore, we show that removal of PHA- or Con A-reactive IgG from IVIg results in loss of inhibitory activity on T cells, indicating that the main mechanism of inhibition is simply a direct binding of the lectin with lectin-reactive IgG, leading to its inability to bind to and activate T cells. The above observations were mostly conducted using the Jurkat T cell line, but key experiments were also performed using primary cells and confirmed the interference of IVIg with mitogenic lectins. In the case of B cells, it is likely that the previously reported inhibitory effects on immunoglobulin secretion result from PWM neutralization rather than by a direct effect of IVIg on stimulated B cells, although this has not been determined experimentally in the present work.
To address correctly the question of whether IVIg acts directly on T cells or B cells, more physiologically relevant activation systems (e.g. anti-CD3 and anti-CD28 or antigen-driven activation) should be used. In some of the previous work discussed above, T cells were activated using anti-CD3 antibodies in combination or not with anti-CD28 antibodies, in the presence of IVIg. Results of these experiments also led the authors to conclude that IVIg directly inhibits T cell functions. However, we obtained evidence indicating that IVIg also interferes with the CD3/CD28 activation system, therefore preventing cell activation (L.P. and R.B., manuscript in preparation), rather than suppressing functions of activated T cells. Recent work from our laboratory using APCs to activate CD4+ T cells with immune complexes of ovalbumin revealed the absence of a direct effect of IVIg on T cell activation or proliferation [17]. Rather, our results showed that IVIg indirectly inhibited the in vivo and in vitro T cell responses by impairing antigen presentation. Similarly, Néron et al. showed that IVIg did not inhibit immunoglobulin secretion by human B cells activated by CD154 (the ligand for CD40) in the presence of cytokines such as IL-2, IL-4 and IL-10 [30], in contrast to the previously reported effects observed using PWM to stimulate B cells in the presence of IVIg [11,22,25]. Another recent report also showed the absence of a direct effect of IVIg on B cells [27]. Altogether, these observations suggest that the effect of IVIg is directed mainly towards the APCs and that the effect on T and B cell functions in IVIg-treated patients described above are consequences of a reduced ability of APCs to deliver activating signals to these cells.
We showed that F(ab′)2 fragments were as effective as IVIg to inhibit IL-2 secretion by Jurkat T cells stimulated with PHA, suggesting that IVIg interacts with lectins in an antigen/antibody manner. Because it is unlikely that PHA-specific IgG were produced in humans by natural immunization against the lectin, we speculated that lectin-reactive IgG could be present in the IVIg fraction containing polyreactive IgG (about 3% of IVIg) [31]. Conversely, IgG are glycoproteins and may interact with lectins through their sugar residues. Indeed, it is well established that all IgG carry a conserved N-linked glycosylation site at Asn297, in the CH2 domain of the constant region [32]. In addition, 15–20% of human serum IgG also bear complex diantennary oligosaccharides attached to the variable regions of their light or heavy chains [33,34]. PHA binds preferentially to galactosylated complex diantennary oligosaccharides [35], and this glycan structure is found mainly on the Fab region of human serum IgG in contrast to oligosaccharides in the Fc region which are hypogalactosylated [34]. This suggests that PHA would bind efficiently to glycans present in the Fab portion but not in the Fc region of the IgG. This hypothesis is supported by our observation that F(ab′)2 and Fc-deglycosylated IVIg but not Fc fragments of IVIg bound to PHA in ELISA. Therefore, to determine what fraction of IVIg interacts with PHA (polyreactive IgG or Fab-glycosylated IgG), we considered the yield of IgG recovered in the IVIg–PHA fraction after chromatography on PHA-Sepharose columns. The IVIg–PHA fraction contained about 80% of the total amount of IgG loaded onto the columns (14·6 mg recovered from 20 mg of IVIg loaded onto the column), indicating that at least 20% of the IgG present in IVIg were bound to or retarded in the PHA-Sepharose column. This amount of lectin-reactive IgG corresponds to the proportion of Fab-glycosylated IgG in human serum [33,34], suggesting that glycans present in the IgG variable region rather than polyreactive IgG are responsible for binding and neutralizing PHA.
Con A and PWM possess oligosaccharide specificities different from those of PHA. These lectins bind preferentially to α-mannose/glucose and dimers of N-acetyl-β-glucosamine, respectively, and are known to recognize different carbohydrates present in the Fc region of IgG [36], in agreement with our observation that Fc-deglycosylated IVIg bound less efficiently to Con A and PWM than intact IVIg in ELISA. Altogether, these observations lead to the general conclusion that IVIg interacts with lectins via specific oligosaccharides present in the Fab or Fc region of IgG, resulting in neutralization of their stimulatory activity.
In conclusion, the observations reported here challenge the previously widely accepted notion that IVIg exerts its anti-inflammatory effects by acting directly on T cells. More probably, the in vivo modulation of T cell populations observed in IVIg-treated patients is a consequence of the effect of IVIg on APCs [17]. Our work also emphasizes the importance of ruling out possible interactions of IVIg with mitogens or other effector components present in a given experimental system before deriving strong conclusions on the mechanisms of action of IVIg based on their apparent immunomodulatory effects observed in vitro.
Acknowledgments
The authors thank Pascal Rouleau and Tony Tremblay for excellent technical assistance. L.P. is the recipient of an Industrial Innovation PhD Scholarship from CRSNG/FQRNT.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Imbach P, Barandun S, d'Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1:1228–31. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–33. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 3.Arnson Y, Shoenfeld Y, Amital H. Intravenous immunoglobulin therapy for autoimmune diseases. Autoimmunity. 2009;42:553–60. doi: 10.1080/08916930902785363. [DOI] [PubMed] [Google Scholar]
- 4.Hughes RA, Dalakas MC, Cornblath DR, Latov N, Weksler ME, Relkin N. Clinical applications of intravenous immunoglobulins in neurology. Clin Exp Immunol. 2009;158(Suppl 1):34–42. doi: 10.1111/j.1365-2249.2009.04025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemieux R, Bazin R, Neron S. Therapeutic intravenous immunoglobulins. Mol Immunol. 2005;42:839–48. doi: 10.1016/j.molimm.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Ballow M. The IgG molecule as a biological immune response modifier: mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory disorders. J Allergy Clin Immunol. 2011;127:315–23. doi: 10.1016/j.jaci.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Abbas AK, Lohr J, Knoechel B, Nagabhushanam V. T cell tolerance and autoimmunity. Autoimmun Rev. 2004;3:471–5. doi: 10.1016/j.autrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Crane IJ, Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit Rev Immunol. 2005;25:75–102. doi: 10.1615/critrevimmunol.v25.i2.10. [DOI] [PubMed] [Google Scholar]
- 9.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–66. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 10.Tsubakio T, Kurata Y, Katagiri S, et al. Alteration of T cell subsets and immunoglobulin synthesis in vitro during high dose gamma-globulin therapy in patients with idiopathic thrombocytopenic purpura. Clin Exp Immunol. 1983;53:697–702. [PMC free article] [PubMed] [Google Scholar]
- 11.Dammacco F, Iodice G, Campobasso N. Treatment of adult patients with idiopathic thrombocytopenic purpura with intravenous immunoglobulin: effects on circulating T cell subsets and PWM-induced antibody synthesis in vitro. Br J Haematol. 1986;62:125–35. doi: 10.1111/j.1365-2141.1986.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 12.Mouzaki A, Theodoropoulou M, Gianakopoulos I, Vlaha V, Kyrtsonis MC, Maniatis A. Expression patterns of Th1 and Th2 cytokine genes in childhood idiopathic thrombocytopenic purpura (ITP) at presentation and their modulation by intravenous immunoglobulin G (IVIg) treatment: their role in prognosis. Blood. 2002;100:1774–9. [PubMed] [Google Scholar]
- 13.Aktas O, Zipp F. Regulation of self-reactive T cells by human immunoglobulins – implications for multiple sclerosis therapy. Curr Pharm Des. 2003;9:245–56. doi: 10.2174/1381612033392152. [DOI] [PubMed] [Google Scholar]
- 14.Yamada H, Morikawa M, Furuta I, et al. Intravenous immunoglobulin treatment in women with recurrent abortions: increased cytokine levels and reduced Th1/Th2 lymphocyte ratio in peripheral blood. Am J Reprod Immunol. 2003;49:84–9. doi: 10.1034/j.1600-0897.2003.01184.x. [DOI] [PubMed] [Google Scholar]
- 15.Achiron A, Margalit R, Hershkoviz R, et al. Intravenous immunoglobulin treatment of experimental T cell-mediated autoimmune disease. Upregulation of T cell proliferation and downregulation of tumor necrosis factor alpha secretion. J Clin Invest. 1994;93:600–5. doi: 10.1172/JCI117012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skansen-Saphir U, Andersson J, Bjork L, et al. Down-regulation of lymphokine synthesis by intravenous gammaglobulin is dependent upon accessory cells. Scand J Immunol. 1998;47:229–35. doi: 10.1046/j.1365-3083.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- 17.Aubin E, Lemieux R, Bazin R. Indirect inhibition of in vivo and in vitro T-cell responses by intravenous immunoglobulins due to impaired antigen presentation. Blood. 2010;115:1727–34. doi: 10.1182/blood-2009-06-225417. [DOI] [PubMed] [Google Scholar]
- 18.Bazin R, Lemieux R, Tremblay T, St-Amour I. Tetramolecular immune complexes are more efficient than IVIg to prevent antibody-dependent in vitro and in vivo phagocytosis of blood cells. Br J Haematol. 2004;127:90–6. doi: 10.1111/j.1365-2141.2004.05105.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosenstreich DL, Farrar JJ, Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976;116:131–9. [PubMed] [Google Scholar]
- 20.Habu S, Raff MC. Accessory cell dependence of lectin-induced proliferation of mouse T lymphocytes. Eur J Immunol. 1977;7:451–7. doi: 10.1002/eji.1830070710. [DOI] [PubMed] [Google Scholar]
- 21.de Vries JE, Caviles AP, Jr, Bont WS, Mendelsohn J. The role of monocytes in human lymphocyte activation by mitogens. J Immunol. 1979;122:1099–107. [PubMed] [Google Scholar]
- 22.Stohl W. Modulation of the immune response by immunoglobulin for intravenous use. I. Inhibition of pokeweed mitogen-induced B cell differentiation. Clin Exp Immunol. 1985;62:200–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo N, Ozawa T, Mushiake K, et al. Suppression of immunoglobulin production of lymphocytes by intravenous immunoglobulin. J Clin Immunol. 1991;11:152–8. doi: 10.1007/BF00918683. [DOI] [PubMed] [Google Scholar]
- 24.Klaesson S, Ringden O, Markling L, Remberger M, Lundkvist I. Immune modulatory effects of immunoglobulins on cell-mediated immune responses in vitro. Scand J Immunol. 1993;38:477–84. doi: 10.1111/j.1365-3083.1993.tb02591.x. [DOI] [PubMed] [Google Scholar]
- 25.Toyoda M, Zhang X, Petrosian A, Galera OA, Wang SJ, Jordan SC. Modulation of immunoglobulin production and cytokine mRNA expression in peripheral blood mononuclear cells by intravenous immunoglobulin. J Clin Immunol. 1994;14:178–89. doi: 10.1007/BF01533367. [DOI] [PubMed] [Google Scholar]
- 26.Tha-In T, Metselaar HJ, Tilanus HW, et al. Superior immunomodulatory effects of intravenous immunoglobulins on human T-cells and dendritic cells: comparison to calcineurin inhibitors. Transplantation. 2006;81:1725–34. doi: 10.1097/01.tp.0000226073.20185.b1. [DOI] [PubMed] [Google Scholar]
- 27.Heidt S, Roelen DL, Eijsink C, Eikmans M, Claas FH, Mulder A. Intravenous immunoglobulin preparations have no direct effect on B cell proliferation and immunoglobulin production. Clin Exp Immunol. 2009;158:99–105. doi: 10.1111/j.1365-2249.2009.03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacMillan HF, Lee T, Issekutz AC. Intravenous immunoglobulin G-mediated inhibition of T-cell proliferation reflects an endogenous mechanism by which IgG modulates T-cell activation. Clin Immunol. 2009;132:222–33. doi: 10.1016/j.clim.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Tawfik DS, Cowan KR, Walsh AM, Hamilton WS, Goldman FD. Exogenous immunoglobulin downregulates T-cell receptor signaling and cytokine production. Pediatr Allergy Immunol. 2011 doi: 10.1111/j.1399-3038.2010.01129.x. DOI: 10.1111/j.1399-3038.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 30.Neron S, Boire G, Dussault N, et al. CD40-activated B cells from patients with systemic lupus erythematosus can be modulated by therapeutic immunoglobulins in vitro. Arch Immunol Ther Exp (Warsz) 2009;57:447–58. doi: 10.1007/s00005-009-0048-3. [DOI] [PubMed] [Google Scholar]
- 31.Lamoureux J, Aubin E, Lemieux R. Autoantibodies purified from therapeutic preparations of intravenous immunoglobulins (IVIg) induce the formation of autoimmune complexes in normal human serum: a role in the in vivo mechanisms of action of IVIg? Int Immunol. 2004;16:929–36. doi: 10.1093/intimm/dxh094. [DOI] [PubMed] [Google Scholar]
- 32.Jefferis R, Lund J, Mizutani H, et al. A comparative study of the N-linked oligosaccharide structures of human IgG subclass proteins. Biochem J. 1990;268:529–37. doi: 10.1042/bj2680529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol Prog. 2005;21:11–16. doi: 10.1021/bp040016j. [DOI] [PubMed] [Google Scholar]
- 34.Holland M, Yagi H, Takahashi N, et al. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim Biophys Acta. 2006;1760:669–77. doi: 10.1016/j.bbagen.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Green ED, Baenziger JU. Oligosaccharide specificities of Phaseolus vulgaris leukoagglutinating and erythroagglutinating phytohemagglutinins. Interactions with N-glycanase-released oligosaccharides. J Biol Chem. 1987;262:12018–29. [PubMed] [Google Scholar]
- 36.Malaise MG, Franchimont P, Bouillene C, Houssier C, Mahieu PR. Increased concanavalin A-binding capacity of immunoglobulin G purified from sera of patients with rheumatoid arthritis. Clin Exp Immunol. 1987;68:543–51. [PMC free article] [PubMed] [Google Scholar]


