Abstract
Sepsis is a systemic inflammatory response to infection and a major cause of morbidity and mortality. Sildenafil (SLD) is a selective and potent inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase PDE5. We aimed to investigate the protective effects of sildenafil on caecal ligation and puncture (CLP)-induced sepsis in rats. Four groups of rats were used, each composed of 10 rats: (i) 10 mg/kg SLD-treated CLP group; (ii) 20 mg/kg SLD-treated CLP group; (iii) CLP group; and (iv) sham-operated control group. A CLP polymicrobial sepsis model was applied to the rats. All groups were killed 16 h later, and lung, kidney and blood samples were analysed histopathologically and biochemically. Sildenafil increased glutathione (GSH) and decreased the activation of myeloperoxidase (MPO) and of lipid peroxidase (LPO) and levels of superoxide dismutase (SOD) in the septic rats. We observed a significant decrease in LPO and MPO and a decrease in SOD activity in the sildenafil-treated CLP rats compared with the sham group. In addition, 20 mg/kg sildenafil treatment in the sham-operated rats improved the biochemical status of lungs and kidneys. Histopathological analysis revealed significant differences in inflammation scores between the sepsis group and the other groups, except the CLP + sildenafil 10 mg/kg group. The CLP + sildenafil 20 mg/kg group had the lowest inflammation score. Sildenafil treatment decreased the serum tumour necrosis factor (TNF)-α level when compared to the CLP group. Our results indicate that sildenafil is a highly protective agent in preventing lung and kidney damage caused by CLP-induced sepsis via maintenance of the oxidant–anti-oxidant status and decrease in the level of TNF-α.
Keywords: lung injury, oxidative stress, polymicrobial sepsis, rat, sildenafil
Introduction
Sepsis is a systemic inflammatory response to infection and a major cause of morbidity and mortality worldwide. Sepsis may result in hypotension and organ dysfunction called septic shock [1]. Sepsis/septic shock is characterized by profound hypotension, progressive metabolic acidosis, systemic inflammatory response syndrome (SIRS), tissue damage and multiple organ dysfunction syndrome (MODS), acute respiratory distress syndrome (ARDS) and/or acute lung injury (ALI), or even death. Although its pathophysiology is not well defined, monocytes orchestrate the innate immunity response to Gram-positive and Gram-negative bacteria by expressing a variety of inflammatory cytokines, including tumour necrosis factor (TNF)-α and interleukin (IL)-6, which are considered to play an essential role in the pathogenesis of sepsis [2–6].
These mediators extend the inflammatory response and can lead to multiple organ dysfunction syndrome [7] and, ultimately, death [8].
Some of these oxidants are known to modulate the expression of various genes that are involved in immune and inflammatory responses [9]. Sepsis and endotoxaemia lead to the production of reactive oxygen species (ROS) [10,11], which have been assumed to play a role in the induction of many proinflammatory cytokines and mediators important in producing the acute inflammatory responses associated with sepsis [12].
Endotoxaemia and sepsis are associated with a reduced endogenous antioxidant capacity, and may therefore result in an oxidant–anti-oxidant imbalance [13]. The proinflammatory effects of ROS include endothelial damage, formation of chemotactic factors, neutrophile reinforcement, cytokine release and mitochondrial injury [14–16], all of which contribute to free radical overload and to oxidant–anti-oxidant imbalance. The processes that are implicated in microvascular dysfunction are followed by organ dysfunction [17]; renal and respiratory functions are the major organs involved in the multiple organ dysfunctions in sepsis [18].
Sildenafil is a selective and potent inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase PDE5 for the cure of sexual dysfunction [19]. This inhibitor preserves alveolar growth and angiogenesis and reduces inflammation and airway reactivity in animal models [20,21]. Inhibition of the metabolism of cGMP results in increased relaxation of the smooth muscle surrounding the arterioles that supply the human corpus cavernosum, acting via a nitric oxide (NO)-dependent mechanism. Inhibition of phosphodiesterase 5 leads to increased concentration of cyclic adenosine monophosphate (AMP) and -GMP locally, which in turn leads to relaxation of pulmonary vascular smooth muscles [22]. Sildenafil induces endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS), which generate nitric oxide (NO). Therefore, the cyclic nucleotides cAMP and cGMP are important second messengers that are known to control many cellular processes, such as inflammation [23,24].
Moreover, sildenafil has been proved to reduce oxidative stress to decrease inflammatory events [25,26]. Another study has shown the renoprotective potential of sildenafil against oxidative stress and inflammation in diabetic rats [27]. When we searched the literature, we found many studies that concur with the ability of sildenafil to affect conditions other than sexual function, but we found no study using sildenafil for preventing CLP-induced organ injury.
Therefore, in this study, we induced sepsis/septic shock in rats with caecal ligation and puncture (CLP, a model of polymicrobial sepsis) and hypothesized that sildenafil could prevent CLP-induced tissue injury in vital organs such as the kidney and the lungs by inhibiting the proinflammatory cytokine response and ROS generation triggered by polymicrobial sepsis.
Materials and methods
Animals
A total of 40 male Wistar rats were used in the experiments. Each rat weighed 220–250 g, and all were obtained from Ataturk University's Experimental Animal Laboratory of Medicinal and Experimental Application and Research Center (ATADEM). Animal experiments and procedures were performed in accordance with national guidelines for the use and care of laboratory animals and were approved by Ataturk University's local animal care committee. The rats were housed in standard plastic cages on sawdust bedding in an air-conditioned room at 22 ± 1°C. Standard rat food and tap water were given ad libitum.
Chemicals
All the chemicals used in our laboratory experiments were purchased from Sigma Chemical Co. (Munich, Germany). Sildenafil (SLD) was obtained from Pfizer (Istanbul, Turkey).
Experimental design
The rats were separated into four groups, each composed of 10 individual rats: (i) 10 mg/kg SLD-treated CLP group; (ii) 20 mg/kg SLD-treated CLP group; (iii) CLP group; and (iv) sham-operated control group. The groups were housed in separate cages.
Sepsis model
A CLP polymicrobial sepsis model was applied to the rats, induced through caecal ligation and two-hole puncture. Anaesthesia was induced through the intraperitoneal administration of thiopental 25 mg/kg. The abdomen was shaved and the peritoneum was opened. Once the diaphragm exposed the abdominal organs, the caecum was isolated and ligated with a 3/0 silk ligature just distal to the ileocaecal valve. Two punctures were made with a 22-gauge needle through the caecum distal to the point of ligation, and the caecum was returned to the peritoneal cavity. The abdominal incision was then closed with a 4/0 sterile synthetic absorbable suture. The wound was bathed in 1% lidocaine solution to ensure analgesia. The sham-operated group received laparotomies, and the rats' caeca were manipulated but not ligated or perforated. All the animals were given 2 ml/100 g body weight of normal saline subcutaneously at the time of surgery and 6 h afterwards for fluid resuscitation. Immediately after the surgical procedure was completed, the rats in the sham-operated and the SLD-treated CLP groups received 10- or 20-mg/kg doses of SLD, which were administered with an oral gavage suspended in saline. There are many sildenafil doses for rats, varying from 0·4 mg/kg to 90 mg/kg, with different administration routes [28–33]. The reason we selected 10- and 20-mg/kg doses of oral sildenafil is that 10 mg/kg/day of sildenafil would result approximately in the same plasma concentration as 50 mg in humans [34]. These doses are very common for rats, and we first aimed to determine if it is protective in CLP-induced organ damage, as well as how the dose affects protection. Therefore, we used 10- and 20-mg/kg oral doses of sildenafil, as have previous authors [35–37]. An equal volume of saline was administered to the sham-operated control group and the CLP group. The rats were deprived of food postoperatively but had free access to water for the next 16 h, until they were killed.
The survival rate in CLP-induced sepsis models varies according to the size of the needle used [38]. Otero-Anton et al. reported that mortality after CLP in rats increased gradually with the size of the caecal puncture. They evaluated 0·5-cm blade incision; 13-gauge, 16-gauge and 18-gauge puncture; and four punctures with a 22-gauge needle. Mortality increased gradually with the puncture size, from 27% with a 22-gauge needle to 95% with the blade incision during a week of observation [38]. In addition, in our previous studies we observed mortality within 12–20 h after sepsis induction with a 12-gauge needle [39–42]. However, in studies performed with 21- and 22-gauge needles, mortality was not as common [38,43,44]. Therefore, we evaluated organ damage (lung and kidney) in this study after sepsis induction with a 22-gauge needle.
All four groups were killed 16 h postoperative with an overdose of a general anaesthetic (thiopental sodium, 50 mg/kg). The lungs and kidneys were removed quickly from all the rats and washed in ice-cold saline. Half the tissues were transferred to a biochemistry laboratory to be kept at −80°C for biochemical analyses, and the other half of the tissues were fixed in 10% formalin solution for histopathological analyses.
Biochemical investigation of lung and kidney tissues
After macroscopic analyses, activities of superoxide dismutase (SOD) and myeloperoxidase (MPO) and amounts of lipid peroxidase (LPO) and glutathione (GSH) enzymes in the rat lung and kidney tissues were determined. To prepare the tissue homogenates, the tissues were ground with liquid nitrogen in a mortar. The ground tissues (0·5 g each) were then treated with 4·5 ml of the appropriate buffer. The mixtures were homogenized on ice using an Ultra-Turrax Homogenizer for 15 min. The homogenates were filtered and centrifuged, using a refrigerated centrifuge at 4°C. These supernatants were then used to determine enzymatic activity. All assays were performed at room temperature in triplicate.
SOD activity
Measurements were made according to the method of Sun et al. [45]. SOD estimation was based on the generation of superoxide radicals produced by xanthine and xanthine oxidase, which react with nitroblue tetrazolium (NTB) to form formazan dye. SOD activity was then measured at 560 nm by the degree of inhibition of this reaction and was expressed as mmol/min/mg/tissue.
MPO activity
MPO activity was measured according to the modified method of Bradley et al. [46]. The homogenized samples were frozen and thawed three times and then centrifuged at 1500 g for 10 min at 4°C. MPO activity was determined by adding 100 µl of the supernatant to 1·9 ml of 10 mmol/l phosphate buffer (pH 6·0) and 1 ml of 1·5 mmol/l o-dianisidine hydrochloride containing 0·0005% (wt/vol) hydrogen peroxide. The changes in each sample's absorbance at 450 nm were recorded on a UV–vis spectrophotometer. MPO activity in all tissues was expressed as µmol/min/mg/tissue.
Determination of LPO
LPO in the tissues was determined by estimating the level of malondialdehyde (MDA) using the thiobarbituric acid test [47]. The rat tissues were excised promptly and rinsed with cold saline. To minimize the possibility of the interference of haemoglobin with the free radicals, any blood adhering to the mucosa was removed carefully. The tissues were weighed and homogenized in 10 ml of 100 g/l KCl. The homogenate (0·5 ml) was added to a solution containing 0·2 ml of 80 g/l sodium lauryl sulphate, 1·5 ml of 200 g/l acetic acid, 1·5 ml of 8 g/l 2-thiobarbiturate and 0·3 ml of distilled water. The mixture was incubated at 98°C for 1 h. After the mixture cooled, 5 ml of n-butanol : pyridine (15 : l) was added. The mixture was centrifuged for 30 min at 896 g. The supernatant was measured at 532 nm, and a standard curve was obtained using 1,1,3,3-tetramethoxypropane. The recovery was more than 90%. The results were expressed as nmol MDA g/tissue.
Total glutathione (GSH) determination
The amount of GSH in the tissues was measured according to the method of Sedlak and Lindsay [48]. The tissues were weighed and homogenized in 2 ml of 50 mm Tris–HCl buffer containing 20 mm erthylenediamine tetraacetic acid (EDTA) and 0·2 m sucrose, pH 7·5. The homogenate was precipitated immediately with 0·1 ml of 25% trichloroacetic acid, and the precipitate was removed after centrifugation at 987.84 g for 40 min at 4°C. The supernatant was used to determine GSH using 5,5′-dithiobis (2-nitrobenzoic acid). Absorbance was measured at 412 nm using a spectrophotometer. The results of GSH levels in the tissues were expressed as nmol mg/tissue.
Histological procedure
Light microscopy
Lung and kidney tissue samples were fixed in 10% buffered formalin for 48 h. After fixation, each lung tissue sample was processed routinely and embedded in paraffin. After embedding, 5-µm sections were taken from the tissue blocks and stained with haematoxylin and eosin (H&E), after which they were photographed for histopathological examination using a light microscope with a digital camera attachment.
Inflammation scoring for lungs and kidneys
Sections were obtained systematically and sampled randomly, and they were then scored depending on the degree of inflammation in the perivascular area as follows: 0: no cell; 1: a few cells; 2: many cells in the peripheral parts of the perivascular area; and 3: numerous cells in the perivascular area [49].
TNF-α cytokine measurement in serum
All the rats were killed 16 h later by an overdose of general anaesthetic (thiopental sodium, 50 mg/kg). Cardiac blood samples were collected immediately and transferred to the laboratory for the estimation of TNF-α levels in serum. Sera from the four rat groups were separated and stored at −80°C until thawing at the time of the assay. TNF-α was measured from one sample with highly sensitive enzyme-linked immunosorbent assay kits (Biosource International, Inc., Camarillo, CA, USA) specific for rat cytokines, according to the manufacturer's instructions. Cytokine assays for each animal and matched controls were run in the same lot.
Statistical analysis
A statistical analysis of oxidant and antioxidant enzymes was carried out using one-way analysis of variance (anova) followed by Duncan's multiple range test (DMRT) using spss software package version 12·0; results were considered significant at P < 0·05. Significance between histopathological scorings was determined with the χ2 test and Fisher's exact test.
Results
Biochemical results for oxidant and anti-oxidant levels of lung tissue in rats
SOD activity, GSH levels, lipid peroxidation levels and MPO enzymatic activity were evaluated in all lung tissues. The results, presented in Table 1, show that SOD activity and GSH levels for the CLP-induced sepsis group were lower than, and MPO and LPO levels were higher than, those of the sham-operated rat group (P < 0·05). Both doses of SLD had preventive effects on the alterations that occurred in the lung tissues after CLP operation. Both doses of SLD increased GSH levels significantly when compared to the CLP group, and the 20-mg/kg dose of SLD increased GSH levels significantly when compared to the sham-operated group. In addition, both doses of SLD were found to decrease the levels of MPO and LPO significantly when compared to the CLP group (P < 0·05). Furthermore, 20-mg/kg sildenafil treatment in the sham-operated rats improved the biochemical status of their lungs.
Table 1.
Effects of sildenafil (SLD) treatments on changes in activities of myeloperoxidase (MPO), superoxide dismutase (SOD) and with levels of lipid peroxidation (LPO) and total glutathione (GSH) in lung tissues of rats
| Treatments (lung tissue) | n | SOD activity (mmol/min/mg tissue) | MPO activity (µmol/min/mg tissue) | Amount of LPO (nmol/g tissue) | Amount of GSH (nmol/mg tissue) |
|---|---|---|---|---|---|
| CLP + SLD (10 mg/kg) | 10 | 124·6 ± 0·4b | 20·5 ± 0·3d | 15·0 ± 0·4c | 4·3 ± 0·1b |
| CLP + SLD (20 mg/kg) | 10 | 121·0 ± 0·6a | 14·2 ± 0·2b | 11·6 ± 0·3b | 5·6 ± 0·1c |
| Sham + SLD (20 mg/kg) | 10 | 137·0 ± 0·4d | 10·0 ± 0·2a | 10·0 ± 0·2a | 6·2 ± 0·1d |
| CLP | 10 | 129·0 ± 0·6c | 27·8 ± 0·1e | 18·4 ± 0·2d | 3·4 ± 0·1a |
| Sham | 10 | 144·7 ± 0·8e | 16·7 ± 0·2c | 15·0 ± 0·1c | 4·4 ± 0·2b |
Means in the same column by the same letter are not significantly different to the Duncan test (α = 0·05). Results are means ± standard error of three measurements. n, number of rats; CLP, caecal ligation and puncture.
Biochemical results for oxidant and anti-oxidant levels of kidney tissue in rats
To explore the effects of anti-oxidant defences on the sepsis process, the anti-oxidant levels (SOD and GSH) were evaluated in all kidney tissues. The levels of oxidant parameters, such as lipid peroxidation levels and MPO enzymatic activity, were also evaluated in all kidney tissues. The results, presented in Table 2, show that SOD activity decreased but the GSH levels increased in the CLP-induced sepsis group. The 10- and 20-mg/kg doses of SLD were found to have an increasing effect on SOD activity when the SLD-treated groups were compared to the CLP control group. Administration of SLD also increased the levels of GSH significantly when the SLD-treated groups were compared to both the sham-operated and the CLP groups (P < 0·05). In the kidney tissues of the CLP-induced septic rats, MPO activity decreased significantly compared to the sham group. Administration of SLD to the CLP-operated rats and the sham-operated rats decreased MPO activity significantly. The lowest MPO activity was found in the sham-operated rats that were treated with 20 mg/kg SLD. Conversely, the CLP operation increased the level of LPO in kidney tissue when compared to the sham operation. Furthermore, 20-mg/kg sildenafil treatment in the sham-operated rats improved the biochemical status of their kidneys.
Table 2.
Effects of sildenafil (SLD) treatments on changes in activities of myeloperoxidase (MPO) and superoxide dismutase (SOD) and with levels of lipid peroxidation (LPO) and total glutathion (GSH) in kidney tissues of rats
| Treatments (kidney tissue) | n | SOD activity (mmol/min/mg tissue) | MPO activity (µmol/min/mg tissue) | Amount of LPO (nmol/g tissue) | Amount of GSH (nmol/mg tissue) |
|---|---|---|---|---|---|
| CLP + SLD (10 mg/kg) | 10 | 126·6 ± 1·2a | 19·0 ± 0·1d | 17·3 ± 0·6c | 4·8 ± 0·1c |
| CLP + SLD (20 mg/kg) | 10 | 125·6 ± 0·3a | 15·0 ± 0·4c | 15·0 ± 0·1b | 6·4 ± 0·2e |
| Sham + SLD (20 mg/kg) | 10 | 139·5 ± 0·7c | 12·1 ± 0·2a | 13·1 ± 0·2a | 5·2 ± 0·2d |
| CLP | 10 | 132·0 ± 0·5b | 25·6 ± 0·3e | 19·0 ± 0·2d | 3·1 ± 0·1a |
| Sham | 10 | 143·0 ± 0·8d | 14·0 ± 0·2b | 14·5 ± 0·2b | 4·0 ± 0·1b |
Means in the same column by the same letter are not significantly different to the Duncan test (α = 0·05). Results are means ± standard error of three measurements. n, number of rats; CLP, caecal ligation and puncture.
Histopathological results for lung tissues
Inflammation scoring
Semiquantitative data analysis of the inflammation score and histopathological evaluation is summarized in Table 3. According to our analysis, significant differences were found in binary comparisons between the sepsis group and the other groups, with the exception of the CLP + sildenafil 10 mg group, in terms of inflammation scores. As seen in Table 3, the mean inflammation score in the CLP group was 2·3, in the CLP + sildenafil 20 mg group it was 1·3 and in the CLP + sildenafil 10 mg group it was 2·1.
Table 3.
Inflammation scores and evaluation of some histopathological changes for all groups' lungs
| Animal number | Mean inflammation score | Venular degeneration | Alveolar degeneration | |
|---|---|---|---|---|
| Sham group | 10 | 0 | – | – |
| CLP group | 10 | 2·3 | ++ | +++ |
| CLP + sildenafil 10 mg group | 10 | 2·1 | ++ | +++ |
| CLP + sildenafil 20 mg group | 10 | 1·7 | ++ | ++ |
CLP, caecal ligation and puncture.
Conventional light microscopic examination
In evaluating the lung tissues in the sham group, vascular structures, such as the pulmonary artery branch, arterioles, terminal bronchioles, interstitium and alveoli, all had a normal appearance (Fig. 1a–d). In addition, in Clara cells in the terminal bronchiole, type 1 and type 2 pneumocytes in the alveolus were observed to be normal in high-magnification H&E-stained sections (Fig. 1b,c).
Fig. 1.
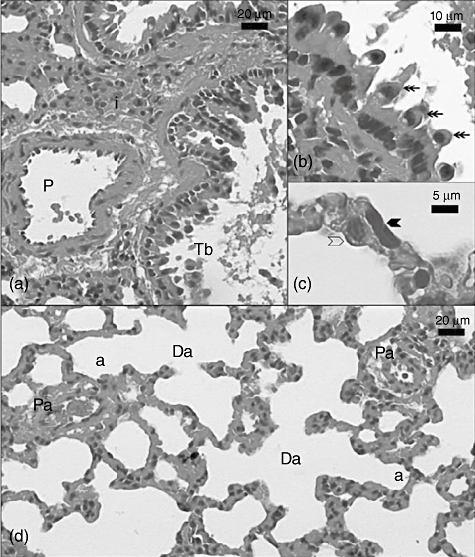
Light micrograph of the sham group lung section. P: pulmonary artery; Tb: terminal bronchiole; i: interstitial; double heated arrow: Clara cells in terminal bronchiol; black arrowhead: type 1 pneumocyte; transparent arrowhead: type 2 pneumocyte; Da: ductus alveolaris; a: alveoli; Pa: pulmonary arteriole. Sections were stained with haematoxylin and eosin.
In the CLP group, inflammation and haemorrhage in the interstitial area were conspicuous (Fig. 2a,d). The inflammation was composed of many lymphocytes and a few eosinophils (Fig. 2d). Inflammation was also seen in both the lamina propria of the terminal bronchioles and the wall of the pulmonary artery (Fig. 2a,c,d). The terminal bronchiole had erythrocytes and inflammatory cells in its lamina (Fig. 2a,c) and its epithelium was covered with hyaline material (Fig. 2d). Haemosiderin remnants were seen in the interalveolar septum and near the pulmonary artery (Fig. 2b and c). In addition, the alveoli had erythrocytes in their sacs and hyaline deposits on their walls (Fig. 2b,c).
Fig. 2.
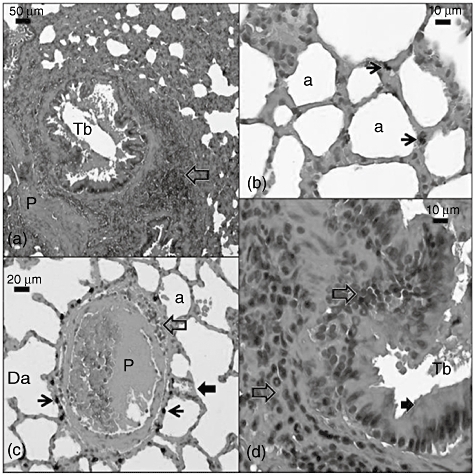
Light micrograph of the caecal ligation and puncture (CLP) group lung section. Tb: terminal bronchiole; transparent arrow: inflammation; P: pulmonary artery; Da: ductus alveolaris; a: alveoli; thin black arrow: haemosiderin remnants; thick black arrow: eosinophilic deposits on the terminal bronchiol epithelia and alveoli. Sections were stained with haematoxylin and eosin.
In the CLP + sildenafil 10 mg group, interstitial inflammation and haemorrhage did not differ from the CLP group (Fig. 3b,c). Our findings of the vascular and bronchial tree structures were also similar to the CLP group (Fig. 3a–e).
Fig. 3.
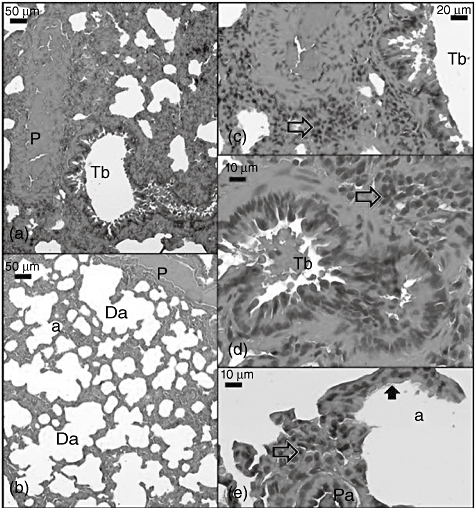
Light micrograph of the caecal ligation and puncture (CLP) + sildenafil 10 mg group lung section. Tb: terminal bronchiole; P: pulmonary artery; transparent arrow: inflammation; Da: ductus alveolaris; a: alveoli; thick black arrow: eosinophilic deposits on the alveolus wall; Pa: arteriole which has hyperchromatic endothelial cells. Sections were stained with haematoxylin and eosin.
When the CLP + sildenafil 20 mg group was evaluated for arteriolar and venular damage, arteriolar inflammation was very low, despite clear damage. The groups' vascular and interstitial pathological changes, such as interstitial haemorrhage, arteriolar obstruction and haemosiderin remnants, were similar, expect for inflammation in the CLP and CLP + sildenafil 10 mg groups (Fig. 4a–d). In addition, aneurism in the pulmonary artery wall was observed.
Fig. 4.
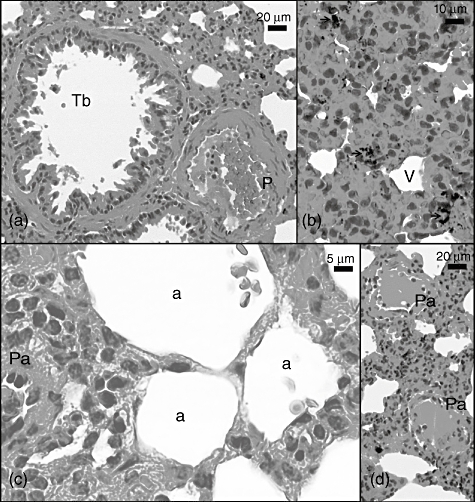
Light micrograph of the caecal ligation and puncture (CLP) + sildenafil 20 mg group lung section. Tb: terminal bronchiole; P: pulmonary artery; a: alveoli; Pa: pulmonary arteriole with degenerated endothelial cells; V: pulmonary vein. Sections were stained with haematoxylin and eosin.
Histopathological results for kidney tissues
Inflammation scoring
Data analysis of the inflammation score for kidneys is summarized in Table 4. Significant differences were found in binary comparisons between the sepsis group and the other groups, but not in the CLP + sildenafil 10 mg group. As seen in Table 4, the mean inflammation score in the CLP group was 2·1, in the CLP + sildenafil 20 mg group it was 1·8 and in the CLP + sildenafil 10 mg group it was 2.
Table 4.
Inflammation scores and evaluation of some histopathological changes for all groups' kidneys
| Animal number | Mean inflammation score | Venular degeneration | Tubular degeneration | |
|---|---|---|---|---|
| Sham group | 10 | 0 | – | – |
| CLP group | 10 | 2·1 | ++ | ++++ |
| CLP + sildenafil 10 mg group | 10 | 2 | ++ | +++ |
| CLP + sildenafil 20 mg group | 10 | 1·8 | ++ | ++ |
CLP, caecal ligation and puncture.
Conventional light microscopic examination
Glomeruli, tubules, interstitium and vascular structures were observed to be normal when kidney tissue sections were evaluated in the sham group (Fig. 5a–d).
Fig. 5.
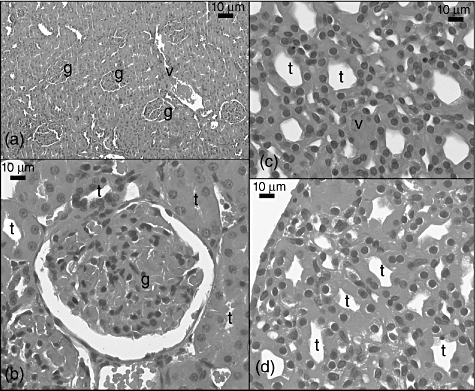
Light micrograph of the sham group kidney sections. g: Glomerulus; v: vessel; t: tubulus.
In the CLP group, the glomeruli showed different histopathological changes via hyperchromasia in intraglomerular mesangial cells (Fig. 6a) and a decrease of Bowman space (Fig. 2b). Tubules with hyperchromatic nuclei were observed (Fig. 6a), and some tubules were composed of only hyaline material (Fig. 6b). An increase of fibroblast, erythrocyte and inflammatory cells was conspicuous in the interstitial area (Fig. 6c,d), and vessel walls were damaged in many areas (Fig. 6a).
Fig. 6.
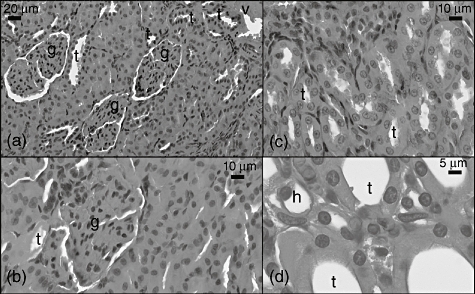
Light micrograph of the caecal ligation and puncture (CLP) group kidney sections. g: Glomerulus, v: vessel, t: tubulus.
In the CLP + sildenafil 10 mg group, glomerular capillary dilatation and segmental degeneration were observed (Fig. 7a). The lumens of the medullar tubules were obstructed, and their cells had more eosinophilic cytoplasm and hyperchromatic nuclei than those of the control group (Fig. 7c). The cytoplasm of these cells also showed vacuolization (Fig. 7d). In addition, some medullar tubules were composed of hyaline material (Fig. 7b), and there were many mesenchymal cells in the interstitial area (Fig. 7b,c).
Fig. 7.
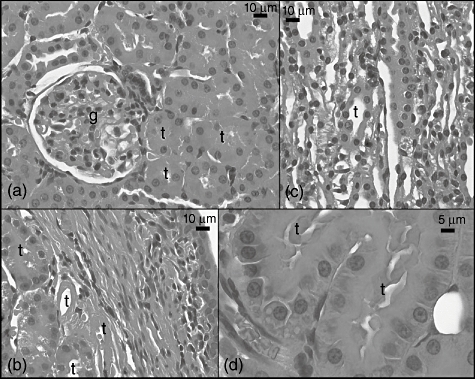
Light micrograph of the caecal ligation and puncture (CLP) 10 mg sildenafil-treated group group kidney sections. g: Glomerulus; v: vessel, t: tubulus.
In the CLP + sildenafil 20 mg group, an increase of extraglomerular mesangial cells and fibroblast that close to glomeruli (Fig. 8a) were seen. However, the glomerular structure was similar to that of the control group. The cortical tubule cells had both eosinophilic cytoplasm and hyperchromatic nuclei (Fig. 8a,b). Increases of fibroblast were conspicuous in the medullar area. There were many mesangial cells in the medulla, as in the CLP + sildenafil 10 mg group. In addition, extravasation of the red blood cells was seen in the medulla (Fig. 8c,d).
Fig. 8.
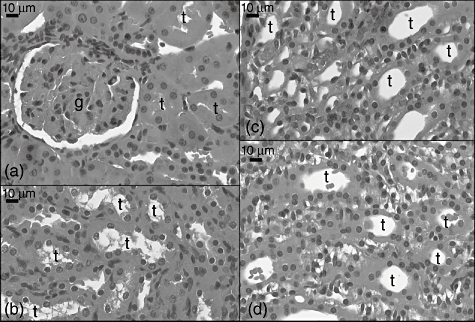
Light micrograph of the caecal ligation and puncture (CLP) 20 mg sildenafil-treated group group kidney sections. g: Glomerulus; v: vessel;, t: tubulus.
Effects of SLD on serum TNF-α levels in CLP-induced sepsis of rats
In the present study, the serum levels of TNF-α, which is an inflammatory cytokine, were studied in the CLP model in the sera of rats (Fig. 9). Levels of TNF-α were found to be increased in the CLP group when compared with the sham-operated animals, as seen in Fig. 9 (P < 0·01). In contrast to the CLP group, the serum levels of TNF-α were found to be decreased by the administration of SLD in septic rats (CLP + SLD groups) (P < 0·01). As shown in Fig. 9, administration of SLD alone in sham-operated rats did not affect the serum levels of TNF-α when compared with the non-treated sham group.
Fig. 9.
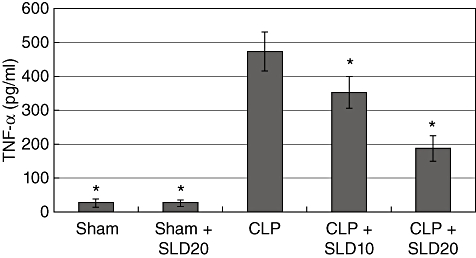
Effects of sildenafil (SLD) on caecal ligation and puncture (CLP)-induced sepsis. Serum tumour necrosis factor-α levels (pg/ml) were compared. Significant at P < 0·001 in Bonferroni test.
Discussion and conclusion
In this present study, we determined that sildenafil has markedly protective effects against CLP, attenuating kidney and lung tissue injury, especially in the vascular bed, and decreasing oxidative stress, as confirmed by biochemical assays and histopathological study. This protection is due primarily to the inhibition of oxidative stress, which is one of the important mechanisms of organ injury of polymicrobial sepsis, and inhibition of the degree of inflammation, as revealed clearly by our finding that pretreatment with sildenafil increased GSH and decreased the activation of MPO and LPO and levels of SOD. We observed a significant decrease in LPO and MPO and a decrease in SOD activity in the sildenafil-treated CLP rats compared with the vehicle-treated sham-operated rats, demonstrating the protective capacity of sildenafil in septic rats. Another result of our study is that sildenafil treatment improves inflammatory cells that accumulate in the lungs and result in lung injury in septic rats. According to our histopathological analysis, significant differences were found in terms of inflammation scores between the sepsis group and the other groups, except in the CLP + sildenafil 10 mg group. The CLP + sildenafil 20 mg/kg group had the lowest inflammation score in our study.
Koksal et al. [50] reported that in caecal ligation and puncture (CLP)-induced sepsis, increased oxidative stress in tissue in parallel with plasma are important mechanisms due to the output of free radicals [50]. Moreover, according to Sakaguchi et al. [51], endotoxin injection resulted in lipid peroxide formation and membrane damage in experimental animals, causing a decreased level of free radical scavengers or quenchers [51]. ROS have been assumed to play a role in the induction of many proinflammatory cytokines and mediators important in producing the acute inflammatory responses associated with sepsis [12]. In our previous studies we determined that kidney, heart, lung and liver tissue exhibited oxidative stress in septic rats [40–42]. The proinflammatory effects of ROS include endothelial damage, formation of chemotactic factors, neutrophil reinforcement, cytokine release and mitochondrial injury [14–16], which all contribute to free radical overload and to oxidant–anti-oxidant imbalance. The processes that are implicated in microvascular dysfunction are followed by organ dysfunction [17]. Dysfunction of very important tissues have been reported during septic shock, as well as ARDS, ALI and acute kidney injury (AKI), which are characterized by the accumulation of a large number of neutrophils in the lungs [52]. Yildirim et al. showed that sildenafil provided a significant decrease in tissue MDA levels in a sildenafil-treated lung fibrosis group, and they also found that endogenous anti-oxidant glutathione was restored in the sildenafil-treated group [24]; these data support our study. A possible explanation for this finding might be that glutathione was conserved due to a lower level of lipid oxidation. Thus, our results showing the inhibition of tissue lipid peroxidation along with the replenishment of GSH content by sildenafil imply that the compound is beneficial in maintaining oxidant–anti-oxidant balance.
In a clinical study, Starkopf et al. demonstrated an increase in lipid peroxidation levels and a decrease in serum anti-oxidant capacity induced by sepsis [53]. In septic shock, the levels and activities of SOD and GSH are due to the oppressive production of free radicals [54]. Therefore, taking these established results into account, we decided to offer insight into the possible mechanism that explains the role of oxidative stress in sepsis. The results are shown in our data, and they are in accordance with our hypothesis that sildenafil exerts ameliorating effects by decreasing LPO and MPO activities as markers of lipid peroxidation. Increased concentrations of LPO and MPO are found in rats with sepsis [55–57], and tissue MPO is a marker of lipid peroxidation levels that increase when septic shock is induced by CLP in rats [58]. GSH is an important constituent of intracellular protective mechanisms against oxidative stress [59]. Ortoloni et al. showed that plasma GSH was decreased in septic shock patients [60]. Another study showed that plasma GSH levels were decreased in children with sepsis [61]. Carbonell et al. showed that depletion of liver GSH potentiated the oxidative stress induced by endotoxins in rats, in which plasma lipid peroxide levels were raised [62]. Ritter et al. showed that MDA and plasma superoxide dismutase levels are markers of early mortality in septic rats [63]. Our study showed increased tissue LPO and MPO levels and decreased GSH and SOD after CLP, consistent with the literature [56]. Another important finding of the present study was that sildenafil attenuated the up-regulation of proinflammatory cytokine TNF-α. Increased serum early release of proinflammatory cytokines is important in the pathogenesis of septic shock [64]. Following tissue infiltration and activation by phagocytic cells, the release of a cascade of pathophysiologically uncontrolled proinflammatory mediators occurs, such as TNF-α[65], which can be responsible for the ongoing interactions of different cell types and can aggravate inflammation and cause multi-system organ failure in experimental models of sepsis and in clinical settings [66]. These data suggest that the ability of these septic rats to produce more inflammatory cytokines in response to CLP-induced sepsis may account in part for a significant increase in the survival of ‘septic-only’ rats. The mechanisms by which CLP exerts a stimulator effect on proinflammatory cytokine levels may involve the activation of this proinflammatory expression.
In conclusion, our results indicate that sildenafil is a highly protective agent in preventing lung and kidney damage caused by CLP-induced sepsis via maintenance of the oxidant–anti-oxidant status and decrease in the level of TNF-α.
Disclosure
None of the authors has a commercial interest, financial interest and/or other relationship with manufacturers of pharmaceuticals, laboratory supplies and/or medical devices or with commercial providers of medically related services.
References
- 1.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–7. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 2.Parrish WR, Gallowitsch-Puerta M, Czura CJ, Tracey KJ. Experimental therapeutic strategies for severe sepsis: mediators and mechanisms. Ann NY Acad Sci. 2008;1144:210–36. doi: 10.1196/annals.1418.011. [DOI] [PubMed] [Google Scholar]
- 3.Matot I, Sprung CL. Definition of sepsis. Intens Care Med. 2001;27(Suppl 1):S3–9. doi: 10.1007/pl00003795. [DOI] [PubMed] [Google Scholar]
- 4.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–44. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths B, Anderson ID. Sepsis, SIRS and MODS. Surgery. 2009;27:446–9. [Google Scholar]
- 6.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins: panacea for sepsis? Lancet Infect Dis. 2006;6:242–8. doi: 10.1016/S1473-3099(06)70439-X. [DOI] [PubMed] [Google Scholar]
- 7.Hietbrink F, Koenderman L, Rijkers G, Leenen L. Trauma: the role of the innate immune system. World J Emerg Surg. 2006;1:15. doi: 10.1186/1749-7922-1-15. doi: 10.1186/1749-7922-1-15. Available at: http://www.wjes.org/content/1/1/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nduka OO, Parrillo JE. The pathophysiology of septic shock. Crit Care Clin. 2009;25:677–702. doi: 10.1016/j.ccc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Jourd'heuil D, Morise Z, Conner EM, Grisham MB. Oxidants, transcription factors, and intestinal inflammation. J Clin Gastroenterol. 1997;25(Suppl 1):S61–72. doi: 10.1097/00004836-199700001-00011. [DOI] [PubMed] [Google Scholar]
- 10.van der Vliet A, Eiserich JP, Shigenaga MK, Cross CE. Reactive nitrogen species and tyrosine nitration in the respiratory tract: epiphenomena or a pathobiologic mechanism of disease? Am J Respir Crit Care Med. 1999;160:1–9. doi: 10.1164/ajrccm.160.1.9807044. [DOI] [PubMed] [Google Scholar]
- 11.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11:745–57. [PubMed] [Google Scholar]
- 12.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol. 1996;157:1630–7. [PubMed] [Google Scholar]
- 13.Spapen H, Zhang H, Demanet C, Vleminckx W, Vincent JL, Huyghens L. Does N-acetyl-L-cysteine influence cytokine response during early human septic shock? Chest. 1998;113:1616–24. doi: 10.1378/chest.113.6.1616. [DOI] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 15.Andrades ME, Ritter C, Dal-Pizzol F. The role of free radicals in sepsis development. Front Biosci (Elite Ed. 2009;1:277–87. doi: 10.2741/E27. [DOI] [PubMed] [Google Scholar]
- 16.Barichello T, Fortunato JJ, Vitali AM, et al. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med. 2006;34:886–9. doi: 10.1097/01.CCM.0000201880.50116.12. [DOI] [PubMed] [Google Scholar]
- 17.Peralta JG, Llesuy S, Evelson P, Carreras MC, Flecha BG, Poderoso JJ. Oxidative stress in skeletal muscle during sepsis in rats. Circ Shock. 1993;39:153–9. [PubMed] [Google Scholar]
- 18.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–69. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 19.Corbin JD, Francis SH, Webb DJ. Phosphodiesterase type 5 as a pharmacologic target in erectile dysfunction. Urology. 2002;60:4–11. doi: 10.1016/s0090-4295(02)01686-2. [DOI] [PubMed] [Google Scholar]
- 20.Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L24–33. doi: 10.1152/ajplung.00245.2007. [DOI] [PubMed] [Google Scholar]
- 21.Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thebaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med. 2005;172:750–6. doi: 10.1164/rccm.200503-510OC. [DOI] [PubMed] [Google Scholar]
- 22.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–36. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 23.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595–7. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 24.Yildirim A, Ersoy Y, Ercan F, et al. Phosphodiesterase-5 inhibition by sildenafil citrate in a rat model of bleomycin-induced lung fibrosis. Pulm Pharmacol Ther. 2010;23:215–21. doi: 10.1016/j.pupt.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Muzaffar S, Shukla N, Srivastava A, Angelini GD, Jeremy JY. Sildenafil citrate and sildenafil nitrate (NCX 911) are potent inhibitors of superoxide formation and gp91phox expression in porcine pulmonary artery endothelial cells. Br J Pharmacol. 2005;146:109–17. doi: 10.1038/sj.bjp.0706305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, et al. Early treatment with cGMP phosphodiesterase inhibitor ameliorates progression of renal damage. Kidney Int. 2005;68:2131–42. doi: 10.1111/j.1523-1755.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 27.Jeong KH, Lee TW, Ihm CG, Lee SH, Moon JY, Lim SJ. Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin-induced diabetic rats. Am J Nephrol. 2009;29:274–82. doi: 10.1159/000158635. [DOI] [PubMed] [Google Scholar]
- 28.Lin G, Huang YC, Wang G, Lue TF, Lin CS. Prominent expression of phosphodiesterase 5 in striated muscle of the rat urethra and levator ani. J Urol. 2010;184:769–74. doi: 10.1016/j.juro.2010.03.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasser JM, Baylis C. Effects of sildenafil on maternal hemodynamics and fetal growth in normal rat pregnancy. Am J Physiol Regul Integr Comp Physiol. 2010;298:R433–8. doi: 10.1152/ajpregu.00198.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali BH, Abdelrahman AM, Al-Salam S, et al. The effect of sildenafil on cisplatin nephrotoxicity in rats. Basic Clin Pharmacol Toxicol. 2011;109:300–8. doi: 10.1111/j.1742-7843.2011.00724.x. [DOI] [PubMed] [Google Scholar]
- 31.Medeiros PJ, Villarim Neto A, Lima FP, Azevedo IM, Leao LR, Medeiros AC. Effect of sildenafil in renal ischemia/reperfusion injury in rats. Acta Cir Bras. 2010;25:490–5. doi: 10.1590/s0102-86502010000600006. [DOI] [PubMed] [Google Scholar]
- 32.Brink CB, Clapton JD, Eagar BE, Harvey BH. Appearance of antidepressant-like effect by sildenafil in rats after central muscarinic receptor blockade: evidence from behavioural and neuro-receptor studies. J Neural Transm. 2008;115:117–25. doi: 10.1007/s00702-007-0806-5. [DOI] [PubMed] [Google Scholar]
- 33.Thorsen LB, Eskildsen-Helmond Y, Zibrandtsen H, Stasch JP, Simonsen U, Laursen BE. BAY 41-2272 inhibits the development of chronic hypoxic pulmonary hypertension in rats. Eur J Pharmacol. 2010;647:147–54. doi: 10.1016/j.ejphar.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Walker DK, Ackland MJ, James GC, et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29:297–310. doi: 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 35.Liebenberg N, Harvey BH, Brand L, Brink CB. Antidepressant-like properties of phosphodiesterase type 5 inhibitors and cholinergic dependency in a genetic rat model of depression. Behav Pharmacol. 2010;21:540–7. doi: 10.1097/FBP.0b013e32833befe5. [DOI] [PubMed] [Google Scholar]
- 36.Guzman DC, Olguin HJ, Brizuela NO, et al. Effect of prostaglandin E1 (PGE1) and sildenafil on serotonin metabolism and some oxidative damage markers in rat prostate gland and brain. Andrologia. 2011;43:266–72. doi: 10.1111/j.1439-0272.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 37.Bae SH, Bae SK, Lee MG. Effect of hepatic CYP inhibitors on the metabolism of sildenafil and formation of its metabolite, N-desmethylsildenafil, in rats in vitro and in vivo. J Pharm Pharmacol. 2009;61:1637–42. doi: 10.1211/jpp/61.12.0008. [DOI] [PubMed] [Google Scholar]
- 38.Otero-Anton E, Gonzalez-Quintela A, Lopez-Soto A, Lopez-Ben S, Llovo J, Perez LF. Cecal ligation and puncture as a model of sepsis in the rat: influence of the puncture size on mortality, bacteremia, endotoxemia and tumor necrosis factor alpha levels. Eur Surg Res. 2001;33:77–9. doi: 10.1159/000049698. [DOI] [PubMed] [Google Scholar]
- 39.Coskun AK, Yigiter M, Oral A, et al. The effects of montelukast on antioxidant enzymes and proinflammatory cytokines on the heart, liver, lungs, and kidneys in a rat model of cecal ligation and puncture-induced sepsis. ScientificWorldJournal. 2011;11:1341–56. doi: 10.1100/tsw.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uyanik A, Unal D, Uyanik MH, et al. The effects of polymicrobial sepsis with diabetes mellitus on kidney tissues in ovariectomized rats. Ren Fail. 2010;32:592–602. doi: 10.3109/08860221003759478. [DOI] [PubMed] [Google Scholar]
- 41.Cadirci E, Altunkaynak BZ, Halici Z, et al. Alpha-lipoic acid as a potential target for the treatment of lung injury caused by cecal ligation and puncture-induced sepsis model in rats. Shock. 2010;33:479–84. doi: 10.1097/SHK.0b013e3181c3cf0e. [DOI] [PubMed] [Google Scholar]
- 42.Albayrak A, Uyanik MH, Odabasoglu F, et al. The effects of diabetes and/or polymicrobial sepsis on the status of antioxidant enzymes and pro-inflammatory cytokines on heart, liver, and lung of ovariectomized rats. J Surg Res. 2011;169:67–75. doi: 10.1016/j.jss.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 43.Koga H, Hagiwara S, Inomata M, et al. The new vitamin E derivative, ETS-GS, protects against cecal ligation and puncture-induced systemic inflammation in rats. Inflammation. 2011 doi: 10.1007/s10753-011-9344-2. doi: 10.1007/s10753-011-9344-2. [DOI] [PubMed] [Google Scholar]
- 44.Gouma DJ, Coelho JC, Schlegel JF, Li YF, Moody FG. The effect of preoperative internal and external biliary drainage on mortality of jaundiced rats. Arch Surg. 1987;122:731–4. doi: 10.1001/archsurg.1987.01400180113022. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 46.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 47.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 48.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 49.Singh B, Shinagawa K, Taube C, Gelfand EW, Pabst R. Strain-specific differences in perivascular inflammation in lungs in two murine models of allergic airway inflammation. Clin Exp Immunol. 2005;141:223–9. doi: 10.1111/j.1365-2249.2005.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koksal GM, Sayilgan C, Aydin S, Oz H, Uzun H. Correlation of plasma and tissue oxidative stresses in intra-abdominal sepsis. J Surg Res. 2004;122:180–3. doi: 10.1016/j.jss.2004.07.246. [DOI] [PubMed] [Google Scholar]
- 51.Sakaguchi S, Kanda N, Hsu CC, Sakaguchi O. Lipid peroxide formation and membrane damage in endotoxin-poisoned mice. Microbiol Immunol. 1981;25:229–44. doi: 10.1111/j.1348-0421.1981.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 52.Dickson S. Sepsis and multiple organ failure anaesthesia. Intens Care Med. 2009;10:165–8. [Google Scholar]
- 53.Starkopf J, Zilmer K, Vihalemm T, Kullisaar T, Zilmer M, Samarutel J. Time course of oxidative stress during open-heart surgery. Scand J Thorac Cardiovasc Surg. 1995;29:181–6. doi: 10.3109/14017439509107227. [DOI] [PubMed] [Google Scholar]
- 54.Goode HF, Webster NR. Free radicals and antioxidants in sepsis. Crit Care Med. 1993;21:1770–6. doi: 10.1097/00003246-199311000-00029. [DOI] [PubMed] [Google Scholar]
- 55.Cuzzocrea S, Mazzon E, Dugo L, et al. Protective effects of n-acetylcysteine on lung injury and red blood cell modification induced by carrageenan in the rat. FASEB J. 2001;15:1187–200. doi: 10.1096/fj.00-0526hyp. [DOI] [PubMed] [Google Scholar]
- 56.Ogilvie AC, Groeneveld AB, Straub JP, Thijs LG. Plasma lipid peroxides and antioxidants in human septic shock. Intens Care Med. 1991;17:40–4. doi: 10.1007/BF01708408. [DOI] [PubMed] [Google Scholar]
- 57.Soriano FG, Liaudet L, Szabo E, et al. Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-deficient mice. Shock. 2002;17:286–92. doi: 10.1097/00024382-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Kharb S, Singh V, Ghalaut PS, Sharma A, Singh GP. Role of oxygen free radicals in shock. J Assoc Physicians India. 2000;48:956–7. [PubMed] [Google Scholar]
- 59.Kannan R, Kuhlenkamp JF, Jeandidier E, Trinh H, Ookhtens M, Kaplowitz N. Evidence for carrier-mediated transport of glutathione across the blood–brain barrier in the rat. J Clin Invest. 1990;85:2009–13. doi: 10.1172/JCI114666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ortolani O, Conti A, De Gaudio AR, Moraldi E, Cantini Q, Novelli G. The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am J Respir Crit Care Med. 2000;161:1907–11. doi: 10.1164/ajrccm.161.6.9903043. [DOI] [PubMed] [Google Scholar]
- 61.Lyons J, Rauh-Pfeiffer A, Ming-Yu Y, et al. Cysteine metabolism and whole blood glutathione synthesis in septic pediatric patients. Crit Care Med. 2001;29:870–7. doi: 10.1097/00003246-200104000-00036. [DOI] [PubMed] [Google Scholar]
- 62.Carbonell LF, Nadal JA, Llanos MC, Hernandez I, Nava E, Diaz J. Depletion of liver glutathione potentiates the oxidative stress and decreases nitric oxide synthesis in a rat endotoxin shock model. Crit Care Med. 2000;28:2002–6. doi: 10.1097/00003246-200006000-00054. [DOI] [PubMed] [Google Scholar]
- 63.Ritter C, Andrades M, Frota Junior ML, et al. Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intens Care Med. 2003;29:1782–9. doi: 10.1007/s00134-003-1789-9. [DOI] [PubMed] [Google Scholar]
- 64.Damas P, Ledoux D, Nys M, et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–62. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zanotti S, Kumar A. Cytokine modulation in sepsis and septic shock. Exp Opin Investig Drugs. 2002;11:1061–75. doi: 10.1517/13543784.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 66.Ayala A, Lomas JL, Grutkoski PS, Chung CS. Pathological aspects of apoptosis in severe sepsis and shock? Int J Biochem Cell Biol. 2003;35:7–15. doi: 10.1016/s1357-2725(02)00099-7. [DOI] [PubMed] [Google Scholar]


