Abstract
Dendritic cells (DCs) are professional antigen-presenting cells capable of initiating primary/adaptive immune responses and tolerance. DC functions are regulated by their state of maturation. However, the molecular pathways leading to DC development and maturation remain poorly understood. We attempted to determine whether inhibition of nuclear factor kappa B (NF-κB), which is one of the pivotal pathways underlying these processes, could induce immunophenotypic and functional changes in lipopolysaccharide-induced mature DCs derived from murine bone marrow. A comparative in vitro study of five clinically used drugs that are known to inhibit NF-κB demonstrated that azithromycin, a macrolide antibiotic, significantly inhibited expression of co-stimulatory molecules (CD40 and CD86) and major histocompatibility complex (MHC) class II by DCs. It also reduced Toll-like receptor 4 expression, interleukin-12 production and the allostimulatory capacity of DCs. These data suggest that azithromycin, as not only an NF-κB inhibitor but also an antibiotic, has potential as a novel drug for manipulation of allogeneic responses.
Keywords: allostimulatory capacity, co-stimulatory molecules, cytokines, dendritic cells, nuclear factor (NF)-κB
Introduction
Dendritic cells (DCs), which are specialized antigen-presenting cells (APCs) derived from CD34+ bone marrow (BM) stem cells, are uniquely well equipped to activate naive T lymphocytes and initiate primary immune responses [1]. DCs can also induce peripheral T cell tolerance under steady-state conditions [2]. This functional change is accompanied by a change in DC immunophenotype. Bacterial products, such as lipopolysaccharide (LPS), and inflammatory cytokines drive the maturation of DCs, which is characterized by up-regulation of major histocompatibility complex (MHC) class II and co-stimulatory molecules CD40, CD80 and CD86. This results in an increased capacity to stimulate T lymphocytes [1,3]. In response to ligation of CD40 by CD154 on antigen-specific T lymphocytes, DCs produce high levels of interleukin (IL)-12, a key cytokine in the development of interferon (IFN)-γ-producing T helper type 1 (Th1) cells [4,5].
Previously we reported that recombinant exoenzyme C3 from Clostridium botulinum specifically inhibits the function of DCs [6]. Despite the well-known important roles of DCs, little is known regarding the molecular mechanisms involved in DC differentiation and maturation. Various investigators demonstrated recently that several pathways, including nuclear factor kappa B (NF-κB), mitogen-activated protein kinase and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin are involved in the maturation and/or survival of DCs [7–11].
NF-κB regulates the transcription of many genes involved in immune responses, including cytokines and growth factors [12,13]. NF-κB is bound to inhibitory protein IκB as an inactive complex in the cytoplasm of many cells. Activation of NF-κB can be mediated by a variety of stimuli, including bacterial lipopolysaccharide (LPS) and tumour necrosis factor (TNF)-α. Several studies demonstrated that NF-κB is required for maturation of DCs [7,8]. However, clinically usable NF-κB inhibitors of DC maturation have not yet been found.
We selected five drugs that are used clinically to treat various diseases and are known to inhibit IκB degradation and hence NF-κB activation. They were 1, 25-dihydroxyvitamin D3 (Vit. D3) [14,15], an angiotensin-converting enzyme (ACE) inhibitor [16], a peroxisome proliferator-activated receptor-γ (PPAR-γ) activator [17,18] and two macrolide antibiotics, clarithromycin and azithromycin (AZM) [19–21]. Sugiyama et al. reported that AZM acts as anti-inflammatory agent by modulating the functions of murine BM-derived DCs in syngeneic immune systems [22]. However, this has not been shown in allogeneic immune systems. Here, in vitro, we analysed the effects of these five drugs on DC maturation and functions, including morphology, cytokine production, expressions of MHC class II, co-stimulatory molecules and Toll-like receptor (TLR)-4, and their allostimulatory capacity. We found that AZM significantly inhibited DC maturation and functions, including allogeneic responses. The present study suggests an attractive role for pharmacological therapy as a means of generating DCs with tolerogenic/regulatory properties. AZM may have potential as a new therapeutic drug for controlling allograft immunity, such as acute graft-versus-host disease and graft rejection in organ transplantation.
Materials and methods
Mice
Female C57BL/6 (H-2 Kb) mice and BALB/c (H-2 Kd) mice aged 6–12 weeks were purchased from Japan SLC, Inc. (Shizuoka, Japan). Institutional approval was obtained for all animal experimentation.
Antibodies and media
Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) used to detect cell surface expression of CD3, CD4, CD11c, CD40, CD80, CD86, MHC class II, and TLR-4-message digest 2 (MD2) by flow cytometry, as well as isotype-matched control mAbs, were purchased from BD Pharmingen and eBioscience (San Diego, CA, USA). RPMI-1640 supplemented with 10% fetal calf serum (FCS), 5 × 10−5m 2-mercaptoethanol (ME) and 10 mm HEPES was used as the culture medium.
Preparation of BM-derived DCs
Bone marrow (BM)-derived DCs were generated as described elsewhere [23,24], with minor modifications. Briefly, BM cells flushed from tibias and femurs of BALB/c mice were seeded at 2 × 106 cells onto a six-well culture plate in culture medium supplemented with 20 ng/ml recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF) (Kirin Brewery Co., Gunma, Japan). The culture medium was changed every 2 days. Loosely adherent clustered cells were used on day 6 as immature DCs (im-DCs). The purity of im-DCs was routinely > 85%, as confirmed by dual positivity for MHC class II and CD11c. Vit. D3 (Sigma, St Louis, MO, USA), ACE inhibitor delapril (Takeda Co. Ltd, Osaka, Japan), PPAR-γ activator troglitazone (Sankyo Co., Tokyo, Japan), clarithromycin (CAM) (Taisho Pharmaceutical Co., Tokyo, Japan) or AZM (Pfizer Inc., Groton, CT, USA) as an NF-κB inhibitor was added to culture wells to the indicated final concentrations at various times. We tested these NF-κB inhibitors at several concentrations to generate BM-derived DCs. The final concentrations of NF-κB inhibitors, except Vit. D3, chosen for the study were 10 times their physiological concentrations shown to have therapeutic effects on several human diseases [25–28]. Vit. D3 (10 nm) [14] was added to culture wells on days 0, 2, 4 and 6. Troglitazone (10 µm), delapril (40 µg/ml) and CAM (20 µg/ml) were added every day (days 0–6). AZM was added according to several schedules: 50 µg/ml on day 6 or on days 0, 3 and 6 and 75 µg/ml on day 6. We also added to culture wells equal amounts of only the respective solvents that were used to dissolve these agents. Im-DCs treated with and without these agents were stimulated with 1 µg/ml LPS from Escherichia coli (serotype 055:B5) (Sigma) or 20 ng/ml TNF-α (BD Pharmingen) for 24 h to develop mature DCs (m-DCs).
Mixed leucocyte reaction (MLR)
The allogeneic MLR assay was performed as described elsewhere [6], with minor modifications. C57BL/6 splenic CD4+ T lymphocytes were enriched by using a SpinSepTM-Murine CD4+ T cell kit (Stem Cell Technologies Inc., Vancouver, Canada) and used as responders. BALB/c BM-derived im-DCs, m-DCs or AZM 50 (days 0, 3, 6)-treated m-DCs as stimulator cells were irradiated with 30 Gy, added in graded doses (from 3 × 102 to 1 × 103) to 1 × 105 responders in 96-well round-bottomed plates (Falcon, Tokyo, Japan) and then incubated for 5 days. [3H]-Thymidine (Amersham, Uppsala, Sweden) incorporation was measured after 12-h pulsed labelling with 1 µCi/well. Results are shown as the mean counts per minute (cpm) of triplicates.
Quantification of cytokines by enzyme-linked immunosorbent assay (ELISA)
Cytokine production was measured in the MLR supernatant using Quantikines M ELISA kits specific for murine IL-12p70, IL-10 and IFN-γ (R&D Systems, Minneapolis, MN, USA). Samples and standards were run in triplicate.
Flow cytometric analysis
DCs, spleen cells and BM cells suspended in phosphate-buffered saline (PBS) were preincubated with FcγR blocking antibody (anti-mouse CD16/CD32; BD Pharmingen) and then incubated with FITC- or PE-labelled mAbs at 4°C for 20 min. After staining, the cells were washed twice with PBS incubated with propidium iodide at room temperature for 5 min and then subjected to fluorescence activated cell sorter (FACS) analysis. Flow cytometry was performed on a FACScan with CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA).
Preparation of nuclear protein fractions and electrophoretic mobility shift assay (EMSA)
Wild-type oligo probe for NF-κB p65 EMSA was end-labelled with γ[-32P] adenosine triphosphate (ATP) using T4 polynucleotide kinase (New England Biolabs, Inc., Beverly, MA, USA). We used the following unlabelled wild-type and mutant competitor double-stranded oligonucleotides (Geneka Biotechnology, Inc., Carlsbad, CA, USA): 5′-AGCTTGGGGTATTTCCAGCCG-3′ (wild-type) and 5′-AGCTTGGCATAGGTCCAGCCG-3′ (mutant) [29]. Although these oligonucleotides had basically been set for human NF-κB p65, they could also be applied to mice because 93% homology with murine NF-κB p65 protein was observed (Geneka Biotechnology). Eleven micrograms of nuclear extract from control im-DCs or AZM-treated or untreated im-DCs stimulated for 2 h with LPS (100 ng/ml) were incubated for 20 min with labelled NF-κB probes at 4°C. DNA–protein complexes were separated on 5% polyacrylamide gels.
Statistical analysis
Analysis of variance (anova) and unpaired two-tailed t-tests were used to determine statistical significance of in vitro data. P < 0·05 was considered statistically significant.
Results
AZM inhibits maturation of DCs
We examined the effects of five NF-κB inhibitors on DC maturation, phenotypically and morphologically. BM-derived immature DCs (im-DCs) were incubated in the presence and absence of each NF-κB inhibitor at the indicated concentrations and times. On day 6, the NF-κB inhibitor-treated and -untreated im-DCs were incubated with LPS or TNF-α to see if they could be induced to mature. Comparative study of the expression of surface molecules on LPS-induced mature DCs (m-DCs) that might be related to allostimulation found that AZM, added at 50 µg/ml on days 0, 3 and 6, inhibited the expression of MHC class II and co-stimulatory molecules (CD40, CD80 and CD86) when Vit. D3 was used as a positive control [30] (Fig. 1a). Conversely, the PPAR-γ activator, ACE inhibitor and clarithromycin did not suppress the expression of MHC class II or co-stimulatory molecules (Fig. 1a). When the expression levels were compared on the basis of the mean fluorescence intensity (MFI), the expression of MHC class II and co-stimulatory molecules but not CD80 were decreased significantly in a dose- and time-dependent manner (Table 1). TLR-4 expression was also decreased in AZM-treated im-DCs stimulated with TNF-α (Fig. 1b). The MFIs of TLR-4 of control m-DCs and AZM-treated m-DCs were significantly different (13·39 ± 1·07 versus 8·56 ± 0·47; P < 0·01, n = 3) (Fig. 1b). Similar to the results for expression of MHC class II and co-stimulatory molecules, the PPAR-γ activator, ACE inhibitor and clarithromycin did not affect expression of TLR-4 (Fig. 1c). We also confirmed that the vehicles used to dissolve the NF-κB inhibitors did not affect the expression of these antigens and showed no toxicity when we added equal amounts of them to culture wells as controls (data not shown). Morphologically, AZM-treated im-DCs (Fig. 1d) were similar to control im-DCs (Fig. 1e). However, in the case of LPS-induced m-DCs, AZM treatment resulted in less prominent dendrite formation, with a round nucleus (Fig. 1f), compared with the control cells (Fig. 1g).
Fig. 1.
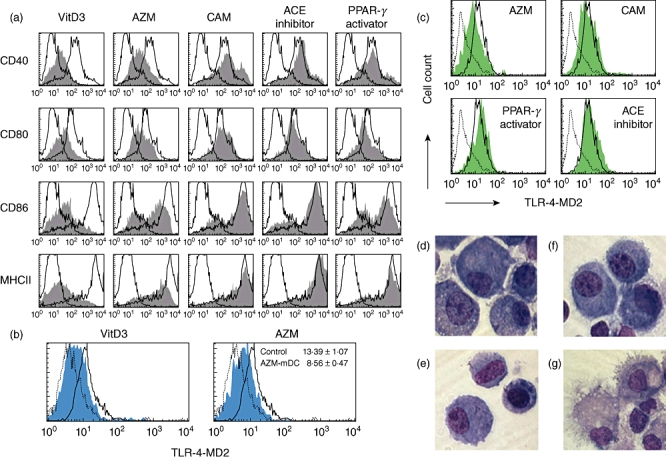
Azithromycin inhibits dendritic cell (DC) maturation. Bone marrow (BM)-derived DCs were generated as described in Materials and methods [using a nuclear factor kappa B (NF-κB) inhibitor at the indicated concentrations and times]. On day 6, maturation was induced by incubation of immature DCs (im-DCs) with lipopolysaccharide (LPS) (a,d–g) or tumour necrosis factor (TNF)-α (b and c). (a–c) Flow cytometric analysis of the expression of the indicated surface molecules on mature DCs (m-DCs). Propidium iodide-negative cells were gated on CD11c+. The staining profiles of control m-DCs (thin line) and NF-κB inhibitor-treated m-DCs (grey) for the indicated surface molecules are shown. Dotted lines indicate isotype controls. We used five kinds of NF-κB inhibitors: 1, 25-dihydroxyvitamin D3 (Vit. D3), used as a positive control in suppression of DC maturation; an angiotensin-converting enzyme (ACE) inhibitor; a peroxisome proliferator-activated receptor-γ (PPAR-γ) activator; clarithromycin (CAM); and azithromycin (AZM). (a) Comparative study of the expression of major histocompatibility complex (MHC) class II CD40, CD80 and CD86 on m-DCs exposed to NF-κB inhibitors. AZM was added to culture wells at 50 µg/ml on days 0, 3 and 6. (b) The expression of Toll-like receptor-4 (TLR-4)-message digest 2 (MD2) on TNF-α-induced m-DCs treated with AZM 50 (days 0, 3, 6) was detected by flow cytometry in comparison with control m-DCs. The mean fluorescence intensity is shown in the upper right corner of the plot. (c) Expression of TLR-4-MD2 on TNF-α-induced m-DCs treated with ACE inhibitor, PPAR-γ activator or CAM was analysed by flow cytometry in comparison with control m-DCs. (d–g) AZM 50-treated (days 0, 3, 6) or untreated DCs were analysed morphologically in cytospin preparations after May–Giemsa staining (original magnification: × 1000). AZM-treated im-DCs (d), control im-DCs (e), AZM-treated m-DCs (f) and control m-DCs (g) are shown. All results are representative of three independent experiments.
Table 1.
Comparison of phenotypes of mature dendritic cells (DCs) cultured with azithromycin (AZM)
| Surface antigen | Control | AZM75 (d6) (P-value)* | AZM50 (d0,3,6) (P-value)* |
|---|---|---|---|
| CD 40 | 296 ± 56 | 141 ± 84 (0·01) | 143 ± 36 (0·001) |
| CD 80 | 211 ± 69 | 175 ± 93 (0·50) | 157 ± 31 (0·16) |
| CD 86 | 1846 ± 634 | 1682 ± 677 (0·70) | 830 ± 277 (0·02) |
| MHC II | 3320 ± 792 | 1781 ± 598 (0·01) | 1654 ± 572 (0·007) |
Data are presented as mean fluorescence intensity plus or minus standard deviation values of five independent experiments. AZM was added at graded concentration and timing; 75 µg/ml on day 6 and 50 µg/ml on days 0, 3 and 6.
P-value relative to results obtained with control. MHC: major histocompatibility complex.
AZM decreases IL-12 production by DCs and inhibits the T lymphocyte stimulatory capacity of DCs
To determine whether AZM might affect the functions of DCs, we first compared IL-12p70 production by AZM-treated and -untreated im-DCs stimulated with LPS. As shown in Fig. 2a, the IL-12p70 concentration was significantly lower in the supernatant of AZM-treated im-DCs (P < 0·001).
Fig. 2.
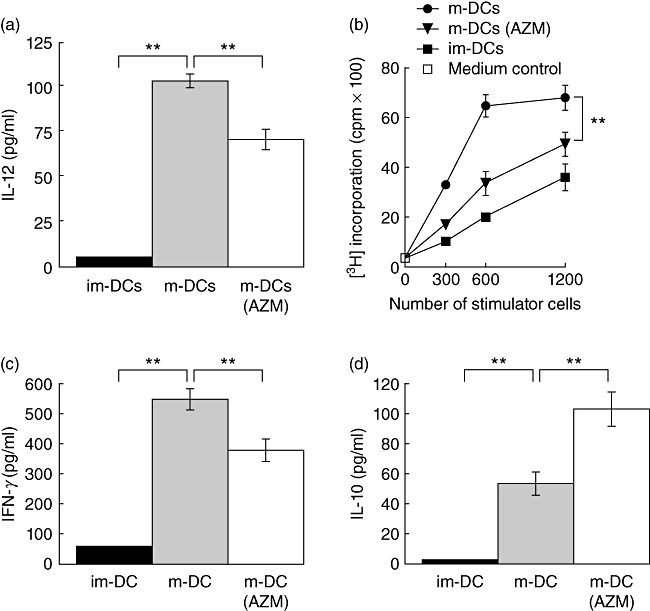
Azithromycin (AZM) inhibits dendritic cell (DC) functions. (a) AZM suppresses interleukin (IL)-12p70 production by DCs. AZM 50-treated (days 0, 3, 6) or untreated immature DCs (im-DCs) were stimulated with lipopolysaccharide (LPS) for 24 h. IL-12p70 concentration in the supernatants was determined by enzyme-linked immunosorbent assay (ELISA). (b) AZM-treated mature DCs (m-DCs) inhibit their allogeneic CD4+ T lymphocyte stimulatory capacity in mixed leucocyte reaction (MLR). Irradiated bone marrow (BM)-derived control im-DCs, m-DCs or AZM 50-treated (days 0, 3, 6) m-DCs from BALB/c mice were used to stimulate 1 × 105 allogeneic splenic CD4+ T lymphocytes from C57BL/6 mice. The ability of DCs to stimulate allogeneic T lymphocytes was assessed by uptake of [3H]-thymidine. Interferon (IFN)-γ (c) or IL-10 (d) production measured by ELISA was decreased in the MLR supernatant. All results are expressed as the mean ± standard deviation of triplicate cultures. Data are representative of three independent experiments. **P < 0·001.
We next asked whether AZM might affect the allogeneic T lymphocyte stimulatory capacity of DCs. To address this question, we performed MLR experiments. [3H]-Thymidine incorporation was suppressed significantly when allogeneic T lymphocytes were stimulated with m-DCs treated with 50 µg/ml of AZM, causing up to 27% reduction of the allostimulatory capacity (Fig. 2b). We also investigated the secretion levels of IFN-γ and IL-10 in the MLR supernatant by enzyme-linked immunosorbent assay. IFN-γ was reduced by 31% when allogeneic T lymphocytes were stimulated with AZM-treated m-DCs compared to untreated m-DCs, indicating that AZM-treated m-DCs decreased Th1 polarization (Fig. 2c). In contrast, secretion of IL-10, which is a key regulator of immune and inflammatory responses, in the MLR setting of allogeneic T lymphocytes stimulated with AZM-treated m-DCs was, on average, two times higher than in the case of stimulation with untreated m-DCs (Fig. 2d). However, the number of T lymphocytes was not significantly different in these wells (data not shown).
AZM decreases NF-κB activation in im-DCs stimulated with LPS
The above results indicate that AZM inhibits not only the maturation but also the functions of DCs. NF-κB was reported to be required for the maturation of DCs [7,8]. We therefore examined the effects of AZM on NF-κB p65 activation in DCs. EMSA was performed on nuclear extracts prepared from im-DCs pretreated with 50 or 75 µg/ml of AZM for varying periods of time and then incubated further with and without LPS for 2 h. In this DNA binding reaction, unlabelled wild-type and mutant competitor oligonucleotides were used in a 100-fold molar excess over labelled NF-κB probe. AZM decreased nuclear NF-κB DNA-binding activity significantly in im-DCs stimulated with LPS in a dose- and time-dependent manner (Fig. 3a,b).
Fig. 3.
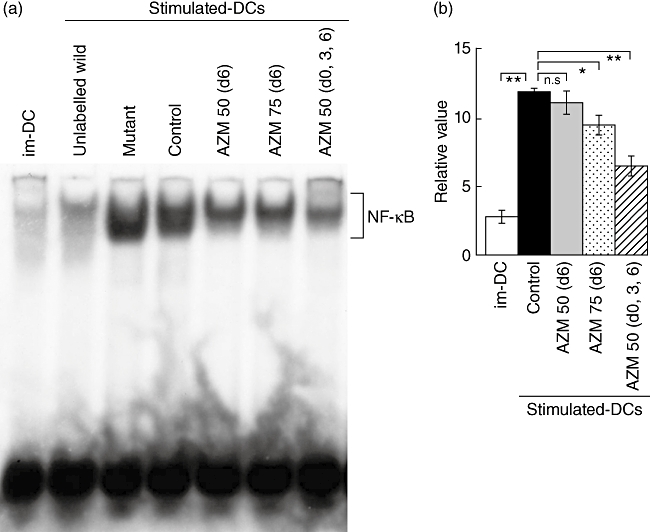
Azithromycin (AZM) inhibits nuclear translocation of nuclear factor kappa B (NF-κB). (a) Electrophoretic mobility shift assay (EMSA) showing the binding of nuclear factors to the NF-κB p65 wild-type oligo probe. The labelled NF-κB probe was incubated with nuclear extract from immature DCs (im-DCs) stimulated with or without lipopolysaccharide (LPS). In lane 1, a labelled NF-κB probe was added to control im-DCs. In lanes 2 or 3, a labelled NF-κB probe with a 100-fold molar excess of unlabelled NF-κB wild-type or mutant competitor oligos was added to im-DCs stimulated with LPS, respectively. In lane 4, labelled NF-κB probe was added to control im-DCs stimulated with LPS. In lanes 5–7, a labelled NF-κB probe was added to AZM-treated im-DCs stimulated with LPS. In lane 5, 50 µg/ml AZM was added on day 6. In lane 6, 75 µg/ml AZM was added on day 6. In lane 7, 50 µg/ml AZM was added on days 0, 3 and 6. (b) The NF-κB DNA–protein complexes were analysed on a Fuji BASS 1000 imaging analyser. The results are representative of three independent experiments. n.s.: not significant' *P < 0·01; **P < 0·001.
Discussion
We found that AZM, a macrolide antibiotic and NF-κB inhibitor, suppresses maturation and allogeneic responses of murine BM-derived DCs in vitro.
AZM is a 15-membered ring macrolide that is used widely for treatment of bacterial infections caused by both Gram-positive and Gram-negative bacteria. AZM is concentrated in lysosomes to an unusual degree because of its dibasic characteristics [31]. Lysosomes in DCs play an important role in antigen presentation: DEC-205, the DC receptor for endocytosis, can recycle and enhance antigen presentation via MHC class II-positive lysosomal compartments [32]. AZM is concentrated inside cells at ratios exceeding 200 : 1. It is highly concentrated in a number of cell types, including polymorphonuclear neutrophils, monocytes and macrophages, which can retain, deliver and, potentially, release AZM at sites of infection [31]. Moreover, Khan et al. reported that AZM inhibited production of IL-1α and TNF-α by LPS-stimulated human monocytes [33]. These functional activities may be important, as in the infected host excessive or unrestricted overproduction of proinflammatory cytokines can be detrimental, as in septic shock [33].
However, little is known with regard to DCs. Recently, Sugiyama et al. reported that macrolide antibiotics, including AZM, act as anti-inflammatory agents by modulating the functions of murine BM-derived DCs [22]. However, in surface marker analysis by flow cytometry, they found that AZM did not inhibit maturation of murine BM-derived immature DCs after LPS stimulation, which contradicts our results (Fig. 1). We think that this discrepancy may be due to a difference in the method of DC pretreatment with AZM, including the higher concentration (10 µg/ml versus 50 or 75 µg/ml) and/or longer incubation time (days 8 and 10 in 11-day culture versus days 0, 3 and 6 or day 6 in 7-day culture) in our study.
IL-10 is well known as a key regulator of anti-inflammatory responses. It can be produced by various cells, including monocytes, subsets of DCs and CD4+ CD25+ regulatory T cells (Treg). Sugiyama et al. also showed that pretreatment with AZM augmented the production of IL-10 by DCs co-cultured with syngeneic T lymphocytes in a murine model [22]. Additionally, some investigators have studied allogeneic immune responses initiated by DCs in the various clinical settings. For example, recent murine studies have shown that interactions between donor T lymphocytes and host DCs are essential for triggering induction of acute graft-versus-host disease (GVHD) following allogeneic bone marrow transplantation (BMT) [34–37].
We examined IL-10 secretion in the MLR supernatants of allogeneic T lymphocytes stimulated with AZM-treated m-DCs (Fig. 2). We detected elevated IL-10 levels in co-cultures of allogeneic T lymphocytes and AZM-treated m-DCs (Fig. 2d). However, we have not confirmed which of those cells, i.e. the allogeneic T lymphocytes stimulated with AZM-treated m-DCs or the AZM-treated m-DCs themselves, secreted the IL-10. Sato et al. generated regulatory DCs, as a subset of potent tolerogenic DCs, by culturing murine BM cells with murine GM-CSF, murine IL-10 and human transforming growth factor (TGF)-β1 for 6 days, followed by LPS stimulation [38]. Those regulatory DCs were characterized by low expression levels of co-stimulatory molecules, moderate levels of MHC molecules, low production of IL-12, high production of IL-10 and suppression of NF-κB activity even after stimulation with LPS [38,39]. The therapeutic effects of regulatory DCs on acute GVHD, organ allograft rejection, allergic airway inflammation, experimental endotoxaemia and bacterial peritonitis have been demonstrated [38–42]. It is tempting to speculate that AZM-treated m-DCs may be functionally related to regulatory DCs, although the method of in vitro induction of DCs is quite different.
In addition to the immunoregulatory effects of AZM, its antibacterial effects may also be important, as bacteria and bacterial products, especially LPS, are associated with inflammatory responses. LPS signalling is mediated by TLR-4 [43]. An et al. reported that TLR-4 mRNA was up-regulated following LPS stimulation of murine im-DCs, which was inhibited by pyrrolidinecarbodithoic acid, an inhibitor of NF-κB [44]. Furthermore, Park et al. showed that a macrolide antibiotic, clarithromycin, induced down-regulation of TLR-4 mRNA in human peripheral blood mononuclear cells stimulated with LPS [45]. Although Park et al. did not show TLR-4 expression on the surface of DCs, our data (Fig. 1b) may be compatible with their findings. Because Sato et al. showed that TLR-4 was internalized from the surface of murine macrophages when they were stimulated with LPS [46], we used TNF-α instead of LPS as a maturation stimulator for im-DCs. We found that AZM inhibited TLR-4 expression significantly (Fig. 1b), and that inhibition may be associated with reduced responses to LPS in vitro.
Taken together, our results suggest that AZM, by inhibiting the NF-κB pathway, blocks DC–T lymphocyte interaction by inactivating DCs with tolerogenic/regulatory properties. Vit. D3 has also been known as inhibitor of differentiation and maturation of DCs in vitro[14,15]. Indeed, Vit. D3 inhibited the expression of MHC class II and co-stimulatory molecules on immature DCs stimulated with LPS more powerful than AZM in the present report. This might be related to the constitutive expression of Vit. D3 receptors on DCs. Therefore, it may be preferable to use Vit. D3 rather than AZM. However, Vit. D3 is difficult to use in the clinical setting because of adverse effects, including hypercalcaemia and renal insufficiency in some patients. Conversely, AZM already has a history of use in the treatment of bacterial infections, so its administration should also reduce the numbers of bacteria, the amount of LPS, and therefore overproduction of proinflammatory cytokines in infected hosts. Some investigators also recently verified that the molecular signalling pathways of DC–T lymphocyte interaction might be novel targets for induction of transplant tolerance or handling of allograft immunity. Further studies of the in vivo effects of AZM in organ transplantation, such as haematopoietic stem cell transplantation, are clearly warranted.
Acknowledgments
We thank Dr Takashi Iwamoto of Chubu College of Life and Health Sciences for technical advice, Dr Koya Shiba of Jikei University School of Medicine for drug information and Miyuki Namikata and Takahiro Ohyachi for technical assistance.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 3.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–7. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 4.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 5.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi M, Azuma E, Ido M, et al. A pivotal role of Rho GTPase in the regulation of morphology and function of dendritic cells. J Immunol. 2001;167:3585–91. doi: 10.4049/jimmunol.167.7.3585. [DOI] [PubMed] [Google Scholar]
- 7.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–80. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimura S, Bondeson J, Foxwell BM, Brennan FM, Feldmann M. Effective antigen presentation by dendritic cells is NF-kappaB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int Immunol. 2001;13:675–83. doi: 10.1093/intimm/13.5.675. [DOI] [PubMed] [Google Scholar]
- 9.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–46. [PubMed] [Google Scholar]
- 10.Haidinger M, Poglitsch M, Geyeregger R, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–31. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 11.van de Laar L, Buitenhuis M, Wensveen FM, Janssen HL, Coffer PJ, Woltman AM. Human CD34-derived myeloid dendritic cell development requires intact phosphatidylinositol 3-kinase-protein kinase B-mammalian target of rapamycin signaling. J Immunol. 2010;184:6600–11. doi: 10.4049/jimmunol.0903089. [DOI] [PubMed] [Google Scholar]
- 12.Falvo JV, Thanos D, Maniatis T. NF-kappa B: a lesson in family values. Cell. 1995;80:529–32. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 13.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–35. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 14.D'Ambrosio D, Cippitelli M, Cocciolo MG, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 16.Hernández-Presa MA, Bustos C, Ortego M, Tuñón J, Ortega L, Egido J. ACE inhibitor quinapril reduces the arterial expression of NF-kappaB-dependent proinflammatory factors but not of collagen I in a rabbit model of atherosclerosis. Am J Pathol. 1998;153:1825–37. doi: 10.1016/s0002-9440(10)65697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aljada A, Garg R, Ghanim H, et al. Nuclear factor-kappaB suppressive and inhibitor-kappaB stimulatory effects of troglitazone in obese patients with type 2 diabetes: evidence of an antiinflammatory action? J Clin Endocrinol Metab. 2001;86:3250–6. doi: 10.1210/jcem.86.7.7564. [DOI] [PubMed] [Google Scholar]
- 18.Appel S, Mirakaj V, Bringmann A, Weck MM, Grünebach F, Brossart P. PPAR-gamma agonists inhibit Toll-like receptor-mediated activation of dendritic cells via the MAP kinase and NF-kappaB pathways. Blood. 2005;106:3888–94. doi: 10.1182/blood-2004-12-4709. [DOI] [PubMed] [Google Scholar]
- 19.Miller SA, Selzman CH, Shames BD, Barton HA, Johnson SM, Harken AH. Chlamydia pneumoniae activates nuclear factor kappaB and activator protein 1 in human vascular smooth muscle and induces cellular proliferation. J Surg Res. 2000;90:76–81. doi: 10.1006/jsre.2000.5847. [DOI] [PubMed] [Google Scholar]
- 20.Ichiyama T, Nishikawa M, Yoshitomi T, Hasegawa S, Matsubara T. Clarithromycin HTFS inhibits NF-kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells. Antimicrob Agents Chemother. 2001;45:44–7. doi: 10.1128/AAC.45.1.44-47.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aghai ZH, Kode A, Saslow JG, et al. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res. 2007;62:483–8. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama K, Shirai R, Mukae H, et al. Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells. Clin Exp Immunol. 2007;147:540–6. doi: 10.1111/j.1365-2249.2007.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 24.Fukao T, Tanabe M, Terauchi Y, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–81. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 25.Shionoiri H, Yasuda G, Abe Y, Yoshimura H, Kaneko Y, Shindo Y. Pharmacokinetics and acute effect on the rennin–angiotensin system of delapril in patients with chronic renal failure. Clin Nephrol. 1987;27:65–70. [PubMed] [Google Scholar]
- 26.Loi CM, Randinitis EJ, Vassos AB, Kazierad DJ, Koup JR, Sedman AJ. Lack of effect of type II diabetes on the pharmacokinetics of troglitazone in a multiple-dose study. J Clin Pharmacol. 1997;37:1114–20. doi: 10.1002/j.1552-4604.1997.tb04295.x. [DOI] [PubMed] [Google Scholar]
- 27.Cheng KL, Nafziger AN, Peloquin CA, Amsden GW. Effect of grapefruit juice on clarithromycin pharmacokinetics. Antimicrob Agents Chemother. 1998;42:927–29. doi: 10.1128/aac.42.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris DL, De Souza A, Jones JA, Morgan WE. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur J Clin Microbiol Infect Dis. 1991;10:859–61. doi: 10.1007/BF01975842. [DOI] [PubMed] [Google Scholar]
- 29.Errin L, Lagow D, Carson D. Synergistic stimulation of MUC1 expression in normal breast epithelia and breast cancer cells by interferon-γ and tumor necrosis factor-α. J Cell Biochem. 2002;86:759–72. doi: 10.1002/jcb.10261. [DOI] [PubMed] [Google Scholar]
- 30.Griffin MD, Lutz WH, Phan VA, Bachman LA, McKean DJ, Kumar R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun. 2000;270:701–8. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 31.Gladue RP, Bright GM, Isaacson RE, Newborg MF. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989;33:277–82. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahnke K, Guo M, Lee S, et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–84. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan AA, Slifer TR, Araujo FG, Remington JS. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents. 1999;11:121–32. doi: 10.1016/s0924-8579(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 34.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–15. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 35.Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–81. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 36.Taylor PA, Ehrhardt MJ, Lees CJ, et al. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD) Blood. 2007;110:3480–8. doi: 10.1182/blood-2007-05-087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–79. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 39.Fujita S, Seino K, Sato K, et al. Regulatory dendritic cells act as regulators of acute lethal systemic inflammatory response. Blood. 2006;107:3656–64. doi: 10.1182/blood-2005-10-4190. [DOI] [PubMed] [Google Scholar]
- 40.Sato K, Yamashita N, Baba M, Matsuyama T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood. 2003;101:3581–89. doi: 10.1182/blood-2002-09-2712. [DOI] [PubMed] [Google Scholar]
- 41.Fujita S, Sato Y, Sato K, et al. Regulatory dendritic cells protect against cutaneous chronic graft-versus-host disease mediated through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2007;110:3793–803. doi: 10.1182/blood-2007-04-086470. [DOI] [PubMed] [Google Scholar]
- 42.Fujita S, Yamashita N, Ishii Y, et al. Regulatory dendritic cells protect against allergic airway inflammation in a murine asthmatic model. J Allergy Clin Immunol. 2008;121:95–104. doi: 10.1016/j.jaci.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 43.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 44.An H, Yu Y, Zhang M, et al. Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology. 2002;106:38–45. doi: 10.1046/j.1365-2567.2002.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JY, Kim HY, Lee JY, et al. Macrolide-affected Toll-like receptor 4 expression from Helicobacter pylori-infected monocytes does not modify interleukin-8 production. FEMS Immunol Med Microbiol. 2005;44:171–6. doi: 10.1016/j.femsim.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Sato S, Nomura F, Kawai T, et al. Synergy and cross-tolerance between Toll-like receptor (TLR)2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]


